Abstract
The redundant target effect (RTE) consists in the speeding of reaction time with single versus multiple targets and can be explained either by a neural coactivation or by a race model. To try to understand the role of the magnocellular and parvocellular systems in the determination of the RTE we carried out three experiments using onset or feature singletons. The former are likely to be mainly processed by the magnocellular system while the latter are mainly processed by the parvocellular system. In experiment 1 we found an RTE both when the target (red disk) was presented in isolation and when it was surrounded by equiluminant green distractors. Thus, the RTE occurred both with onset and feature singletons. However, with the former, the RTE could be accounted for by neural coactivation while with the latter it could be accounted for by a probabilistic explanation. In experiment 2 we tried to ascertain the role of distractors in yielding a probabilistic RTE: we used either targets in isolation or surrounded by distractors of lower luminance and found an RTE that could be explained by neural coactivation for both kinds of targets. This ruled out an effect of distractors per se in determining a probabilistic RTE. Finally, in experiment 3 we used targets of lower luminance than either the background or the distractors. We found that the RTE could be accounted for by neural coactivation with targets alone while it was probabilistic with distractors. Overall, these results show that stimuli presumably processed by the magnocellular system yield redundancy gains that result from a neural coactivation mechanism. In contrast, stimuli presumably processed by the parvocellular system are compatible with a probabilistic redundancy gain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the seminal work of Todd (1912) evidence has accumulated that, when an observer is asked to react as quickly as possible to visual onsets, reaction time (RT) is faster when two target stimuli are simultaneously presented instead of just one (e.g., Cavina-Pratesi et al. 2001; Diederich and Colonius 1991; Giray and Ulrich 1993; Miller 1986; Raab 1962; Schwarz and Ischebeck 1994). This phenomenon is known as the ‘redundant target effect’ (RTE), and the difference in RT between single- and double-targets is called the redundancy gain. The RTE has been accounted for by two different models, namely the race model (Raab 1962) and the neural co-activation model (Miller 1982). According to the former, the RTE occurs because information from each target is conveyed to a central processor in separate and independent channels whose speed of processing is a random variable. Presenting multiple stimuli increases the probability that on a given channel a stimulus is processed quicker than on an average channel. By contrast, the co-activation account assumes the existence of a neural mechanism in which signals from each channel are summed and, as a result, are processed more quickly. This mechanism has a threshold that is reached faster with multiple signals than with single ones.
To qualify the nature of the RTE, Miller (1982) devised a mathematical method (race inequality test) that enables one to establish whether the redundancy gain can be explained by probability summation rather than by neural coactivation. Miller’s race inequality test uses the cumulative frequency distribution (CFD) of RTs in the single- versus double-target condition and sets an upper limit for the CFD when redundant targets are presented. If this limit is violated, then a probabilistic explanation is no longer tenable and the RTE can be ascribed to a neural co-activation mechanism (Miller 1982, 1986). When the results from normal observers are considered, the evidence supporting neural or probabilistic explanations of the RTE is controversial with some studies showing a violation of Miller’s inequality (e.g., Cavina-Pratesi et al. 2001; Forster et al. 2002; Iacoboni and Zaidel 2003; Miniussi et al. 1998; Savazzi and Marzi 2002), and others not (e.g., Corballis 1998, 2002; Corballis et al. 2002; Murray et al. 2001; Pollmann and Zaidel 1999; Roser and Corballis 2003). Many studies of simple RT do not show redundancy gain exceeding probability summation in normal subjects—although there is typically violation of probability summation (the race model) in the split brain (Corballis 1998, 2002; Reuter-Lorenz et al. 1995). Corballis (2002) found little or no violation of the race model, either under luminance contrast or equiluminance conditions, in normal subjects. A study by Roser and Corballis (2003) did find some evidence of neural coactivation in normal controls using red and green stimuli, but it was dwarfed by that shown in the split-brained subjects. There is some evidence that variations in stimulus location might play a role—although there was little evidence for violations of the race model in a study in which the stimuli could appear at four different locations (Corballis et al. 2002). For a recent discussion of coactivation versus race model, see Miller and Ulrich (2003). Undoubtedly, violations of the race model have been more consistently found when the RTE was assessed in brain-damaged patients than in normal subjects. Marzi et al. (1996) found a reliable redundancy gain attributable to neural coactivation in patients with unilateral left extinction when the two targets were presented to opposite visual fields. The RTE occurred even when one stimulus in a pair was presented to the left hemifield and therefore was not perceived. Intriguingly, Reuter-Lorenz et al. (1995), Marzi et al. (1997), and Corballis (1998) have reported that patients with a complete section of the corpus callosum show a redundancy gain that is consistent with neural coactivation. These results have led to the hypothesis that the RTE is mediated by neural summation in the superior colliculus (SC), a subcortical visual center that might subserve inter-hemispheric transfer after commisurotomy (Corballis 1998; Iacoboni et al. 2000; Miniussi et al. 1998). Further, evidence for a collicular locus for the RTE comes from the experiment of Tomaiuolo et al. (1997) who found a reliable RTE in hemispherectomy patients, even when one stimulus in a pair was presented to the hemianopic hemifield contralateral to the ablated hemisphere and was therefore undetected.
The SC is known to play a crucial role in spatial attention and oculomotor orienting; in a recent fMRI study in humans, Gitelman et al. (2002) found an activation of the SC specifically related to an exploratory attentional contingency. An analysis of effective connectivity demonstrated that the SC was significantly influenced by the activity in a network of cortical regions including the right frontal eye fields and bilateral parietal and occipital cortices. Another important characteristic of the SC is that its neurons respond to input from different sensory modalities, thus acting as an important center for multisensory integration (Stein 1998; Stein et al. 1993). The fact that a redundancy gain has been observed for bimodal targets delivered in the auditory and visual modality (Gielen et al. 1983; Nickerson 1973), and in the visual and tactile modality (Forster et al. 2002), is consistent with the hypothesis that the SC is one of the possible substrate for the neural summation underlying the RTE together with related cortical areas such as extrastriate (Iacoboni et al. 2000) and premotor (Iacoboni and Zaidel 2003) cortex. In the attempt to identify the information processing stage at which the RTE takes place (perceptual versus motor), Miniussi et al. (1998) had their participants react as quickly as possible to single- vs. double-target presentation while event-related potentials (ERPs) were recorded from the scalp. The behavioral results were in favor of a neural coactivation model, and, most importantly, electrophysiological results showed the RTE to be associated with a clear modulation of the P1 component of the ERP, thought to be generated from extrastriate visual cortex and to subserve early perceptual processing. Given that the extrastriate cortex is heavily interconnected with the SC these data again support a collicular contribution to the RTE and a coactivation at a visual stage. This possibility has also been raised in a recent paper by Savazzi and Marzi (2002) who reported a reliable redundancy gain for targets pairs in which one of the two stimuli had a luminance below detection threshold and was not perceived by the observers. The authors hypothesized that the redundancy gain could be accounted for by a possibly lower threshold of activation of the SC neurons as compared to neurons of the geniculo-striate pathway. Summation between sub- and suprathreshold stimuli could thus take place at the level of the SC. Further evidence in favor of a role of the SC as a site of coactivation comes from a recent experiment by Savazzi and Marzi (report in preparation). They found that when using short wavelength visual stimuli that are known to be invisible to SC neurons the redundancy gain observed was probabilistic while it violated the race inequality with white or long-wavelength stimuli. This result is in keeping with that of Corballis (1998) who found in split-brain subjects that the observed neural redundancy gain was greatly diminished when the stimuli were equiluminant with the background and therefore undetectable by the SC that is known not to receive color opponent input. Several hypotheses on the information-processing stage of neural coactivation have been put forward and some of them have argued for a pre-motor or motor locus; for an extended discussion see Cavina-Pratesi et al. 2001. For example, Diederich and Colonius (1987) argued that the redundancy gain may occur at the motor level with a faster programming or execution of response elicited by double as opposed to single stimuli. They used a bimanual response and bimodal stimuli, and found that intermanual differences in RT and redundancy gain depended to a similar extent on the interstimulus interval between the redundant stimuli. Given that intermanual differences are likely to be related to response execution, their results were consistent with a motoric stage for the RTE. In contrast to a motoric stage, however, Mordkoff et al. (1996), using response force and the lateralized readiness potential (LRP) as dependent variables in a RTE paradigm, could not find a redundancy gain in these measures. Finally, more recently, Miller et al. (2001), by reanalyzing the data from a single-cell study of Lamarre et al. (1983) in a monkey performing a RTE with bimodal stimuli, obtained evidence that the neural correlate of the redundancy gain did not concern the motoric stage of the response. These results point to a perceptual or pre-motor rather than motoric stage for the RTE.
A main motivation for the present study was the consideration that the RTE has been often studied with one versus two onset singleton targets appearing over a blank background. In this regard, it is well established that an onset singleton can be a very salient event in the visual field, usually grabbing attention automatically (Jonides and Yantis 1988; Yantis and Jonides 1990). However, a singleton can also be defined on the basis of its static properties (Folk et al. 1992), such as for example in the case of a red element amidst green elements, or a vertical line amidst horizontal lines. If the difference between the physical properties defining the singleton and the non-singleton elements (usually called ‘distractors’ in the visual search literature, e.g., Wolfe 1998) is large enough, the singleton element is said to stand out from the display (Treisman and Gelade 1980), i.e., it is immediately visible to the observer (Duncan and Humphreys 1989; Nothdurft 2000). The distinction between onset and feature singletons is of particular relevance when the anatomical and functional organization of the human visual system are considered. It has long been known that visual information is sent to cortical visual areas along two separate pathways, the magnocellular (M) pathway and the parvocellular (P) pathway (Livingstone and Hubel 1987). The M-pathway, which is virtually color blind, is mainly concerned with detection of luminance changes and motion-signal processing, and is specialized for object localization. By contrast, the P-pathway, which is mainly devoted to the analysis of fine details and isoluminant color-defined targets, is thought to be concerned with object recognition. The M- and P-pathways have partially different cortical target areas, since the former mainly projects to the dorsal stream in the parietal cortex whereas the latter has its main projections to the ventral stream in the inferior temporal cortex (Ungerleider and Mishkin 1982). Exceptions to this rule have been found; see, for example, recent evidence that the two pathways partially converge already at the level of the primary visual cortex (Vidyasagar et al. 2002). Because of this functional distinction, it seems reasonable to hypothesize that onset singleton detection is fundamentally associated with magnocellular system activity whereas feature (color) singleton detection is related to the parvocellular system.
On the grounds of the different neural mechanisms presumably involved in the detection of onset as compared to feature singletons, and because the RTE has been most often studied by presenting onset singletons, the present experiments were aimed at addressing whether the RTE can also be observed when the target is a feature singleton, and what its summation mechanism might be. To this end, participants were tested in an RTE task in which the same target was displayed either in isolation or among distractors. As mentioned above, when onset singletons were used as target in previous studies, the SC, which receives mainly M input, has been repeatedly implicated as the locus of neural summation in the redundancy gain (e.g., Savazzi and Marzi 2002). Given that SC neurons do not have opponent properties (Marrocco and Li 1977; Schiller and Malpeli 1977), by presenting the target as a color singleton among distractors we should prevent target detection to be subserved by the SC. This would allow us to ascertain whether the RTE possibly found under this condition is still consistent with neural summation. The presentation of a feature color singleton amidst equiluminant distractors should largely restrict neural activity for target identification to the P-system. Thus, while we have abundant evidence that the RTE with onset singletons is related to neural coactivation, RTE with feature singletons might in principle have a different mechanism.
The present study consists of three experiments aimed at comparing the effect of onset and feature singletons on the RTE. The basic task consisted of responding as quickly as possible to one or two targets presented either with or without distractors. When the target was presented in isolation the paradigm replicated the classic experimental condition used in previous RTE studies; instead, when the target was presented among homogeneous distractors the paradigm became a simple visual search task in which the target stood out from the display (Treisman and Gelade 1980; Wolfe 1998).
Experiment 1
The aim of this experiment was to study the RTE when the target was defined either by luminance change or by feature contrast. There is evidence in the literature that the RTE can be observed also when the target does not consist of a sudden onset. For example, Schwarz and Ischebeck (1994) found an RTE with lines of different orientation. In the study of Pollmann and Zaidel (1999), the target was defined either as an inverted T among upright Ts (experiment 1), or a as tilted T among upright Ts (experiment 2). In this regard, the visual search literature has consistently demonstrated that in the latter case the target stands out whereas in the former it does not (Wolfe 1994). In both the experiments of Pollmann and Zaidel (1999), the results were consistent with a probabilistic explanation, that is, there was no violation of Miller’s inequality test. However, in both cases the target was always presented among distractors, and therefore any possible difference in redundancy gain between an onset singleton target and a feature singleton target could not be addressed.
To circumvent this problem we presented the target (a red disk) either in isolation or among a fixed number of equiluminant distractors (green disks).
Method
Participants
Twelve right-handed students (five males) from the University of Verona participated in the study after having given their informed consent. The experiment was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants had normal or corrected-to-normal vision and were not aware of the purpose of the experiment.
Apparatus
Stimulus displays were presented on a Sony Trinitron Multiscan E530 color monitor driven by a personal computer equipped with a graphics board (640×480, 60 Hz). The monitor was placed at eye level on a table in front of the participant. The participants sat with the head positioned on a headrest, so that the distance between the eyes and the screen was approximately 57 cm.
Stimuli
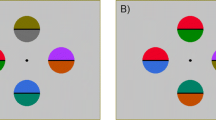
In this and the following experiments the target and the distractors consisted of disks subtending 1.5° of visual angle. The disks were equally spaced around a fixation point on an imaginary circle of 15° of diameter (see Fig. 1). In the no-distractor condition, the display consisted of one or two target disks, whereas in the distractor condition the display consisted of eight disks (either one target and seven distractors, or two targets and six distractors). When two targets were present they always occupied diametrically opposite positions in the two hemifields. All stimulus combinations were equiprobable. The target was red whereas the distractors were green, with both colors set at equiluminance (4.4 cd/m2) by means of a Minolta chromameter CL-200. The background of the screen was black and had a luminance of 0.001 cd/m2; testing took place in a dimly lit room (about 1 cd/m2). Stimulus duration was 150 ms.
Example of the stimulus displays used in the three experiments of the present study. In Experiment 1 one or two red targets were presented either among green distractors (left panels) or in isolation (right panels). In Experiment 2 one or two light-gray targets were presented either among dark-gray distractors (left) or in isolation (right). In Experiment 3 one or two dark-gray (upper panels) or light-gray (lower panels) targets were presented among green distractors (left panels) or in isolation (right panels)
Design
Two within-participant factors, Target (two levels: single vs. double) and Distractor (two levels: absent vs. present) were analyzed for RT as the variable. Each participant performed a total of 384 trials divided into three separate blocks. To avoid automatization of the response upon stimulus presentation, 72 catch trials, consisting of the presentation of eight distractors without targets, were presented randomly among regular trials. Each testing session began with the presentation of a single block of practice trials (28).
Procedure
Participants were instructed to respond as quickly as possible by pressing the spacebar of the computer keyboard with their dominant hand (right in all cases) when they detected the target, and to refrain from responding on catch trials. In this and in the other two experiments, RTs shorter than 140 ms or longer than 650 ms were considered as anticipations and retards, respectively, and were excluded from statistical analyses. They represented a minuscule proportion (about 1%) in all three experiments.
Results
The main effect of Target was significant, F (1,11)=47.9, P<0.0001, with RTs to single targets slower (at 375.3 ms) than those to double targets (351.8 ms), whereas both the main effect of Distractor, F (1,11)=2.308, P>0.15, and the first-order interaction of Target-by-Distractor, F (1,11)=0.343, P>0.5, were not. This means that the RT difference between the single- and double-target condition produced a reliable redundancy gain both when the target appeared among distractors (22.7 ms) and when it appeared in isolation (24.2 ms).
Test of the race model
In order to assess whether the redundancy gain observed was related to neural coactivation or to probability summation, we applied Miller’s race inequality test. Since we expected different mechanisms to elicit the RTE when the target was presented with or without distractors, the analysis was performed separately for the distractor present and absent conditions. As shown in Fig. 2, race inequality (Miller 1982) was violated when the target appeared in isolation, whereas no violation occurred when the target appeared among distractors.
Violation of the race inequality test for the two distractors conditions (absent vs. present) in experiment 1. Gray rectangle indicates the area in which the distributions are significantly different from zero, as assessed by one-sample t-tests. Violation of the race inequality test is evident only when the targets were presented without distractors
Discussion
The results showed a reliable RTE when the target was presented among distractors, confirming the findings of Pollmann and Zaidel (1999). However, the novel aspect of the present results is that the mechanism underlying the RTE varied depending upon whether the target was presented alone or with distractors. When the target was presented in isolation, the redundancy gain violated race inequality and therefore was consistent with the neural coactivation model (Miller 1982). In contrast, with distractors the redundancy gain was consistent with the probabilistic model (Raab 1962). Why should the mechanism of summation vary between an onset and a feature singleton target? We believe that a possible explanation might be found when the mechanisms used by the visual system for extracting the salient element in a display are considered. When a singleton color target is presented amidst homogeneous equiluminant distractors, its saliency is determined on the basis of feature differences among the display elements. When target and distractors have the same luminance, the SC cannot separate the signal of the target from those of the distractors, and redundancy gain is subserved presumably by cortical areas receiving P-pathway input and belonging to the ventral stream, such as the extrastriate cortex, which has been shown to be crucially involved in visual search with equiluminant stimuli (De Weerd et al. 2003). Under these conditions we found a probabilistic redundancy gain. In contrast, when the target is presented in isolation, its saliency is mainly determined by activity within the M-pathway, presumably at such an early stage as the SC or later on in processing at cortical areas pertaining to the dorsal stream, such as posterior parietal cortex (PPC) (Gottlieb et al. 1998), and this is compatible with a neural coactivation mechanism of redundancy gain. An important question is obviously why the two presumed sites of redundancy gain yield a different mechanism of summation.
Experiment 2
The results from experiment 1 suggest that neural coactivation is subserved by the SC only when the target is defined on the basis of visual transients. Yet, it should be noted that in this experiment onset singletons differed from feature singletons not only because in the former condition there was a visual transient but also because the target was the only element present in the display. Therefore, it might be that the main difference between the two conditions was the presence of distractors, which per se could have yielded a probability summation independent of the type of stimuli. To address this issue we modified the experimental conditions of experiment 1 by presenting the target either in isolation or among distractors of lower luminance. If our hypothesis about the role of visual transients in neural coactivation is correct, in experiment 2 the redundancy gain should violate the Miller’s inequality test both when the target is presented in isolation and when is presented among distractors with a lower luminance.
Method
Participants
Twelve right-handed students (six males) from the University of Verona served as participants, after having given informed consent. The experiment was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants had normal or corrected-to normal vision and were not aware of the purpose of the experiment. None of them participated in the previous experiment.
Apparatus, design and procedure
The apparatus, experimental design and procedures were as in experiment 1.
Stimuli
The stimuli were the same as in experiment 1 except for the following differences. In order to maximize the difference in visual transients between the target and the distractors, the former was light gray (3.8 cd/m2) and the latter were dark gray (0.6 cd/m2). The stimuli were presented over a dark-gray background (1.8 cd/m2). Figure 1, middle row, presents an example of the stimulus display.
Results
RTs were entered into a repeated-measures analysis of variance (ANOVA) in which the factors were Target (single vs. double) and Distractor (absent vs. present). We found significant main effects of Target, F (1,11)=156.4, P<0.0001, with RTs to single targets (325.8 ms) slower than those to double targets (301.6 ms), and of Distractor, F (1,11)=7.1, P<0.021, with RTs to targets in isolation faster (310.5 ms) than those to targets among distractors (316.9 ms), whereas the Target-by-Distractor interaction was not significant, F (1,11)=0.01, P>0.9. It is important to point out that the redundancy gain was significant both when targets appeared among distractors (24.0 ms) and when they appeared in isolation (24.3 ms).
Analysis of the race model
As in experiment 1, testing of the race inequality was performed separately for the distractors present and absent conditions. In accordance with our prediction, the race inequality test (Miller 1982) was violated in both conditions (Fig. 3).
Violation of the race inequality test for the two distractors conditions (absent vs. present) in experiment 2. Gray rectangles indicate the areas in which the distributions are significantly different from zero. Violation of the race inequality test is evident both when the targets were presented without distractors and among black distractors
Discussion
The results of experiment 2 clearly indicated that it was not the presence of distractors per se that is crucial for obtaining a redundancy gain based on neural coactivation. Instead, the key factor is the strength of the visual transient elicited by the target relative to those elicited by the distractors. In other words, the probability of observing an RTE consistent with a neural coactivation model seems to depend on whether the target is detectable on the basis of the strength of the visual transient elicited upon presentation. In keeping with that, violation of the race model was observed not only when the target appeared in isolation, but also when it appeared with distractors of lower luminance than the target.
In experiment 1, the target and the distractors had different colors but the same luminance. Importantly, electrophysiological studies have reported that there are no projections to the SC from color-opponent cells in the retina (Marrocco and Li 1977; Schiller and Malpeli 1977). Hence, the red target and the green distractors were indistinguishable on the basis of their visual transients at the SC level. Therefore, neural summation was the same whether the display consisted of one red target and seven green distractors, or two red targets and six green distractors. By contrast, it is well established that luminance increments, such as abrupt onsets, are able to elicit differential responses in the neurons of the SC (Munoz and Wurtz 1995), which is typically involved in automatic orienting of attention and saccadic eye movements to visual transients such as a peripheral flash of lights (Kustov and Robinson 1996). In experiment 2, not only the target was defined as an onset transient (with distractors as offset transients), but it also had a greater luminance contrast with respect to the background than the distractors and this is in keeping with having found a coactivation mechanism for the observed redundancy gain.
Experiment 3
A further important question is whether evidence for neural coactivation could also be found when the target had a lower luminance than the distractors. To this aim, for a fixed luminance level of distractors, we directly compared targets with higher or lower luminance.
Method
Participants
Twelve right-handed students (four males) from the University of Verona served as participants after having given informed consent. The experiment was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants had normal or corrected-to-normal vision and were not aware of the purpose of the experiment. None of them participated in the previous experiments.
Apparatus, design and procedure
The apparatus, experimental design and procedures were as in experiment 1.
Stimuli
The stimuli were the same as in experiment 1 but with the following exceptions. The target could be either light-gray (3.8 cd/m2) or dark-gray (0.6 cd/m2), whereas the distractors were green (1.1 cd/m2). The redundancy conditions consisted of two light-gray targets or two dark-gray targets. Target and distractors were presented over a gray background (1.8 cd/m2). Examples of stimulus displays are shown in Fig. 1 (bottom two rows).
Results
RTs were entered into a repeated-measures ANOVA in which the main factors were Target number (single vs. double), Target color (light vs. dark), and Distractor (absent vs. present). The following main effects were significant: Target number, F (1,11)=160.3, P<0.0001, with RTs to single targets (372.5 ms) slower than those to double targets (345.7 ms); Target color, F (1,11)=7.8, P<0.017, with RTs to light-gray targets (353.7 ms) faster than those to dark-gray targets (364.4 ms); and Distractor, F (1,11)=8.5, P<0.014, with RTs to targets in isolation faster (352.8 ms) than those to targets among distractors (365.4 ms). The only significant interaction was Target color × Distractor, F (2,22)=27.9, P<0.0001, with RTs for dark-gray targets in isolation similar (365.1 ms) to those with distractors (363.8 ms); RTs for light-gray targets in isolation were faster (340.4 ms) than for targets with distractors (367.1 ms).
Analysis of the race model
As shown in Fig. 4, the race inequality test (Miller 1982) was violated in all conditions except for dark gray targets with distractors.
Violation of the race inequality test for the two target conditions (white vs. black) as a function of the two distractors conditions (absent vs. present) in experiment 3. Gray rectangles indicate the areas in which the distributions are significantly different from zero. When the targets had a greater luminance than the distractors (White targets), violation of the race inequality test is evident regardless of whether the distractors were presented or not. By contrast, when the targets had a lower luminance than the distractors (Black targets), violation of the race inequality test is evident only when the distractors were absent
Discussion
Experiment 3 had the purpose of ascertaining whether an RTE could be found even when the target had a lower luminance than the distractors. When the target was presented without distractors, and was defined as a luminance decrement of some regions of the background, the results showed an RTE that violated the race inequality test, thus supporting the neural coactivation model (Miller 1982). Because the target had a lower luminance than the background, this suggests that neural summation can occur even when visual transients are elicited by OFF signals, thus extending the role of visual transients for the neural summation process to luminance decrements. Overall, the results from experiments 1 and 2 have indicated that when distractors are present evidence for neural co-activation could be found only when the target had a greater luminance than the distractors. In experiment 3, a violation of the race model was found only when the target was light-gray among green distractors, but not when it was dark-gray among green distractors. In the latter condition the target had a lower luminance than the distractors, and therefore it should have produced a stronger OFF signal than the green elements. However, in spite of that, neural summation did not take place.
Altogether the results indicate that neural summation occurs for visual transients elicited by luminance decrements when the only visual transient present in the display is that elicited by the target. One possibility to explain the lack of evidence for the neural summation when both the target and the distractors are defined as luminance decrements might rely on the fact that the difference in the strength of visual transients was too small to allow the target signal to be distinguished from the distractors signals.
General discussion
In this study we investigated the RTE by presenting the target either in isolation or among a fixed number of distractors. The target was clearly distinguishable from the distractors on the basis of a simple feature like color (experiment 1) or luminance (experiments 2 and 3), and this made the target stand out from the display (Treisman and Gelade 1980). The presence of distractors was manipulated in order to have the target appearing either as an isolated visual transient (an onset/offset singleton) or as a static feature singleton (a red item among green items). This distinction is important, since the presence of a salient feature singleton is mainly associated with activity in cortical areas such as the extrastriate visual cortex (De Weerd et al. 2003) belonging to the ventral stream and receiving mainly P-pathway input whereas a salient onset (or offset) singleton can probably be detected early-on in visual processing by a subcortical structure such as the SC receiving M-pathway input.
Experiment 1 showed that the RTE was consistent with a neural coactivation model when the red target appeared in isolation, whereas it was consistent with a probabilistic model when the red target appeared among equiluminant green distractors. In experiment 2 a light-gray target was presented either in isolation or among dark-gray distractors; in both cases, the RTE could be explained by neural coactivation. Finally, experiment 3 showed that a target with a lower luminance than the background yielded an RTE consistent with the neural model when without distractors but consistent with the race model when presented among distractors of a higher luminance.
Overall, the present results suggest that, when the target can be detected on the basis of the activity within the M-system and the dorsal stream, the RTE is mediated by neural summation, likely to be at the SC level. By contrast, when the RTE is presumably mediated by cortical areas involved in saliency computation based on static feature detection, such as extrastriate visual areas belonging to the P-system and the ventral stream, then its mechanism is probabilistic. Why should the mechanism of redundancy gain be different when subserved by one or the other visual stream? Below, we will try to discuss some possibilities.
Let us start with the case of the target being a red element amidst green elements. Under this condition the target can be detected on the basis of activity within the ‘saliency map’, a biased representation of the visual field in which the more prominent location is signaled by the highest peak of activation within the map (Wolfe 1994). If a unique salient element is present in the display, mechanisms of saliency computation working either by local inhibition (Koch and Ullman 1985) or by feature contrast detection (Julesz 1986) can detect the feature singleton very quickly and efficiently, as demonstrated by the fact that RT does not vary as a function of the number of distractors in the display (Treisman and Gelade 1980; Wolfe 1998). However, activation at each location within the saliency map presents a certain degree of variability that reflects the fact that signals in the nervous system are probabilistic in nature. Hence, if RTs for target detection are based on the output of neural mechanisms involved in the processes of saliency computation, having two targets increases the probability that one of the two correspondent peaks of activation reaches the threshold necessary to initiate the manual response faster than with a single target. The probabilistic model is therefore entirely consistent with the neural mechanisms for saliency computation of a feature singleton target.
We now turn to consider the case of the target being a singleton in the luminance domain, that is, when a target appears either in isolation (as an ON or an OFF signal) or when it has a greater luminance than the distractors. Because of the M-pathway projections to the SC, we propose that the SC can mediate the RTE by producing a neural summation whenever the target is distinguishable on the basis of the transient channels activity. On the grounds of the neuroanatomical and functional connections of the SC with motor-related areas, it seems plausible to hypothesize that the SC can modulate the speed of the manual response for target detection. While the SC superficial layers have neurons that have exclusively visual functions, the deep layers have neurons that receive afferents from visual and sensori-motor cortical regions (e.g., frontal eye fields, PPC), and are involved in the translation of sensory signals into motor commands for the eyes (Schall 1995) as well as for the hands (Lunenberger et al. 2001). Thus, one possibility is that the contribution of the SC to the redundancy gain is to subserve neural coactivation at a premotor level. However, the possibility of a perceptual locus cannot be excluded since a recent study by Cavina-Pratesi et al. (2002) has shown that the requirement of a motor response is not necessary for the RTE to take place since these authors found a redundancy gain in a stop-signal paradigm by comparing the efficacy of single and double visual signals in inhibiting the motor response. In addition, the above-mentioned ERP results of Miniussi et al. (1998) have provided substantial neural evidence for a perceptual locus of the RTE
In conclusion, the present study has been concerned with the conditions in which the RTE could be explained by a probabilistic hypothesis and conditions in which it was consistent with a neural coactivation hypothesis. Overall the results support the possibility that the RTE is related to neural coactivation when the target is detectable on the basis of activity of transient channels within the M-pathway, particularly at the SC level. Presumably, the SC is endowed with the neural basis for summation at the level of its neuronal pools. By contrast, when the target is a feature singleton, the RTE is better accounted for by probabilistic models, in agreement with the fact that the results of the neural mechanisms involved in saliency computation are noisy and vary stochastically over time (Wolfe 1994). Presumably, the cortical areas involved in static feature detection, in contrast to the SC, are not endowed with the neuronal machinery suitable for the kind of neural coactivation underlying the redundancy gain. Interestingly, Corballis (2002) reached a broadly similar conclusion about redundancy effects in the magnocellular and parvocellular systems, although based on correlations rather than on presence or absence of race-model violations (see final paragraph of that study).
Finally, a note of caution is in order as far as the distinction between neural and probabilistic summation is concerned. Violation of the inequality is evidence for neural coactivation (given that an additional assumption of context independence is satisfied; see Colonius 1990); non-violation, however, would still be compatible with some moderate amount of neural coactivation that does not exceed the upper bound given by the inequality. Thus, the present data do not unambiguously suggest that two entirely different mechanisms are at work for dynamic and for static discontinuities. What is clear, however, is the fact that the former, in contrast to the latter, triggers a response mechanism that cannot exclusively be based on probability summation. Of course, this consideration does not invalidate the interpretation in terms of the P- and M-systems.
References
Cavina-Pratesi C, Bricolo E, Prior M, Marzi CA (2001) Redundancy gain in the stop signal paradigm: Implications for the locus of activation in simple reaction time. J Exp Psychol Hum Percept Perform 27:932–941
Colonius H (1990) Possibly dependent probability summation of reaction time. J Math Psychol 34:253–275
Corballis MC (1998) Interhemispheric neural summation in the absence of the corpus callosum. Brain 121:1795–1807
Corballis MC (2002) Hemispheric interactions in simple reaction time. Neuropsychologia 40:423–434
Corballis MC, Hamm JP, Barnett KJ, Corballis PM (2002) Paradoxical interhemispheric summation in the split brain. J Cogn Neurosci 14:1151–1157
De Weerd P, Desimone R, Ungerleider LG (2003) Generalized deficits in visual selective attention after V4 and TEO lesions in macaques. Eur J Neurosci 18:1671–1691
Diederich A, Colonius H (1987) Intersensory facilitation in the motor component? A reaction time analysis. Psychol Res 49:23 –29
Diederich A, Colonius H (1991) A further test of the superposition model for the redundant-signals effect in bimodal detection. Percept Psychophys 50:83–86
Duncan J, Humphreys GW (1989) Visual search and stimulus similarity. Psychol Rev 87:272–300
Folk CL, Remington RW, Johnston, JC (1992) Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform 18:1030–1044
Forster B, Cavina-Pratesi C, Aglioti SM, Berlucchi G (2002) Redundant target effect and the intersensory facilitation from visual-tactile interactions in simple reaction time. Exp Brain Res 143:480–487
Gielen SC, Schmidt RA, Van den Heuvel PJ (1983) On the nature of intersensory facilitation of reaction time. Percept Psychophys 34:161–168
Giray M, Ulrich R (1993) Motor coactivation revealed by response force in divided and focused attention. J Exp Psychol Hum Percept Perform 19:1278–1291
Gitelman DR, Parrish TB, Friston KJ, Mesulam M (2002) Functional anatomy of visual search: regional segregation within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage 15:970–982
Gottlieb JP, Kusunoki M, Goldberg M (1998) The representation of visual salience in monkey parietal cortex. Nature 391:481–484
Iacoboni M, Zaidel E (2003) Interhemispheric visuo-motor integration in humans: the effect of redundant targets. Eur J Neurosci 17:1981–1986
Iacoboni M, Ptito A, Weekes NY, Zaidel E (2000) Parallel visuomotor processing in the split brain: cortico-subcortical interactions. Brain 123:759–769
Jonides J, Yantis S (1988) Uniqueness of visual onset in capturing attention. Percept Psychophys 43:346–354
Julesz B (1986) Texton gradients: the texton theory revised. Biol Cybern 54:245–251
Koch C, Ullman S (1985) Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol 4:219–227
Kustov AA, Robinson DL (1996) Shared neural control of attentional shifts and eye movements. Nature 384:74–77
Lamarre Y, Busby L, Spidalieri G (1983) Fast ballistic arm movements triggered by visual, auditory, and somesthesic stimuli in the monkey. I. Activity of precentral cortical neurons. J Neurophysiol 50:1343–1358
Livingstone MS, Hubel DH (1987) Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci 7:3416–3468
Lunenburger L, Kleiser R, Stuphorn V, Miller LE, Hoffmann KP (2001) A possible role of the superior colliculus in eye-hand coordination. Prog Brain Res134:109–125
Marrocco RT, Li RH (1977) Monkey superior colliculus: properties of single cells and their afferent inputs. J Neurophysiol 4:844–860
Marzi CA, Smania N, Martini MC, Gambina G, Tomelleri G, Palamara A, Alessandrini F, Prior M (1996) Implicit-redundant targets effect in visual extinction. Neuropsychologia 34:9–22
Marzi CA, Fanini A, Girelli M, Ipata AE, Miniussi C, Smania N, Prior M (1997) Is extinction following parietal damage an interhemispheric disconnection phenomenon? In: Thier P, Karnath HO (eds) Parietal lobe contribution to orientation in 3D space, Experimental Brain Research Series. Springer-Verlag, Heidelberg, Berlin, New York, pp 431–445
Miller J (1982) Divided attention: evidence for coactivation with redundant signals. Cogn Psychol 14:247–279
Miller J (1986) Time course of coactivation in bimodal divided attention. Percept Psychophys 40:331–343
Miller JO, Ulrich R (2003) Simple reaction time and statistical facilitation: a parallel grains model. Cogn Psychol 46:101–151
Miller JO, Ulrich R, Lamarre Y (2001) Locus of the redundant signals effect in bimodal divided attention. Percept Psychophys 63:555–562
Miniussi C, Girelli M, Marzi CA (1998) Neural site of the redundant target effect: Electrophysiological evidence. J Cogn Neurosci 10:216–230
Mordkoff JT, Miller J, Roch AC (1996) Absence of coactivation in the motor component: evidence from psychophysiological measures of target detection. J Exp Psychol Hum Percept Perform 22:25–41
Munoz DP, Wurtz RH (1995) Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and built-up cells. J Neurophysiol 73:2334–2348
Murray MM, Foxe JJ, Higgins BA, Javitt DC, Schroeder CE (2001) Visuo-spatial neural response interactions in early cortical processing during a simple reaction time task: a high-density electrical mapping study. Neuropsychologia 39:828–844
Nickerson RS (1973) Intersensory facilitation of reaction time: energy summation or preparation enhancement? Psychol Rev 80:489–509
Nothdurft HC (2000) Salience from feature contrast: variation with texture density. Vision Res 40:3181–3200
Pollmann S, Zaidel E (1999) Redundancy gains for visual search after complete commissurotomy. Neuropsychology 13:246–258
Raab D (1962) Statistical facilitation of reaction time: energy summation or preparation enhancement? Psychol Rev 80:489–509
Reuter-Lorenz PA, Nozawa G, Gazzaniga MS, Hughes HC (1995) Fate of neglected targets: a chronometric analysis of redundant target effects in the bisected brain. J Exp Psychol Hum Percept Perform 21:211–230
Roser M, Corballis MC (2003) Interhemispheric neural summation in the split brain: effects of stimulus colour and task. Neuropsychologia 41:830–846
Savazzi S, Marzi CA (2002) Speeding up reaction time with invisible stimuli. Curr Biol 12:403–407
Schall JD (1995) Neural basis of saccade target selection. Rev Neurosci 6:63–85
Schiller PH, Malpeli JG (1977) Properties and tectal projections of monkey retinal ganglion cells. J Neurophysiol 40:428–445
Schwarz W, Ischebeck A (1994) Coactivation and statistical facilitation in the detection of lines. Perception 23:157–168
Stein BE (1998) Neural mechanisms for synthesizing sensory information and producing adaptive behaviors. Exp Brain Res 123:124–135
Stein BE, Meredith MA, Wallace MT (1993) The visually responsive neuron and beyond: multisensory integration in cat and monkey. Prog Brain Res 95:79–90
Todd JW (1912) Reaction to multiple stimuli. Science Press, New York
Tomaiuolo F, Ptito M, Marzi CA, Paus T, Ptito A (1997) Blindsight in hemispherectomized patients as revealed by spatial summation across the vertical meridian. Brain 120:795–803
Treisman A, Gelade G (1980) A feature-integration theory of vision. Cogn Psychol 12:97–136
Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield JW (eds) Analysis of visual behavior. MIT Press, Cambridge MA, pp 549–586
Vidyasagar TR, Kulikowski JJ, Lipnicki DM, Dreher B (2002) Convergence of parvocellular and magnocellular information channels in the primary visual cortex of the macaque Eur J Neurosci 16:945–956
Wolfe JM (1994) Guided Search 2.0: a revised model of visual search. Psychol Bull Rev 1:202–238
Wolfe JM (1998) Visual search. In: Pashler H (ed) Attention. Psychology Press, Hove, pp 13–73
Yantis S, Jonides J (1990) Abrupt visual onset and selective attention: voluntary vs. automatic allocation. J Exp Psychol Hum Percept Perform 16:121–134
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turatto, M., Mazza, V., Savazzi, S. et al. The role of the magnocellular and parvocellular systems in the redundant target effect. Exp Brain Res 158, 141–150 (2004). https://doi.org/10.1007/s00221-004-1884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-1884-3