Abstract
Hippocampal place cells are selectively active when a rat occupies restricted locations in an environment, and head direction cells fire selectively when the rat’s head is pointed in a particular direction in allocentric space. Both place cells and head direction cells are usually coupled, and they are controlled by a complex interaction between external landmarks and idiothetic cues. Most studies have investigated this interaction by rotating the landmarks in the environment. In contrast, a recent study translated the apparatus relative to the landmarks in an environment and found that most place cells maintained the same preferred location on the apparatus regardless of the location of the apparatus in the room. Because head direction cells are insensitive to the rat’s location in an environment, the distal landmarks may influence the place field firing locations primarily by controlling the bearing of the head direction cell system. To address this question, ensembles of CA1 place cells and head direction cells of the anterior thalamus were recorded simultaneously, as a rectangular or circular track was moved to different locations in a room with distinct visual landmarks. Most place cells maintained their firing fields relative to the track when the track was translated, and head direction cells maintained the same preferred firing direction. When the distal landmarks were rotated around the track, the firing fields of place cells and the preferred directions of head direction cells rotated with the cues. These results suggest that the precise firing locations of place cells are controlled by an interaction between local and idiothetic cues, and the orientation of the CA1 ensemble representation relative to the distal landmarks may be controlled indirectly by the distal landmarks’ influence over the bearing of the head direction cell system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hippocampus is a brain structure that plays a critical role in navigation and in episodic memory (O’Keefe and Nadel 1978; Vargha-Khadem et al. 1997; Tulving and Markowitsch 1998). In rodents, the most salient correlate of hippocampal neuronal firing is the spatial location of the animal, leading O’Keefe and Nadel (1978) to suggest that the hippocampus was the locus of a “cognitive map” of the environment, used for spatial navigation and as a framework to organize the items and events of experience. Nonspatial correlates of hippocampal pyramidal cells have also been reported, and recent results have suggested that such nonspatial correlates may be superimposed on the spatial firing of such cells (O’Keefe 1976; Wiebe and Staubli 1999; Moita et al. 2003). Thus, understanding the sources of information that generate the properties of hippocampal place cells may facilitate an understanding of both navigational and episodic-memory functions of the hippocampus.
Early reports on the properties of place cells demonstrated that their firing fields (place fields) were controlled by the rotation of salient landmarks in the periphery, in that rotation of the distal landmarks caused the preferred firing locations of the place cells to rotate correspondingly (O’Keefe and Conway 1978; Muller and Kubie 1987). In contrast, the local cues on the behavioral apparatus had little or no influence on the place fields, as the place fields maintained the same preferred location relative to the external laboratory landmarks when the apparatus was rotated in between recording sessions. In these experiments, the local cues on the behavioral apparatus may not have been as salient as the peripheral landmarks. In subsequent experiments with more salient local cues, place fields were shown to be controlled by the local cues as well as by the peripheral landmarks (Young et al. 1994; Gothard et al. 1996; Shapiro et al. 1997; Save and Poucet 2000; Zinyuk et al. 2000; Knierim and McNaughton 2001; Brown and Skaggs 2002; Knierim 2002a). Moreover, in some cases, the place fields appeared to be controlled by idiothetic (self-motion) cues that arise from the vestibular system, motor efference copy, optic flow, or other sources of input generated as the animal moves about its environment (Sharp et al. 1995; Wiener et al. 1995; McNaughton et al. 1996; Knierim et al. 1998; Knierim 2002b; Stackman et al. 2002). Thus, the preferred firing locations of hippocampal place cells are controlled by a complex interaction between idiothetic cues and external sensory cues, both local to the animal’s recording environment and in the periphery.
An important input into the hippocampus derives from the head direction cell system. Head direction cells fire selectively when the animal’s head is pointed in a particular direction in allocentric space (Ranck 1985; Taube et al. 1990; Taube 1998). They are sensitive to direction only in the yaw axis (horizontal plane); head direction in the pitch or roll axes (±90°) does not affect their firing. Head direction cells and place cells are usually coupled, in that a rotation of the head direction cell’s tuning curve relative to the external environment will either cause an equivalent rotation of place fields of the hippocampus or a remapping of the place cell representation (Knierim et al. 1995). Like place cells, head direction cells are controlled by a complex interaction between external landmarks and idiothetic cues (Taube and Burton 1995; Blair and Sharp 1996; Goodridge et al. 1998; Knierim et al. 1998; Zugaro et al. 2003). Lesions to brain areas that contain head direction cells cause both navigation deficits and also make the firing locations of hippocampal place cells less stable (Taube et al. 1992; Warburton et al. 2000; Calton et al. 2003).
Most studies of the influence of idiothetic cues and external landmarks on the firing of place cells and head direction cells have rotated the landmarks in the environment. Few studies have addressed the effects of translating the apparatus relative to the peripheral cues in an environment. Anecdotal observations have suggested that place cells can be bound to the local apparatus or to the external laboratory framework in such cases, but these findings were not analyzed quantitatively (O’Keefe 1979; O’Keefe and Burgess 1996; Lever et al. 2002). Knierim and Rao (2003) explicitly addressed this question by recording hippocampal place cells on a rectangular or circular track that was moved between sessions to different locations in the three-dimensional space of the laboratory. They found that the large majority of place fields were bound to the track when it was moved, with a minor influence of the relative location of the distal landmarks on the firing locations of the place fields. When these landmarks were rotated around the apparatus, however, the place fields rotated correspondingly, in agreement with the classic literature on the effects of cue rotations. Because it is generally accepted that head direction cells are insensitive to the animal’s location in an environment, but are controlled by the rotation of distal landmarks (Ranck 1985; Taube et al. 1990; Taube 1998), Knierim and Rao (2003) suggested that distal landmarks may not directly define place field firing locations, but may exert strong control over them indirectly by controlling the bearing of the head direction cell system (McNaughton et al. 1996; Muller et al. 1996; O’Keefe and Burgess 1996; Knierim et al. 1998; Burgess et al. 2000). Head direction cells were not recorded in that experiment, however, and it is conceivable that they might have changed their preferred firing directions in between recording sessions, as has been reported in other experiments (Knierim et al. 1995; Golob et al. 2001). For example, Knierim et al. (1995) showed that when a rat was repeatedly disoriented before a recording session, head direction cells could change their preferred directions relative to the external landmarks even when the recording environment was stable. Similarly, Golob et al. (2001) reported unexpected changes in the preferred direction of head direction cells in ~25% of trials in which the rats were introduced into a recording chamber from different starting points when performing a spatial reference memory task. If head direction cell tuning curves were similarly unstable in the experiment of Knierim and Rao (2003), then the lack of effect of relative translation of the distal landmarks on place cells could not be attributed to the head direction cell system. Thus, the present experiment recorded simultaneously from place cells of CA1 and head direction cells of the anterior thalamus to test whether the two cell types were coupled in this experimental manipulation. As predicted, head direction cells and place cells were both controlled by rotation of the distal landmarks; when the track was translated to different locations in the room, head direction cells maintained stable tuning curves and place cells generally maintained their preferred firing locations in the track-based, rather than room-based, coordinate frame.
Materials and methods
Subjects
Five male Long-Evans rats, aged 5 months (weighing approximately 600 g at arrival), were housed individually on a 12:12 h reversed light-dark cycle and were maintained at 80–90% of their free-feeding weights. Recordings were performed during the dark portion of the light-dark cycle. Animal care, surgical procedures, and euthanasia were performed in accordance with National Institutes of Health (NIH) and University of Texas Health Science Center at Houston Institutional Animal Care and Use Committee (IACUC) guidelines.
Recording techniques
Under surgical anesthesia, a custom-built recording drive allowing the independent manipulation of 20 electrodes was implanted over the right hemisphere. An anterior bundle contained 11 tetrodes aimed at the anterior dorsal nucleus (ADN) of the thalamus and a posterior bundle contained 7 tetrodes aimed at the CA1 layer of the hippocampus. Each bundle also contained a reference electrode for differential recording. During surgery, electrode recordings were made to identify the location of the septal pole of the hippocampus; the front of the anterior bundle was positioned 0.3 mm anterior to that point and 1.3–1.6 mm from the midline. This procedure was a more reliable method to center the electrode array over the ADN than relying on position relative to bregma. The posterior bundle was always located 1.6 mm posterior and 0.3 mm lateral to the center of the anterior bundle. After 7 days of postsurgical recovery, the tetrodes were slowly advanced over the course of several days as the rat sat quietly in a towel-lined dish next to the recording electronics and computer outside the behavioral room. The tetrodes of the posterior bundle were lowered to the CA1 pyramidal cell layer by monitoring the depth profile of hippocampal sharp waves (Buzsaki 1986) and observing the increase of multiunit activity. Head direction cell activity was monitored by rotating the rat passively on a turntable after each tetrode adjustment, listening for the characteristic directional tuning of these cells. Recordings were performed with the Cheetah Data Acquisition System (Neuralynx, Tucson, AZ). Neural signals were amplified (2–5K), filtered (600 Hz to 6 kHz), digitized at 32 kHz, and stored on a PC computer. The headstage had a circular array of LEDs (five red LEDs in front and five blue LEDS in back) and a boom arm that extended two green LEDS 15 cm behind the headstage. The output of a color CCD camera (model 1300; Cohu, San Diego, CA) mounted on the ceiling was captured by a video frame grabber (DT3120; Data Translation, Marlboro, MA) at 30 Hz. At each frame, the position of the rat was defined as the center of mass of all blue and red pixels (the LEDs over the rat’s head) and the head direction was defined as the angle between the center of mass of the blue and red pixels and the center of mass of the green pixels (the LEDs on the boom arm).
Training
Two types of apparatus were used in the present experiments (Fig. 1): a rectangular track (10-cm-wide arms, 45×50 cm outside dimensions) and a circular track (45 cm inner diameter, 63 cm outer diameter). The tracks stood on blocks 13 cm above a small rectangular table (61×51 cm). The tracks, which contained no salient surface cues, were wiped clean with 70% alcohol before the first recording session of each day. Lighting was provided by a single 25-W bulb centered on the ceiling. A circle of black curtains (275 cm diameter) reaching from ceiling to floor surrounded the track. Attached to the curtain, the visual cues consisted of a triangular, dotted piece of cardboard, a striped cardboard circle, a piece of Styrofoam, a string of three plastic saucers, and a large white paper drape hung on the curtain. A hula-hoop was placed on the floor leaning against the white paper drape. A white noise generator was placed directly beneath the small table on which the tracks stood, to mask external sounds. During training sessions, the rat was carried directly into the room on a pedestal, the headstage was connected to the recording cable, and the animal was placed on the track at a random starting point. The task consisted of running clockwise on the track for chocolate sprinkles placed at arbitrary locations on the track (about two rewards per lap), such that no local areas of the track were preferentially associated with the reward. The experimenter moved around the track frequently so as not to become a stable landmark for the rat. Occasional attempts by the rat to move counterclockwise were discouraged by blocking its progress with a piece of cardboard.
Experimental protocol
Each day before the behavioral sessions, baseline data were collected from the rat during sleep or awake immobility for 20–30 min next to the computer outside the behavior room. These data were compared with a baseline session at the end of the day to help assess the overall recording stability. After the baseline session, the rat was placed in a covered box and, after 30 s, was taken in the box on a brief walk in the computer room and around the track, in order to disrupt the rat’s ability to maintain a strong sense of direction between the behavioral area and the external environment (Knierim et al. 1995; Jeffery and O’Keefe 1999; Knierim and Rao 2003). The rat was then placed on a pedestal kept in the center of the track, the headstage was connected to the recording cables, and the rat was placed on the track at a random starting point while the pedestal was removed. After 15 laps on the track, the rat was placed back on the pedestal, the cables were disconnected, and the rat was placed back in the covered box and again taken on a brief random walk. Standard sessions were performed with the track in the center of the room, with the distal cues positioned as during training. For the rectangular track sessions, the recording sequence was: (1) standard 1, (2) east translation (40–58 cm from the standard location), (3) west translation (76-112 cm from the east location), (4) standard 2, (5) north translation (23–28 cm from the standard location), (6) south translation (48–76 cm from the north location), and (7) standard 3. For the circular track sessions, the recording sequence was as follows: (1) standard 1, (2) east translation (36–56 cm from the standard location), (3) west translation (67–104 cm from the East location), (4) standard 2, (5) 45° clockwise rotation of distal landmarks, (6) 45° further clockwise rotation of distal landmarks, and (7) standard 3. Each rat underwent 1 day of the rectangular track protocol followed by 1 day of the circular track protocol.
Data analysis
The tetrode allows the isolation of single units based primarily on the relative amplitudes of signals recorded simultaneously at four slightly different locations. Additional waveform characteristics, such as spike width, are also used. Waveform characteristics were plotted as a scatter plot of one of the electrodes versus another. Individual units formed clusters of points on such scatter plots, and the boundaries of these clusters were defined with the use of a custom interactive program running on a PC workstation. Isolation quality of the cell was rated on a subjective scale of 1 (very well isolated) to 4 (marginally isolated), based on the size of the waveforms relative to background and on the closeness and degree of potential overlap between neighboring clusters. These ratings were made completely independently of the place field qualities of the cell or of its response to the cue manipulations. All cells rated “marginally isolated” were excluded from analysis.
Head direction cell tuning curves were generated by dividing the number of spikes fired when the rat faced a particular direction (in bins of 10°) by the total amount of time the rat spent facing that direction. For CA1 place cells, the circular and rectangular tracks were linearized to generate one-dimensional firing rate arrays by dividing the track into equally sized position bins and, for each bin, dividing the number of spikes fired by the amount of time the rat occupied that bin. Spikes that occurred when the rat’s head was positioned off the track were excluded, as these off-track positions were not sampled consistently across sessions. Similarity of the head direction cell tuning curves and the one-dimensional place field rate maps across sessions was quantified by calculating the Pearson product-moment correlation between the corresponding firing rate arrays of the two sessions. Correlations were computed if the CA1 cell met the following place field criteria for at least one of the two sessions being correlated: (1) the spatial information score was ≥0.9 bit; (2) the cell fired ≥50 spikes, a minimum number to ensure reliability of the spatial information score; and (3) the statistical significance of the information score was P<.01 (Skaggs et al. 1993, 1996). Each rat experienced 2 days of recording, the first day on the rectangular track and the second day on the circular track. Data were analyzed separately for the rectangular track and for the circular track. For statistical comparison, the correlation scores (r) were converted to a normal distribution by
and analysis of variance (ANOVA) tests were performed on the transformed z-scores.
Rotation analysis
To compare how the directional tuning curves or place fields of individual cells may have rotated between sessions, the Pearson product-moment correlation between a cell’s firing rate arrays in the two sessions was measured, and then the firing rate bins of the second session were shifted by one bin (equal to a 5° shift for CA1 or a 10° shift for head direction cells). The firing rate array of the first session was correlated with the shifted array of the second session, and then the second array was again shifted by 5°/10°. This was repeated 71 times for place cells and 35 times for head direction cells, and the shifted angle that produced the highest correlation was taken as the amount that the place field or directional tuning curve had shifted between the two sessions. The same criteria as above were applied to determine which place cells to include in each correlation pair. No statistical tests were performed on individual cells to determine if the highest correlation values were significant. Instead, circular statistics were employed to evaluate the mean rotation angles of the sample (Zar 1999).
Results
Multiple single units from CA1 and ADN were simultaneously recorded in four rats. In one additional rat, data were obtained only from the thalamus. Histological analysis revealed that all place cell recording sites were localized to the CA1 layer of the hippocampus (Fig. 2A). For four of the five rats, head direction cell recording sites were localized to the ADN of the thalamus (Fig. 2B). For one rat, the tetrodes advanced at a slight angle and missed the ADN; deeper in the thalamus, however, head direction cells were recorded in a region near the border of the anterior ventral (AVN) and the ventral anterior (VAN) nuclei (Fig. 2C). For circular track sessions, 30 place cells from CA1, 11 head direction cells from ADN, and 2 head direction cells from AVN/VAN were analyzed in detail. For rectangular track sessions, 35 place cells from CA1, 10 head direction cells from ADN, and 2 head direction cells from AVN/VAN were analyzed in detail. Head direction cells from the AVN/VAN region have not been reported, to our knowledge. The two cells recorded from this region had spike widths (measured from peak to valley) of 156 and 250 μs, mean firing rates of 7.8 and 7.4 Hz, maximum firing rates (at preferred direction) of 55.5 and 45.3 Hz, and the width of their directional tuning curves at half-height were 45° and 50°, respectively.
Circular track
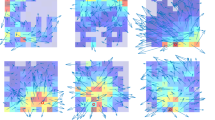
Figure 3 shows the CA1 place fields and head direction cell tuning curves for six simultaneously recorded neurons when the circular track was translated in the environment (sessions 1–4) and when the distal landmarks were rotated around the apparatus (sessions 5–7). The first session was a standard session with the circular track placed in the center of the room, followed by sessions in which the track was moved to the east, moved to the west, and then back to the standard location. The CA1 place fields were completely bound to the track coordinates, regardless of the location of the track in the room. The head direction tuning curves also maintained their preferred firing directions, regardless of the translation of the track. When the distal landmarks were rotated around the track, however, both place fields and head direction tuning curves rotated with the cues, demonstrating that both sets of cells were sensitive to the orientation of the set of distal landmarks.
Representative examples of effect of circular track translation and distal cue rotation. Simultaneously recorded ensembles of CA1 place fields (cells 1–3) and head direction (HD) tuning curves (cells 4–6) remained bound to the track regardless of the translation (sessions 1–4) and rotated their place fields or preferred directions along with the 45° rotations of the distal landmarks (sessions 5–7). The place cell firing rate maps are plotted with a gray scale, with the lightest gray signifying locations that the rat visited in which the cell did not fire and black signifying the pixel with the maximum firing rate. The maximum rates for each cell and each session are as follows: cell 1 (95–10 2.1 of Table 3)—session 1: 19.25 Hz, session 2: 25.25 Hz, session 3: 19.6 Hz, session 4: 24.14 Hz, session 5: 18.61 Hz, session 6: 16.26 Hz, session 7: 17.04 Hz; cell 2 (95–10 4.1 of Table 3)—session 1: 7.4 Hz, session 2: 0.85 Hz, session 3: 2.08 Hz, session 4: 5.3 Hz, session 5: 5.1 Hz, session 6: 3.2 Hz, session 7: 4.5 Hz; cell 3 (95–10 5.1 of Table 3)—session 1: 7.8 Hz, session 2: 7.06 Hz, session 3: 12.34 Hz, session 4: 15.48 Hz, session 5: 8.7 Hz, session 6: 11.68 Hz, session 7: 17.47 Hz. Head direction cells are plotted in polar coordinates. The axes for each cell are scaled as follows: cell 4 (95–10 9.1 of Table 3) ±40 Hz; cell 5 (95–10 11.1 of Table 3) ±26 Hz; cell 6 (95–10 13.1 of Table 3) ±54.5 Hz
The similarity of place field locations and head direction tuning curves across sessions was quantified by calculating the correlation of firing rate arrays between sessions. Figure 4 shows the distribution of correlations for different session pairs. For statistical analysis, the correlations were transformed to a normal distribution (see Materials and methods) in order to run multifactor ANOVAs. We analyzed the track translation sessions (Fig. 4A) separately from the cue-rotation sessions (Fig. 4B). For track translations, there was a significant main effect of cell type [CA1 vs head direction (HD), F(1,98)=148.22, P<.0001] but no significant effect of session translation and no significant interaction. These results indicate that the head direction tuning curves were more highly correlated between sessions than were the linearized place field arrays, but that neither type of cell was affected by the location of the track in the room (standard vs east vs west). Given the known variability in place field firing properties on a trial-by-trial basis (Fenton and Muller 1998) and the various forms of remapping that characterize hippocampal representations but not head direction representations (Muller et al. 1996), the significant main effect between place cells and head direction cells is not surprising. In contrast to the translation sessions, the cue rotation sessions (Fig. 4B) produced main effects of both cell type (CA1 vs HD, F(1,101)=56.34, P<.0001) and rotation (standard vs first rotation vs second rotation, F(2,101)=222.32, P<.0001), as well as a significant interaction between these factors (F(2,101)=40.27, P<.0001). Thus, the between-session correlations of both place fields and head direction tuning curves were significantly affected by the cue rotations.
Spatial correlation frequency distributions of CA1 place fields and head direction tuning curves between circular track sessions. For each cell, the correlation of its place field or head direction tuning curve was calculated in track-based coordinates between two sessions. A Effect of circular track translation. The median correlations between the two standard sessions (STD1–STD2) before and after the east–west translation were 0.68 (CA1) and 0.98 (HD); between the standard 1 and east sessions were 0.68 (CA1) and 0.98 (HD); and between the east session and the west session were 0.73 (CA1) and 0.97 (HD). These results indicate that most of the place cells and head direction cells maintained the same place fields and preferred directions, although a minority of them changed between the sessions. B Effect of distal cue rotations. The median correlations between the standard session (STD2) and the first 45°-rotation session were 0.40 (CA1) and 0.45 (HD); and between the standard session (STD2) and the second 45°-rotation session were 0.10 (CA1) and 0.16 (HD). These results indicate that most of the place fields and head direction tuning curves rotated along with the cues (see Fig. 5). However, the median correlations between STD2–STD3 was 0.80 (CA1) and 0.98 (HD), indicating that the place cells and head direction cells returned to their original preferred locations and directions when the cues returned to the original bearing
The cue rotation sessions produced significant decreases in the between-session correlations of place fields and head direction tuning curves because of the simple fact that the place fields and head direction tuning curves rotated with the cues. Figure 5 shows the amount that each place field or head direction tuning curve rotated after the first and second 45°-rotation sessions. The mean rotation angle between the standard to first rotation was 30.08° for place fields and 36.17° for head direction cells; the mean rotation angle between first rotation to second rotation was 44.30° for place fields and 44.05° for head direction cells, showing that the distal cues controlled the firing of place fields and head direction cells after the rotation. In some cases there was a partial remapping of place fields after the cue rotations, resulting in place field rotations that were not tied to the cue rotations. Regardless of whether the cue rotation produced a corresponding rotation of the place fields and head direction tuning curves or partial remapping of place fields, these results demonstrate that the cells were influenced by rotations of the distal landmarks and controlled by them in ways similar to those reported in the literature (O’Keefe and Conway 1978; Muller and Kubie 1987; Taube et al. 1990).
Amount of rotation by each place field or head direction tuning curve following distal cue rotation. Place cells are grouped in 5° bins and head direction cells are grouped in 10° bins. The mean rotation angles between the first 45° rotation and the standard session preceding it were 30.08° (CA1) and 36.17° (HD); the mean angles between first 45° rotation and a further 45° rotation were 44.30° (CA1) and 44.05° (HD). These results demonstrate that the cells on average rotated their fields and directions in the same direction as the distal landmarks. The 0° reference for the second rotation is plotted relative to the 45° reference of the previous session, to illustrate how the place fields and head direction tuning curves rotated relative to the laboratory frame of reference. Each point refers to a single cell
The correlation and rotation analyses of CA1 place fields produced results that were similar to those reported previously (Knierim and Rao 2003), an important replication to allow the legitimate comparison with the results from head direction cells in the present study. For a complete comparison with the prior study, we also categorized place cells according to whether they met criteria for having a place field in both sessions (maintain), having a place field after translation only (gain), having a place field before translation only (lose), or having no place field in either of the sessions (no field) under comparison (Table 1). The CA1 cells that met the place field criteria in both sessions (maintain) had a median correlation of 0.82, showing that the firing locations were highly correlated on the track. In only one case was the median correlation <0.4. This analysis complements the correlation analysis, which can be insensitive to certain effects, such as changes in the place field firing rate. That is, a strong place cell in one session may fire only a few spikes in another session but, if the spikes are in the same location, the correlation between the two sessions will be high. This analysis also provides a more complete picture of the varied responses of place fields that cannot always be captured by the correlation measure alone. The exact numbers in each category are not of particular interest, as this type of unit classification will unavoidably lead to arbitrary assignments (for example, because the cut-off for being defined as a significant place field was an information score of 0.90, a cell that had a score of 0.89 in one session and 0.90 in the other session will be classified as having a place field in only one session, even though they are marginally different). Rather, the important point is whether the relative number of cells in each category changes between the standard sessions and the translated sessions. A χ2 analysis demonstrated that there was no effect of translation on the categorization scheme (χ2=0.935, n.s.). Thus, this analysis supports the correlation analysis and replicates the original finding of Knierim and Rao (2003).
Rectangular track
Figure 6 shows the CA1 place fields and head direction tuning curves for six simultaneously recorded cells as the rectangular track was translated to five different locations in the environment. The first session on the rectangle was a standard session, in which the track was located in the center of the room at the same position occupied during the training trials. In subsequent sessions, the track was translated to the east, to the west, back to the standard location, to the north, to the south, and back to the standard location. The CA1 place fields fired on the same locations on the track regardless of where the track was located in the room. Similarly, the preferred firing direction of the head direction cells remained mostly unaltered regardless of the location of the rectangular track in the room.
Representative examples of effect of rectangular track translation. Simultaneously recorded ensembles of CA1 place fields (cells 1–3) and head direction tuning curves (cells 4–6) remained bound to the track regardless of the translation (sessions 1–7). The place cell firing rate maps are plotted with a gray scale, with the lightest gray signifying locations that the rat visited in which the cell did not fire and black signifying the pixel with the maximum firing rate. The maximum rates for each cell and each session are: cell 1 (113–09 1.1 of Table 4)—session 1: 14.54 Hz, session 2: 6.39 Hz, session 3: 2.23 Hz, session 4: 3.5 Hz, session 5: 14.95 Hz, session 6: 5.8 Hz, session 7: 11.35 Hz; cell 2 (113–09 1.2 of Table 4)—session 1: 14.43 Hz, session 2: 10.2 Hz, session 3: 13.25 Hz, session 4: 16.23 Hz, session 5: 12.72 Hz, session 6: 12.13 Hz, session 7: 15.23 Hz; cell 3 (113–09 1.4 of Table 4)—session 1: 15.95 Hz, session 2: 10.06 Hz, session 3: 8.4 Hz, session 4: 12.68 Hz, session 5: 5.08 Hz, session 6: 4.52 Hz, session 7: 5.66 Hz. Head direction cells are plotted in polar coordinates. The axes for each cell are: cell 4 (113–09 14.1 of Table 4) ±69 Hz; cell 5 (113–09 15.1 of Table 4) ±100.5 Hz; cell 6 (113–09 11.1 of Table 4) ±82.2 Hz
The distribution of correlations for the rectangular track session was quantified (Fig. 7) similarly to the circular track sessions. Because all session types were translation manipulations, a multifactor ANOVA was run on the transformed correlations for all conditions shown in Fig. 6. As with the circular track, there was a significant main effect of cell type (CA1 vs HD, F(1,217)=157.07, P<.0001). In contrast to the circular track, however, there was a significant main effect of session type (F(5,217)=5.38, P<.0001), with no significant interaction between the two factors. It is clear from Fig. 7 that the lowest correlations came when the track was shifted from east to west, the largest single shift between sessions. These data are similar to those reported by Knierim and Rao (2003), although the difference did not reach statistical significance in that study. Even though the correlations tended to be lower in the east–west translations, the majority of place fields still had high correlations in those sessions. Finally, to test for differences in correlations between the circular and rectangular track sessions, a three-way ANOVA on the transformed correlations comparing cell types (CA1 or HD) versus apparatus (circle or rectangle) versus sessions (standard 1–standard 2, standard 1–east, east–west) revealed no significant main effect of apparatus (P<.54). The main effects of cell types (P<.0001) and sessions (P<.002) were highly significant. No interactions were significant, although the apparatus × cell type interaction approached significance (P<.09). Thus, for the translation session types that were run with both tracks, there was no significant difference between circular track and rectangular track sessions in the responses of the place cells and head direction cells.
Spatial correlation frequency distributions of CA1 place fields and head direction tuning curves between rectangular track sessions. For each cell, the correlation of its place field or head direction tuning curve was calculated in track-based coordinates between the two sessions. The median correlations between two standard sessions (STD1–STD2) before and after the east–west translation were 0.81 (CA1) and 0.98 (HD); between standard 1 and east session were 0.85 (CA1) and 0.98 (HD); between east session and west session were 0.73 (CA1) and 0.94 (HD); between the north session and the standard session (STD2) preceding it were 0.90 (CA1) and 0.98 (HD); between the north and the south sessions were 0.82 (CA1) and 0.94 (HD); and between STD2–STD3 were 0.87 (CA1) and 0.97 (HD). These results indicate that most of the place cells and head direction cells maintained the same place fields and preferred directions, although a minority of them changed between sessions
A categorization analysis, similar to the circular track data, was performed on the rectangular track data. Table 2 shows the categorization of CA1 cells. The CA1 cells that met the place field criteria in both sessions (maintain) had a median correlation of 0.88, showing that the firing locations were highly correlated on the track. In only two cases was the median correlation <0.4. A χ2 analysis revealed no significant effect of the translations on the distribution of response types (χ2=10.22, n.s.).
Consistency of place field positions on track in different sessions
If a place field were tied to a restricted position in the room based on the external landmarks, the place cell should fire only when the track occupied that position. To test this, the number of track positions in which the cell fired at the same location on the track was counted for each place field (Fig. 8). The circular track occupied three different positions in the room during the experiment (standard, east, and west). A cell that fired at the same location on the track in all three room positions would thus get a score of 3, whereas a cell that fired at the same location only at two room positions would get a score of 2. Similarly, the rectangular track occupied five positions (standard, east, west, north, and south), so a cell that fired at the same location on the track in all room positions would get a score of 5, a cell that fired at the same location on the track in only 4 of the 5 sessions would get a 4, and so on. If a cell had more than one subfield on the track, each subfield was scored separately. For the circular track 78% of the fields had a score of 3, as their cells fired at the same location on the track for all the room positions, and 96% of the cells had a score of 2 or higher. For the rectangular track, 71% of the fields had a score of 5, as their cells fired at the same location on track for all the five positions in the room, and 91% of the fields had score of 3 or higher. Thus, in agreement with Knierim and Rao (2003), even when a minority of place cells remapped the track in between recording sessions (Tables 1, 2, gain cells), the new place fields were not bound to a single position in the room, but tended to become incorporated into the track-bound place field representation.
Number of positions in the room where each place cell fired at the same location on the track. If a cell had multiple subfields, each subfield was counted separately. The circular track occupied three room positions (standard, east, and west). If a cell fired at the same location on the track for all three room positions, it received a score of 3; if it fired in only one of the three track positions, it received a score of 1. The rectangular track occupied five room positions (standard, east, west, north, and south). Thus, if a cell fired at the same location on the track for all five room positions, it received a score of 5. A majority of place cells fired at the same location on the track, even when the track was in several different positions in the room
Rotation analysis of place fields and head direction tuning curves
Although the correlation histograms of Figs. 4 and 7 show that place fields and head direction tuning curves were highly correlated between translation sessions, this analysis may be insensitive to small changes in the place fields or tuning curves of the neurons. It is of interest to know if such changes occur, and whether the changes are consistent within a simultaneously recorded ensemble. A rotation analysis was performed on the place field plots and head direction tuning curves to find the angle at which the firing rate arrays for each cell were maximally correlated. Tables 3 and 4 present the data for the circular track and rectangular track sessions, respectively. CA1 cells were included for a given comparison only if the cell met the place field criteria for each session of the pair; thus, each cell did not necessarily contribute a data point for every session pair. The head direction cells maintained the same direction (±10°) on most translation sessions. (Because the tuning curves were measured in 10° bins, a shift of 10° is not necessarily significant, but may result from binning artifacts.) There was a greater amount of variability in the rotation of the place fields, but the place fields of an ensemble did not rotate on average (i.e., fields that rotated slightly clockwise were counterbalanced by fields that rotated slightly counterclockwise). A small number of place fields rotated by large amounts; these are presumed to be cells that remapped the track, developing a new place field at an arbitrary location. In the one translation session in which the head direction ensemble rotated significantly counterclockwise (Table 4, rat 113–09, east–west translation), 5/8 place fields also rotated similarly. (Although this data set contributed to the low correlations seen in the east–west frequency distribution of Fig. 7, the correlations were low even when this data set was excluded from the analysis.) To quantify the variability of rotation angles in individual ensembles, the circular standard deviation of the rotation angles was calculated for each place cell and head direction cell ensemble (Zar 1999). For place cells, the average standard deviation for an ensemble was 11.14° and for head direction cells the average standard deviation was 2.57°. A Mann-Whitney test showed that the median standard deviation for place cell ensembles was significantly higher than that for head direction cell ensembles (P<.0001).
If single cues had strong control over the place fields and head direction tuning curves, this might have led to systematic changes in the bearings of these properties between sessions. For example, if the linear sides of the rectangular track were a potent directional cue, the head direction cells may have been less variable in the rectangular track sessions versus the circular track sessions. Similarly, if one of the distal landmarks dominated (for example, the cue on the north wall), then the preferred direction of head direction cells that fired when the rat faced that cue might have systematically changed as the track moved east or west relative to that cue. Tables 3 and 4 show no obvious trends to suggest any difference in the rotation angles between the circular track and rectangular track sessions, or between the various translation sessions, however. This result suggests that the bearing of the place fields and head direction tuning curves is controlled by the distal cue set as a whole, rather than by any single cue.
Reorientation of preferred firing direction
In most sessions, the head direction cell tuning curves were stable throughout the whole session. In one rat (rat 86), however, the head direction tuning curves rotated during the first few laps in 2 of the 14 sessions (one circular track session and one rectangular track session); both of these cells were recorded from the AVN/VAN border. Figure 9A shows the first case, when the rectangular track was located in the south position in the room. Both head direction cells that were recorded rotated their tuning curves during the first four to five laps of that session. In each case, the tuning curve was initially oriented approximately 180° away from the normal preferred direction of the cells in the other six sessions. The tuning curves rotated smoothly (although not necessarily at constant velocity) until they realigned with their normal preferred directions by lap 5, after which they remained stable. The graph at the far right shows the position of the rat on the track as a function of time when the cell fired. Because the rat ran unidirectionally on the track, the position is highly correlated with head direction, and these plots show clearly the incremental changes in firing directions of the cells over the first part of the session. Figure 9B shows similar behavior of the same two cells recorded the next day on the circular track, when the track was located in the east location in the room. (On this day, the preferred directions of the cells were shifted slightly counterclockwise from the preferred directions of the previous day.) Unfortunately, no place cells were recorded from this rat in these sessions, so we are unable to determine whether place fields rotated along with the head direction tuning curves. These data demonstrate that the head direction tuning curves can become temporarily decoupled from the external landmarks, but still maintain the same directions relative to other head direction cells, and can change their preferred directions over time until they become realigned with the external landmarks (Knierim et al. 1998).
Reorientation of head direction tuning curves in two simultaneously recorded head direction cells from the border region of AVN and VAN, during rectangular (A) and circular (B) track sessions. The tuning curves were initially oriented approximately 180° away from the normal preferred direction of the other six sessions. They rotated smoothly counterclockwise until they realigned with their normal preferred directions by lap 5, after which they remained stable. The graph at the far right shows the position of the rat on the track as a function of time when the cell fired. Because the rat ran unidirectionally on the track, the position is highly correlated with head direction, and these plots show the incremental changes in firing directions of the cells over the first part of the session. The axes for each cell are scaled as follows: A cell 1 (86–13 9.1 of Table 4) ±180 Hz; cell 2 (86–13 8.1 of Table 4) ±90 Hz; B cell 1 (86–14 9.1 of Table 3) ±135 Hz; cell 2 (86–14 8.1 of Table 3) ±52.5 Hz
Discussion
Place cells and head direction cells have been shown to be a coupled system, in that rotation of the head direction cell tuning curves is accompanied by equivalent rotations of the firing fields of simultaneously recorded place cells or remapping of the place field representation (Knierim et al. 1995, 1998). These rotations can be caused by rotations of the peripheral landmarks in an environment, but they can also occur spontaneously, as both sets of cells become decoupled from all external sensory cues while remaining closely coupled to each other. The present paper demonstrates the close coupling between these systems when there is a relative translation between the behavioral apparatus and the peripheral landmarks, rather than a rotation. The majority of place fields maintained their firing locations on the track regardless of the position of the track in the room, showing their insensitivity to the configurations of distal landmarks that were available to the animal at different places in the room. Similarly, head direction cell tuning curves were also largely unaffected by the apparatus translations, in agreement with prior reports that head direction tuning curves were independent of the animal’s location within an environment (Ranck 1985; Taube et al. 1990; Taube 1998). When the distal landmarks were rotated around the circular track, however, both place fields and head direction cell tuning curves rotated correspondingly, showing that the distal cues had control over the bearing of both neural representations. Thus, place cells and head direction cells behaved similarly in the differential effectiveness of relative translations versus relative rotations of the local apparatus and the distal landmarks.
Local views of distal landmarks do not change much as the animal moves in its environment. Thus, landmarks located at a distance do not provide precise information about location; environmental geometry, local cues on the apparatus, and idiothetic cues are more accurate sources of precise location information (O’Keefe and Nadel 1978; Burgess et al. 2000; Hartley et al. 2000). Because of the relatively unvarying nature of distal landmarks, they are instead well-suited to providing information about direction or orientation in an environment, regardless of location (O’Keefe and Nadel 1978; Cressant et al. 1997, 1999; Save and Poucet 2000; Zugaro et al. 2001). Knierim and Rao (2003) suggested that the place cell representation of an environment is constructed primarily on idiothetic cues and local apparatus information (for example, corners), and the representation is oriented relative to the distal landmarks by the head direction cell system. This interpretation was limited, however, because Knierim and Rao (2003) did not record head direction cells in their experiment, and it was conceivable that the head direction cells may not have behaved as predicted (Knierim et al. 1995; Golob et al. 2001). The present study was initiated to address this possibility. Importantly, CA1 place cells were also recorded in this experiment, to ensure that the original results of Knierim and Rao (2003) were replicated in the current animals. Indeed, the data from the present study are consistent with the interpretation of Knierim and Rao (2003) that place cells derive their major spatial information from local apparatus cues and self-motion cues, and the role of the distal landmarks is to orient the spatial representation relative to the external world, likely mediated by the head direction cell system.
One prediction from this interpretation is that a lesion to the head direction cell system would abolish the control of distal landmarks over place cells, rendering place fields insensitive not only to translations of the apparatus but also to rotations of the distal landmarks. Furthermore, if idiothetic cues were disrupted and there were no local directional cues on the track, it would predict that the hippocampal representation would rotate relative to the distal landmarks even in sessions in which the landmarks remained stable between sessions. Relevant data come from Calton et al. (2003), who recorded the activity of place cells in a gray, high-walled cylinder after lesions to the ADN or the postsubiculum (PoS). In this experiment, a single, white cue card provided the only salient directional reference. Calton et al. (2003) reported that lesions to the ADN produced a mild impairment of the control of place fields by the cue card, whereas lesions of the PoS produced a profound rotational instability of place field locations relative to the cue card. The results of the PoS lesions are thus consistent with the notion that head direction cells mediate the control of distal landmarks over place fields. Because ADN lesions have been shown to abolish head direction cell properties in PoS (Goodridge and Taube 1997), however, one might predict that the ADN lesion should also have a strong effect on place field stability. There are potential explanations for this difference. First, head direction cells have been reported in a number of brain areas that are associated with the hippocampus, including the retrosplenial cortex (Chen et al. 1994), and in the hippocampus itself (Leutgeb et al. 2000). It is not known whether ADN lesions abolish directional tuning properties of these cells as well, and it is thus possible that these cells were able to orient the place field representation relative to the cue card in the ADN-lesioned animals of Calton et al. (2003). Specifically, it is conceivable that head direction cells in the retrosplenial cortex (Chen et al. 1994) may be able to control place cells via connections with the entorhinal cortex in ADN-lesioned rats, whereas lesions to the PoS may cause downstream changes to the entorhinal cortex that hinder the ability of retrosplenial head direction cells to control place cells by this route. Second, the cue card used in the study by Calton et al. (2003) has properties of both a local landmark and a distal landmark. It is local in that it is directly accessible to the rat: the rat can touch, sniff, and otherwise interact with it. It is a distal cue in the sense that it is located at the periphery of the chamber. Such cues located at the periphery control place cells and head direction cells more strongly than items placed near the center of the chamber (Cressant et al. 1997, 1999; Zugaro et al. 2001). Other studies have demonstrated, however, that under appropriate conditions, place cells can be controlled by both proximal and distal cues simultaneously (Shapiro et al. 1997; Knierim 2002a). Thus, the place fields in the ADN-lesioned animals of Calton et al. (2003) may have been controlled by the cue card because the local cue properties of the card exerted direct control over place cells in a way that purely distal cues (i.e., cues on the walls of the environment that the rat does not have direct access to) are incapable. It might be useful to perform lesions of the head direction cell system using the current experimental design, in which there is a clear, operational distinction between local cues on the track and distal cues on the wall, to test whether abolishment of the head direction cell signal causes the complete loss of the ability of the distal landmarks to control the hippocampal place fields. Finally, the possibility of involvement of PoS non-head direction cells in cue control over place fields cannot be ruled out. Lesions of ADN may only abolish PoS head direction cell activity, sparing non-head direction cells that may play a role in preserved cue control.
The discovery of head direction cells in the border region between the AV and VA thalamic nuclei is a novel finding. We were not able to assign the recording site to either nucleus unambiguously. Calton et al. (2003) reported that head direction cells have not been found in the AVN, although Taube (1995) reported that some recording sites near the ADN/AVN border were ambiguous. The fact that our penetration traversed most (or all) of the AVN before encountering the head direction cells is consistent with this report that AVN may not contain head direction cells, but we cannot rule out the possibility that these cells were in the AVN. The AVN receives similar input as the ADN and it projects to the retrosplenial cortex (Price 1995), which has been shown to contain head direction cells (Chen et al. 1994). The VA nucleus has major connections with rostral premotor areas and the basal ganglia (Price 1995; McFarland and Haber 2002). Head direction cells have been recorded in the striatum (Wiener 1993; Ragozzino et al. 2001), and this may be a route by which the VA nucleus receives head direction information. Because idiothetic cues are a major input onto head direction cells, this pathway may be a mechanism by which motor efference cues exert control over head direction cells (Stackman et al. 2003). Further experiments are necessary to confirm this possibility and to precisely define which nucleus contains the head direction cells.
The partial remapping that occurred in the place fields is an important detail in understanding fully the effects of track translation on the hippocampal representation. In general, the remapping phenomenon is complex and often variable. The factors that induce the phenomenon are not well characterized, but they include a variety of contextual changes to the external, sensory environment and to internal, cognitive factors (Bostock et al. 1991; Knierim et al. 1995; Markus et al. 1995; Anderson and Jeffery 2003; Knierim 2003). The partial remapping that occurred in a minority of cells (gain and lose cells in Tables 1, 2) is clear evidence that, at the system level, the hippocampus was sensitive to the changes in the track locations. We envision that the rat’s perception of the new track locations caused some alteration in hippocampal inputs (either bottom-up sensory inputs or top-down cognitive inputs) that caused a minority of place cells to join or delete themselves from the present hippocampal representation. The resulting representation was then oriented relative to the distal landmarks by the head direction cell system. Of paramount importance is the result that most of the cells that remapped in a given session maintained their new place fields on the track in subsequent sessions in different parts of the room (resulting in the paucity of place fields that occurred in only one position of the track in the room; Fig. 8). Thus, even though different sessions presumably altered hippocampal inputs to cause a substantial minority of cells to remap, the remapping cannot be explained by the place fields being tied to room cues or to the coordinate frame of the room.
Finally, the demonstration of slowly rotating head direction tuning curves (Fig. 9) is an important replication of similar effects that have been reported in other experimental environments (Knierim et al. 1995, 1998). In the previous experiments, the cells were recorded as albino rats foraged in a visually impoverished environment (a gray cylinder with a single polarizing cue card on the wall). The poor vision of these rats and the impoverished environment may have been special factors that allowed the head direction cells to drift within the session, as if the animal were running in the dark. In the present experiment, pigmented, Long-Evans rats, which possess better vision than albino rats, were recorded in an environment with multiple visual landmarks. Thus, even under these conditions, the head direction cells can become decoupled from the landmarks and drift for many seconds. (Although it is interesting that the only two cases in which we saw such drift in this experiment came from the head direction cells at the AVN/VAN border, it is not known whether this was a chance result or whether such cells are more susceptible to drift than cells in the ADN.) Similar to the previous studies, we interpret these results as evidence in favor of a model that the head direction cell system is an internally coherent system that can be independent of visual landmarks. Through experience in an environment, however, the landmarks gain control over the head direction cells, presumably through a Hebbian learning process, thus allowing the landmarks to correct for errors in the head direction system (McNaughton et al. 1991, 1996; Goodridge et al. 1998; Knierim 2005). When the system is grossly misaligned with the landmarks (presumably as the result of the disorientation procedure followed here), the system can sometimes act as if there are no landmarks at all, and it drifts as error accumulates from the idiothetic cues used to maintain the proper representation of head direction. When the system drifts into the preferred direction relative to the landmarks, the landmarks regain control over the cells. This slow rotation is different from the fast corrections reported by Zugaro et al. (2003). We presume that such fast corrections also occurred in many of our rats before the recording system was plugged in, as they were all disoriented before each recording session but only one rat demonstrated the slow rotation phenomenon in only two sessions. The conditions under which the landmarks are able or unable to rapidly reorient the head direction cell system (and presumably the place cell system) may provide important clues to the nature of the interaction between external landmarks, idiothetic cues, and learning in the properties of these neurons.
References
Anderson MI, Jeffery KJ (2003) Heterogeneous modulation of place cell firing by changes in context. J Neurosci 23:8827–8835
Blair HT, Sharp PE (1996) Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci 110:643–660
Bostock E, Muller RU, Kubie JL (1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1:193–205
Brown JE, Skaggs WE (2002) Concordant and discordant coding of spatial location in populations of hippocampal CA1 pyramidal cells. J. Neurophysiol 88:1605–1613
Burgess N, Jackson A, Hartley T, O’Keefe J (2000) Predictions derived from modelling the hippocampal role in navigation. Biol Cybern 83:301–312
Buzsaki G (1986) Hippocampal sharp waves: their origin and significance. Brain Res 398:242–252
Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS (2003) Hippocampal place cell instability after lesions of the head direction cell network. J Neurosci 23:9719–9731
Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL (1994) Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res 101:8–23
Cressant A, Muller RU, Poucet B (1997) Failure of centrally placed objects to control the firing fields of hippocampal place cells. J Neurosci 17:2531–2542
Cressant A, Muller RU, Poucet B (1999) Further study of the control of place cell firing by intra-apparatus objects. Hippocampus 9:423–431
Fenton AA, Muller RU (1998) Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc Nat Acad Sci U S A 95:2717–2719
Golob EJ, Stackman RW, Wong AC, Taube JS (2001) On the behavioral significance of head direction cells: neural and behavioral dynamics during spatial memory tasks. Behav Neurosci 115:285–304
Goodridge JP, Taube JS (1997) Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J Neurosci 17:9315–9330
Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS (1998) Cue control and head direction cells. Behav Neurosci 112:749–761
Gothard KM, Skaggs WE, Moore KM, McNaughton BL (1996) Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci 16:823–835
Hartley T, Burgess N, Lever C, Cacucci F, O’Keefe J (2000) Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10:369–379
Jeffery KJ, O’Keefe JM (1999) Learned interaction of visual and idiothetic cues in the control of place field orientation. Exp Brain Res 127:151–161
Knierim JJ (2002a) Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci 22:6254–6264
Knierim JJ (2002b) The path-integration properties of hippocampal place cells. In: Sharp PE (ed) The neural basis of navigation: evidence from single cell recording. Kluwer, New York, pp 41–58
Knierim JJ (2003) Hippocampal remapping: implications for spatial learning and navigation. In: Jeffery KJ (ed) The neurobiology of spatial behaviour. Oxford University Press, Oxford, pp 226–239
Knierim JJ (2005) Coupling between head direction cells and place cells: influences of landmarks, self-motion, and intrinsic circuitry. In: Wiener SI, Taube JS (eds) Head direction cells and the neural mechanisms underlying directional orientation. MIT Press, Cambridge, MA (in press)
Knierim JJ, McNaughton BL (2001) Hippocampal place-cell firing during movement in three-dimensional space. J Neurophysiol 85:105–116
Knierim JJ, Rao G (2003) Distal landmarks and hippocampal place cells: effects of relative translation versus rotation. Hippocampus 13:604–617
Knierim JJ, Kudrimoti HS, McNaughton BL (1995) Place cells, head direction cells, and the learning of landmark stability. J Neurosci 15:1648–1659
Knierim JJ, Kudrimoti HS, McNaughton BL (1998) Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J Neurophysiol 80:425–446
Leutgeb S, Ragozzino KE, Mizumori SJ (2000) Convergence of head direction and place information in the CA1 region of hippocampus. Neuroscience 100:11–19
Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J (2002) Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416:90–94
Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA (1995) Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci 15:7079–7094
McFarland NR, Haber SN (2002) Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci 22:8117–8132
McNaughton BL, Chen LL, Markus EJ (1991) “Dead reckoning”, landmark learning, and the sense of direction: a neurophysiological and computational hypothesis. J Cogn Neurosci 3:190–202
McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL (1996) Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol 199:173–185
Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT (2003) Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron 37:485–497
Muller RU, Kubie JL (1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7:1951–1968
Muller RU, Ranck JB Jr, Taube JS (1996) Head direction cells: properties and functional significance. Curr Opin Neurobiol 6:196–206
O’Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109
O’Keefe J (1979) A review of the hippocampal place cells. Prog Neurobiol 13:419–439
O’Keefe J, Burgess N (1996) Geometric determinants of the place fields of hippocampal neurons. Nature 381:425–428
O’Keefe J, Conway DH (1978) Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res 31:573–590
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Price JL (1995) Thalamus. In: Paxinos G (ed) The rat nervous system. Academic, San Diego, pp 629–648
Ragozzino KE, Leutgeb S, Mizumori SJY (2001) Dorsal striatal head direction and hippocampal place representations during spatial navigation. Exp Brain Res 139:372–376
Ranck JB Jr (1985) Head direction cells in the deep cell layer of dorsal presubiculum in freely moving rats. In: Buzsaki G, Vanderwolf CH (eds) Electrical activity of archicortex. Akademiai Kiado, Budapest, pp 217–220
Save E, Poucet B (2000) Involvement of the hippocampus and associative parietal cortex in the use of proximal and distal landmarks for navigation. Behav Brain Res 109:195–206
Shapiro ML, Tanila H, Eichenbaum H (1997) Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus 7:624–642
Sharp PE, Blair HT, Etkin D, Tzanetos DB (1995) Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J Neurosci 15:173–189
Skaggs WE, McNaughton BL, Gothard KM, Markus EJ (1993) An information-theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL (eds) Advances in neural information processing systems. Morgan Kaufman, San Mateo, CA, pp 1030–1037
Skaggs WE, McNaughton BL, Wilson MA, Barnes CA (1996) Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6:149–172
Stackman RW, Clark AS, Taube JS (2002) Hippocampal spatial representations require vestibular input. Hippocampus 12:291–303
Stackman RW, Golob EJ, Bassett JP, Taube JS (2003) Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90:2862–2874
Taube JS (1995) Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15:70–86
Taube JS (1998) Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55:225–256
Taube JS, Burton HL (1995) Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol 74:1953–1971
Taube JS, Muller RU, Ranck JB Jr (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10:420–435
Taube JS, Kesslak JP, Cotman CW (1992) Lesions of the rat postsubiculum impair performance on spatial tasks. Behav Neural Biol 57:131–143
Tulving E, Markowitsch HJ (1998) Episodic and declarative memory: role of the hippocampus. Hippocampus 8:198–204
Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M (1997) Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277:376–380
Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP (2000) Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci 12:1714–1726
Wiebe SP, Staubli UV (1999) Dynamic filtering of recognition memory codes in the hippocampus. J Neurosci 19:10562–10574
Wiener SI (1993) Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci 13:3802–3817
Wiener SI, Korshunov VA, Garcia R, Berthoz A (1995) Inertial, substratal and landmark cue control of hippocampal CA1 place cell activity. Eur J Neurosci 7:2206–2219
Young BJ, Fox GD, Eichenbaum H (1994) Correlates of hippocampal complex-spike cell activity in rats performing a nonspatial radial maze task. J Neurosci 14:6553–6563
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Zinyuk L, Kubik S, Kaminsky Y, Fenton AA, Bures J (2000) Understanding hippocampal activity by using purposeful behavior: place navigation induces place cell discharge in both task-relevant and task-irrelevant spatial reference frames. Proc Natl Acad Sci U S A 97:3771–3776
Zugaro MB, Berthoz A, Wiener SI (2001) Background, but not foreground, spatial cues are taken as references for head direction responses by rat anterodorsal thalamus neurons. J Neurosci 21:RC154(1–5)
Zugaro MB, Arleo A, Berthoz A, Wiener SI (2003) Rapid spatial reorientation and head direction cells. J Neurosci 23:3478–3482
Acknowledgements
We thank Dr. Inah Lee and Geeta Rao for assistance with data collection, analysis, and manuscript preparation. This work was supported by grants from the Public Health Service (R01 NS39456 and K02 MH63297) and by the Lucille P. Markey Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoganarasimha, D., Knierim, J.J. Coupling between place cells and head direction cells during relative translations and rotations of distal landmarks. Exp Brain Res 160, 344–359 (2005). https://doi.org/10.1007/s00221-004-2016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2016-9