Abstract
Movements by a standing person are commonly associated with adjustments in the activity of postural muscles to cause a desired shift of the center of pressure (COP) and keep balance. We hypothesize that such COP shifts are controlled (stabilized) using a small set of central variables (muscle modes, M-modes), while each M-mode induces changes in the activity of a subgroup of postural muscles. The main purpose of this study has been to explore the possibility of identification of muscle synergies in a postural task using the framework of the uncontrolled manifold (UCM) hypothesis employing the following three steps in data analysis: (i) Identification of M-modes: Subjects were asked to release a load from extended arms through a pulley system, resulting in a COP shift forward prior to load release. Electromyographic (EMG) activity of eleven postural muscles on one side of the body was integrated over a 100 ms interval corresponding to the early stage of the COP shift, and subjected to a principal component (PC) analysis across multiple repetitions of each task. Three PCs were identified and associated with a ‘push-back M-mode’, a ‘push-forward M-mode’ and a ‘mixed M-mode’. (ii) Calculation of the Jacobian of the system, which relates changes in the magnitude of M-modes to COP shifts using regression techniques: Subjects performed three different tasks (releasing different loads at the back, voluntarily shifting body weight forward and backward, at different speeds) to verify if the relationship between magnitudes of M-modes and COP shifts is task or direction specific. (iii) UCM analysis: Three tasks were chosen (load release in the front, arm movement forward and backward) which were associated with an early shift in COP. A manifold was identified in the M-mode space corresponding to a certain average (across trials) shift of the COP and variance per degree of freedom within the UCM (VUCM) and orthogonal (VORT) to the UCM was computed. Across subjects, VUCM was significantly higher than VORT when analysis at the third step was performed using a Jacobian computed based on a set of tasks associated with a COP shift in the same direction but not in the opposite direction. This result confirms our hypothesis that the M-modes work together as a synergy to stabilize a desired shift of the COP. Forward and backward COP shifts are associated with different synergies based on the same three M-modes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Hughlings Jackson (1889), it has been recognized that the central nervous system (CNS) does not control muscles independently, but unites them in groups. Bernstein (1967) proposed that the CNS uses muscle synergies as a means of solving the ‘motor redundancy’ problem. Gelfand and Tsetlin (1966) viewed muscle synergies as a particular example of structural units, which are task-specific ensembles of elements within a neuromotor system.
The notion of muscle synergies is commonly used in both basic and clinical research including studies of control of vertical posture (Bouisset et al. 1977; Crenna et al. 1987; Massion et al. 1992; Allum and Honegger 1993; Sabatini 2002; Holdefer and Miller 2002). The term postural synergy is frequently used, often loosely, suggesting a co-variation of EMG or kinematic indices over the time course of a postural adjustment or across several repetitions of a task with a postural component. In particular, short-latency reactions of standing subjects to an unexpected rotation or translation of the force platform have been described using the notions of the ankle strategy and of the hip strategy (Horak and Nashner 1986; Horak et al. 1990) implying two postural synergies used predominantly by subjects during slow (the ankle strategy) and fast (the hip strategy) translations of the force plate. The hip strategy is also predominantly used by elderly individuals. More complex patterns of adjustments have also been described including a “multi-link strategy” (Allum et al. 1989). Furthermore, kinematic synergies related to postural adjustments have been studied (Alexandrov et al. 1998b; Vernazza-Martin et al. 1999).
Consistent with ideas of Gelfand and Tsetlin (1966), a new computational approach to the study of synergies has been proposed: the uncontrolled manifold (UCM) hypothesis (Schöner 1995; Scholz and Schöner 1999; reviewed in Latash et al. 2002b). The UCM hypothesis assumes that the controller (CNS) acts in a state space of control variables. The control variables are not immediately observable and their number may be smaller than the number of involved elements, such as joints or muscles, depending on the level of analysis. In other words, the dimensionality of the space of control variables is smaller than the dimensionality of the state space of elements. The controller selects in the former space a sub-space (a manifold, UCM) corresponding to a value of a performance variable that needs to be stabilized. Then, it arranges co-variations among the control variables such that their variance has relatively little effect on the selected performance variable, i.e. it is mostly confined to the UCM.
If several attempts at a task are analyzed, variance of the control variables across the attempts can be partitioned into two components, within the UCM and orthogonal to it. If the control variables are indeed organized into a synergy stabilizing that performance variable, their variance orthogonal to the UCM is expected to be smaller as compared to the variance within the UCM. In other words, the controller allows relatively high variability of control variables (and elements) as long as this variability does not affect the desired value of the performance variable.
To perform the UCM analysis for a particular task, one needs to move through the following steps:
-
1.
Identification of independent control variables (ICV): These are the control variables that are independently manipulated by the CNS to stabilize performance variables. For example, in multi-finger force production studies, individual finger forces cannot be considered independent because of the phenomenon of enslaving (Kilbreath and Gandevia 1994; Zatsiorsky et al. 1998, 2000). Hence, UCM analysis of finger coordination in such tasks has been based on a different set of variables, force modes, defined in a special experimental series (Scholz et al. 2002). Force modes are the hypothetical independent control variables whereas the actual measured forces of each finger depend on a command to this finger (its force mode), as well as on commands to other fingers.
-
2.
Identification of relations between the ICV with a selected performance variable (the Jacobian of the system): This stage of analysis starts with the formulation of a control hypothesis, i.e. a hypothesis about a particular performance variable, which is supposed to be stabilized by a synergy. For example, in earlier kinematic studies, features of the trajectory of the endpoint of a kinematic chain were assumed to be stabilized, and the Jacobian was defined by the geometry of the moving effector (Scholz and Schöner 1999). In multi-finger force production experiments, the control hypotheses assumed that the total force (or total moment) produced by a set of fingers was stabilized, and the Jacobian linked its changes to changes in force modes (Scholz et al. 2002).
-
3.
UCM Analysis: A manifold (UCM) corresponding to a value of the performance variable is determined. Several attempts at a task are analyzed and the variance, computed across tasks is partitioned into two components, one within the manifold and the other, orthogonal to it. The former is supposed to be significantly larger than the latter. In multi-finger force production studies (Scholz et al. 2002), force profiles of individual fingers were recorded and subjected to such an analysis across trials at different phases of the task.
Previous studies using the UCM approach (Scholz and Schöner 1999; Scholz et al. 2000, 2002) were done at the level of mechanical variables, such as joint angles and finger forces. With this experiment, our aim is to expand the use of this approach to a ‘more physiological’ variable, EMG, to identify muscle synergies associated with postural tasks.
We are going to move through the above three steps using the notion of muscle modes (M-modes) (Krishnamoorthy et al., in press), which are jointly activated muscle groups that are formed for particular tasks and can be seen across variations in task parameters. M-modes are assumed to be orthogonal dimensions in the control space such that a control signal can be represented as a vector in the M-mode space. Further, we introduce a control hypothesis that the CNS arranges co-variations among changes in magnitudes of M-modes to stabilize a certain task-specific center of pressure (COP) shift. This hypothesis is based on a body of literature that views coordinates of COP as an important variable for postural control (Collins and De Luca 1993; Winter et al. 1998; Zatsiorsky and Duarte 2000; Baratto et al. 2002). Muscle synergies are defined as co-variations of control variables (M-modes) that stabilize a particular value of COP shift.
In the present study, regression techniques are used to relate variations in the magnitude of the M-modes to variations in the COP shift. Finally, we compute a UCM in the M-mode space corresponding to a certain average (across trials) shift of the COP and compare variances per degree of freedom within the UCM (VUCM) and orthogonal (VORT) to the UCM.
We hypothesized that the magnitude of the COP shift would be stabilized not by a fixed, optimal combination of the M-modes but by co-variations of the changes in magnitudes of M-modes across trials at the same task. If this hypothesis is confirmed, i.e. the variance within the corresponding UCM is significantly higher than variance orthogonal to the UCM (VUCM >VORT), the following conclusions can be made: (1) the control hypothesis on stabilization of COP shift by co-variations of the magnitudes of M-modes is confirmed; (2) postural control can be described as a process of organizing task-specific synergies as combinations of elements (M-modes) in a relatively low-dimensional space; and (3) the UCM approach can be used to identify muscle synergies based on EMG indices.
A priori, we could not predict whether similar or different combinations of M-modes (synergies) would be used for forward and backward COP shifts. Thus, another goal of the study was to use the UCM method to verify if a single synergy or two different synergies are used in tasks that require COP shifts in different directions.
Materials and methods
Subjects
Eight unpaid healthy subjects, four male and four female, of mean age 29 years (±4.5 SD), mean weight 60.63 kg (±7.2 SD) and mean height 1.68 m (±0.1 SD) without any known neurological or motor disorder, participated in the experiment. All subjects were right-handed based on their preferential hand use during eating and writing. The subjects gave written informed consent according to the procedure approved by the Office for Regulatory Compliance of the Pennsylvania State University.
Apparatus
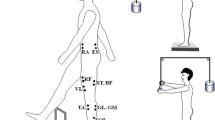
A force platform (AMTI, OR-6) was used to record the moment around a frontal axis (My), and the vertical component of the reaction force (Fz). An oscilloscope (Tektronics TDS 210) showed the time pattern of My to the subject and the experimenter. A uni-directional accelerometer (Sensotec) was taped to the dorsal aspect of the subject’s hand, just under the metacarpophalangeal joint of the middle finger or the thumb, depending on the task. The axis of sensitivity of the accelerometer was directed along the required motion. Disposable self adhesive electrodes (3 M) were used to record the surface EMG activity of the following eleven leg and trunk muscles: tibialis anterior (TA), gastrocnemius lateralis (GL), gastrocnemius medialis (GM), soleus (SOL), vastus lateralis (VL), vastus medialis (VM), rectus femoris (RF), biceps femoris (BF), semitendinosus (ST), rectus abdominis (RA) and erector spinae (ES) (see Fig. 1). The electrodes were always placed on the left side of the subject’s body on the muscle bellies, with their centers approximately 3 cm apart. Signals from the electrodes were amplified (×3000) and band-pass filtered (60–500 Hz). Data were recorded at a sampling frequency of 1000 Hz with a 12-bit resolution. A Gateway 450 MHz PC with customized software based on the LabView-4 package was used to control the experiment and collect the data.
The experimental setup: Subjects stood on a force platform. In trials when subjects were required to release the load in front, the load was held directly in the hands (LR F ) and when the load was to be released behind the subject, the subject held a handle, which was connected to the load through the pulley system (LR B ). Location of some of the EMG electrodes is also shown (GL lateral head of gastrocnemius, SOL soleus, ST semi-tendinosus, ES erector spinae, TA tibialis anterior, VL vastus lateralis, RF rectus femoris, RA rectus abdominis)
In some conditions, the subject held a load (20×20×10 cm) between his/ her hands, by pressing on the sides of the load or via a pulley system (Fig. 1).
Procedure
One group of tasks was associated with anticipatory postural adjustments (APAs, for review see Massion 1992) and involved COP shifts as an implicit component. These tasks required the subject to release a load (LR) from extended arms (Aruin and Latash 1996) or to perform a fast bilateral arm movement (Belen’kii et al. 1967). The other group of tasks explicitly required the subject to voluntarily shift his/ her COP (VS) using visual feedback provided by the oscilloscope (Danion et al. 1999).
Load release (LR) task
In the initial position, the subject stood on the force platform with his/ her feet side-by-side, at hip width. This position was marked on the platform and was reproduced across trials. In trials where the 3 kg load was released in front of the subject (LRF), the subject pressed on the sides of the load with extended hands. When the same load was to be released at the back (LRB), the subject pressed on the sides of the horizontal handle, which in turn was attached to the load through the pulley system (Fig. 1). In another condition, the subject was asked to drop a variable load at the back (LRBV), with the load mass ranging from 2 kg to 7 kg (3–11.5% of subject’s body mass, on average) in increments of 0.5 kg. Subjects were instructed to release the load with a quick, small amplitude, bilateral shoulder abduction movement.
Arm movement (AM) task
The initial position was the same as in the LR task, except that the subject’s hands were now hanging loosely by his/her side. Subjects were asked to perform a fast, bilateral arm flexion movement (AMF) or bilateral arm extension movement (AMB) over a nominal distance of 40°. However, subjects were allowed to select a comfortable distance and reproduce it.
Voluntary sway (VS) task
The initial position was the same as in the AM task. The subject was required to move his/ her body weight towards the toes (VSF) or the heels (VSB). In different trials they were asked to produce this movement at different speeds, self-selected by the subjects. Subjects watched the oscilloscope, which showed them the current value of My. The initial position of the subject was marked on the oscilloscope. The required My shift was also marked and was approximately 10 Nm, which corresponded to a COP shift of about 1–2 cm depending on the subject’s body weight.
For each trial, data were collected over 3 s. Subjects were instructed to stand as quietly as possible in the initial position before the beginning of the trial. The subjects heard a computer generated beep 500 ms after data collection had begun, which indicated to them that they could initiate the required action. Subjects were reminded not to initiate their actions immediately after the beep, but to wait for about a second.
The order of the conditions was pseudo-randomized across subjects. A rest period of 6 s between trials and a rest period of 2 min between two conditions was given. Sufficient rest periods (about 1 min) were given between sets of trials, such that fatigue was never an issue. Prior to each condition, two practice trials were given.
Different variations of the three tasks were used at different steps of analysis (Steps 1, 2 and 3 described in the “Introduction”). In all there were seven series of experiments:
Step 1: identification of M-modes
- Series 1:
-
Releasing the 3-kg load behind the subject (LRB): 50 trials (two sets of 25)
This particular task was selected based on results from our previous study (Krishnamoorthy et al., in press), as leading to the most reproducible M-modes across subjects.
Step 2: computation of the Jacobian matrix
Three series of experiments were selected to analyze the relations between changes in magnitudes of M-modes and the corresponding COP shifts, i.e. to define the Jacobian matrix:
- Series 2:
-
Releasing different loads (2–7 kg in increments of 0.5 kg) behind the subject (LRBV): 2 repetitions with each load for a total of 22 trials;
- Series 3:
-
Shift of COP voluntarily towards the toes at varying speeds (VSF), 22 trials; and
- Series 4:
-
Shift of COP voluntarily towards the heel at varying speeds (VSB), 22 trials (only seven subjects performed this task);
First, we used the load release task as for the identification of M-modes, but with varying weights, LRBV. In this series, forward COP shifts were an implicit task component; they occurred prior to the release of the load and were associated with APAs. Second, we used a task associated with explicit COP shifts in the same direction, forward (VSF). Third, voluntary sway backwards (VSB) was used to check if relations between COP shifts and magnitudes of M-modes were direction-specific.
Step 3: UCM analysis
At this step, we used a set of tasks associated with APAs leading to COP shifts.
- Series 5:
-
Releasing the 3 kg load in front of the subject (LRF): 25 trials (this condition was more fatiguing than the LRB because the subject acted against the combined weight of the arms and the load. Hence, the series were split into two sets of 15 and 10 trials);
- Series 6:
-
Fast arm movement forward (AMF): 25 trials; and
- Series 7:
-
Fast arm movement backward (AMB): 25 trials.
In addition, two control trials were performed: The subject was asked to hold a load of 5.3 kg in front of the body and behind the body (through the pulley system) for 5 s. These data were used for EMG normalization as described in the next subsection.
Data processing
All signals were processed off-line, filtered with a 50 Hz low-pass, fourth order, zero-lag Butterworth filter using LabView 4. All EMG signals were rectified. Individual LR and AM trials were viewed on a monitor screen and aligned according to the first change in the signal of the accelerometer (movement initiation) that could be identified by visual inspection at optimal resolution. This moment will be referred to as “time zero” (t0). VS trials were aligned by the first visible shift of My.
Changes in the background muscle activity associated with the early phase of the COP shift were quantified as follows. In the LR and AM trials, rectified EMG signals were integrated from 100 ms prior to t0 to t0 (∫EMG). In these trials, My shift started, on average, 80 ms prior to t0 (cf. Aruin and Latash 1995). Since VS trials were aligned by the earliest My shift, to have comparable intervals of EMG integration across tasks, EMG were integrated from −20 ms to +80 ms with respect to t0 in the VS task (Krishnamoorthy et al., in press). These integrals were corrected by subtracting integrated activity from –500 to –450 ms prior to t0 multiplied by two (the baseline EMG activity, ∫EMGbl).
In order to compare the ΔIEMG indices across muscles and subjects, we normalized them by the integrals of EMGs collected in the control trials as follows: ΔIEMG indices for dorsal (ventral) muscles were divided by integrals of EMG over 100 ms in the middle of the control trial, IEMGcontrol, during holding the load in front of (behind) the body:
Coordinates of the center of pressure (COP) in the anterior-posterior directions were calculated using the following approximation:
COP shift corresponding to the EMG activity calculated above was computed as follows:
where My1/Fz1 was computed at time, t0+50 ms and My2/Fz2 is the average COP position between −150 ms and −100 ms with respect to t0 (50 ms prior to the period of EMG integration).
Statistics
Standard statistical methods were used. Data are mostly presented as means and standard errors.
Step 1: defining M-modes using principal component analysis (PCA)
For the LRB series, in each subject, we have ΔIEMGnorm data matrices with the size 50×11 (50 rows corresponding to repetitions and 11 columns corresponding to muscles). The correlation matrix between the ΔIEMGnorm was subjected to PCA, using procedures from Statistica 6.0 (StatSoft, Inc.). The correlations were computed among the columns. The factor analysis module with principal component extraction was employed.
For each subject, the obtained eigen-values and PCs of the matrix were then considered. Based on the percentage of total variance accounted by individual PCs (see later) and on analysis of the scree plots, only the first three PCs (M-modes) were selected for further analysis. The eigenvectors of the three PCs were used in further data processing.
Step 2: defining the Jacobian using multiple regression
Linear relations between changes in the M-modes magnitudes and the COP shifts were assumed and the corresponding multiple regression equations computed. The coefficients of the regression equations were arranged in a matrix that is in essence a Jacobian matrix, J. Series 2, 3 and 4 were used to generate linear estimates of the Jacobians. The columns of the J are coefficients relating changes in magnitude of M-modes (ΔMMMs) to COP shift. Three tasks (LRBV, VSB, VSF) were used to define three separate Jacobians (J LRBV, J VSB, and J VSF). This was done to check whether the Jacobians were task-specific and/or COP direction specific.
ΔIEMGnorm data (22×11) for each of the series (LRBV, VSF and VSB) were multiplied with the eigenvectors (11×3) obtained at Step 1 and further summed up to yield three ΔMMMs (22×3) for each trial. A multiple regression analysis was then performed using these ΔMMMs as the independent variables and the corresponding ΔCOP shift as the dependent variable (see Step 2, Procedure). Optimal sets of coefficients were defined for each subject and for each of the three series using:
With this approach, the Jacobian matrices are reduced to (3×1) vector-columns.
Step 3: UCM analysis
For each trial of series 5, 6 and 7, ΔIEMGnorm were computed and transformed into ΔMMMs as in Step 2. The hypothesis that ΔCOP is stabilized, accounts for one degree of freedom (DOF; d=1). The space of ΔMMMs has dimensionality n=3. Thus, the system is redundant with respect to the task of stabilizing ΔCOP. The mean contribution of each M-mode to ΔCOP was calculated. Since the model relating ΔMMMs to ΔCOP is linear, the mean values were subtracted from each computed value and the residuals were further analyzed as follows.
The uncontrolled manifold represents combinations of M-modes that are consistent with a stable value of ΔCOP. The UCM is calculated as the null space of the Jacobian matrix. The null space of J is the set of all vector solutions x of the system of equations Jx=0. The null space is spanned by the basis vectors, ε i , which have 2 DOFs. The vector of individual mean-free ΔMMMs was resolved into its projection onto the null space:
and component orthogonal to the null space:
The amount of variance per DOF within the UCM is:
and orthogonal to the UCM is:
We used the Wilcoxon signed rank test to compare if there was a significant difference between VUCM and VORT across subjects. A non-parametric test was used because of the relatively small sample size and high variability across subjects.
Results
General EMG patterns
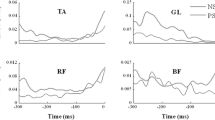
When a subject stood and held a load in front of the body, there was increased background activity of dorsal muscles (GL, GM, SOL, BF, ST, and ES). Prior to load release, a drop in this activity was typically seen, commonly accompanied by bursts of activity in the ventral muscles (TA, RA, VL, VM, and RF). Conversely, prior to load release behind the body, the background activity in ventral muscles typically dropped, and there could be EMG bursts in the dorsal muscles. Figure 2A illustrates typical EMG patterns in a representative subject for a LRB trial.
EMG activity in the 11 postural muscles during a trial of load release at the back, LRB (A), voluntary sway forward, VSF (B), and arm movement forward, AMF (C), for a typical subject. Vertical dashed lines correspond to time zero, t0. EMG was integrated over the 100 ms interval before t0 (GL lateral head of gastrocnemius, GM medial head of gastrocnemius, SOL soleus, BF biceps femoris, ST semi-tendinosus, ES erector spinae, TA tibialis anterior, VL vastus lateralis, VM vastus medialis, RF rectus femoris, RA rectus abdominis)
Fast arm movements forward AMF (backwards, AMB) were preceded by an increase in the background activity of ventral (dorsal) muscles accompanied by a decrease in the activity of dorsal (ventral) muscles. Figure 2C illustrates typical EMG patterns in a representative subject for an AMF trial. Early EMG changes (APAs) were variable across subjects; some subjects did not show clear bursts or episodes of EMG suppression in some muscles.
In trials involving voluntary sway forward (VSF), a drop in the background activity of ventral muscles and bursts of activity in dorsal muscles usually accompanied an early anterior shift of the COP. In the VSB trials, there was an increase in the background activity of ventral muscles and a drop in the activity of dorsal muscles. Figure 2B shows typical EMG patterns during a VSF trial. These EMG patterns also varied across subjects.
Identification of M-modes: results of PCA
The indices of integrated muscle activity associated with an early shift of the COP (ΔIEMG indices, see the Methods) for all muscles were measured in each trial of Series 1 that involved 50 repetitions of LRB task. These were normalized by the integrated muscle activity during control trials (ΔIEMGnorm indices). The ΔIEMGnorm indices for each subject were subjected to a PCA. Consistent with the previous study (Krishnamoorthy et al., in press), across all subjects, we found that principal components from PC4 onwards not only explained little variance in the ΔIEMGnorm space, but these components also had at most one muscle with significant loading, and were poorly reproducible across subjects. There were three consistent PCs accounting on average for about 62% (±1%) of the total variance. The average amount of variance explained by PC1 was 32% (±1%), by PC2 was 17% (±1%) and by PC3 was 12% (±0.5%) across all subjects. Table 1 shows the loadings of all the muscles on the three PCs for a representative subject in the LRB condition. The significant loadings (loadings above ±0.5; see Hair et al. 1995) are in bold. Muscles typically seen in the three PCs were:
- PC1:
-
GL, GM, SOL, BF, ST, ES—”push-back M-mode” or M1-mode.
- PC2:
-
VL and/or VM, RF, TA—”push-forward M-mode” or M2-mode.
- PC3:
-
TA, RA, VM, GL—“mixed M-mode” or M3-mode.
The groups are named based on the general effect of the changes in muscle activity in a group on the center of mass displacement. The muscles indicated in italics in the third synergy, did not show up consistently in the PC indicated, but were sometimes in one of the other M-modes.
Identifying the Jacobians: results of multiple regression procedure
To identify the relations between changes in the magnitudes of the three M-modes (MMMs) and associated COP shifts (ΔCOP), we used series 2, 3, and 4. In these series, the subjects were asked to produce sets of trials, which induced early COP shifts of different magnitude. Three series were used to identify three possible sets of coefficients between MMMs and ΔCOP (three Jacobians), one associated with an implicit early COP shift forward (LRBV), the second associated with an explicit COP shift forward (VSF), and the third associated with an explicit COP shift backwards (VSB).
Table 2 presents a summary of the regression coefficients for the three conditions for all subjects. Note that the three Jacobians (J LRBV, J VSB, and J VSF) vary significantly in the magnitudes of coefficients. Numbers in bold in Table 2 are significant predictors of ΔCOP. In general, M1-mode was a significant predictor in series LRBV and VSF. M2-mode was a significant predictor in LRBV and VSB, although only in some of the subjects. M3-mode was a significant predictor in VSB and rarely in LRBV and VSF. The three M-modes accounted for 79% (±6%) of the total variance in ΔCOP for the LRBV series, 85% (±3%) of the total variance in ΔCOP for the VSB series and 88% (±3%) of the total variance in ΔCOP for the VSF series.
We also used these data to confirm the results of identification of M-modes described in the previous section. To do this, variance in the space of integrated EMG indices was partitioned into components within the space of M-modes and orthogonal to that space. Then, each variance component was divided by the number of DOFs in each of the two subspaces. On average, variance per DOF in the M-mode space was twice as high as orthogonal to the M-mode space. Each subject showed higher variance per DOF within the M-mode space in each of the three tests; this difference was statistically significant (p<0.01) as confirmed by Wilcoxon signed-rank tests.
UCM analysis
Data from three series (series 5, 6, and 7 in the Methods) were used to perform analysis of the structure of variability in the space of M-modes. These series were associated with implicit early shifts of the COP during APAs. One of them involved an action similar to that used to identify M-modes and their relations to ΔCOP, namely releasing the load held in front of the body (LRF). The other two series involved a completely different action, fast bilateral arm movement forward (AMF) or backwards (AMB). Total variance in the M-mode space across repetitions was partitioned into two components, one of which (VUCM) was within an uncontrolled manifold (UCM) computed using one of the Jacobians defined at the previous step, while the other one (VORT) was orthogonal to the UCM.
Figure 3 shows the log-transformed ratios of VUCM to VORT averaged across subjects with standard error bars, for each of the three series LRF, AMF and AMB, using each of the Jacobians, J LRBV, J VSB, J VSF. The top, middle and bottom panels show results for the LRF, AMF and AMB series respectively. The bars with upward slanted lines in all three panels are results of using the Jacobian from the LRBV condition (J LRBV). The middle bars in all panels are results from using J VSB and the bars with downward slanted lines are results from using J VSF.
Results of UCM analysis across subjects for the series load release in the front, LRF, arm movement forward, AMF, and arm movement backward, AMB. The bars represent ratio of VUCM to VORT after natural log transformation. The bars with upward slanted lines are results from using J LRBV, the unfilled bars from J VSB and the bars with downward slanted lines from J VSF
VUCM is significantly higher than VORT if the log-transformed ratio of the two is significantly different for zero. Relatively high average values of the ratio were observed when data from series associated with an early shift of the COP forward (backwards) were processed using Jacobians computed also based on series with an early COP shift forward (backwards). However, these values were not different from zero when data from a series associated with a COP shift in a certain direction were processed using a Jacobian from a series with an early COP shift in the opposite direction. In particular, Wilcoxon tests have confirmed that the ratio was significantly higher than zero when the data from the LRF and AMF series were processed using J VSB and when the data from the AMB series were processed using J VSF. The ratios were not significantly different from zero when the other combinations of series and Jacobians were used.
Discussion
The main purpose of this study has been to explore the possibility of identification of muscle synergies in a postural task using the framework offered by the uncontrolled manifold hypothesis (Scholz and Schöner 1999; Latash et al. 2002b). One of the major advantages of the UCM-hypothesis is that it allows testing different control hypotheses, i.e. hypotheses on variables that may or may not be selectively stabilized by the coordinated activity of a set of motor elements forming a motor synergy. Earlier studies have shown that analysis of the structure of variability within the UCM-hypothesis is indeed able to distinguish among competing control hypotheses using studies of kinematic and kinetic variables (Scholz and Schöner 1999; Scholz et al. 2000, 2002; Latash et al. 2001, 2002a, 2002b). However, as mentioned in the Introduction, applying the UCM-hypothesis to EMG signals is far from being trivial because of the several necessary steps that need to be taken to partition the variance in the space of muscle activation patterns (EMG space) into components that affect and do not affect a hypothesized performance variable.
We selected for this study a set of postural tasks partly based on our previous experience with such tasks (Aruin and Latash 1995; Shiratori and Latash 2000) and partly because maintenance of the vertical posture has commonly been associated with the generation of adequate patterns of shifts of the center of pressure (Collins and De Luca 1993; Winter et al. 1998; Zatsiorsky and Duarte 2000; Baratto et al. 2002). Note that the control hypothesis that patterns of activation of postural muscles are organized to stabilize a particular pattern of the COP shift is not the only possible one. Other performance variables can be considered such as position of the center of mass of the body (cf. Gollhofer et al. 1989; Vernazza et al. 1996) or position of the head (cf. Pozzo et al. 1990; Simoneau et al. 1992; Ledebt et al. 1995).
Within the current study, however, we tested only one control hypothesis, namely that the CNS organizes co-variations of control variables to stabilize a COP shift. Within the selected time window of 100 ms, actual displacements of the joints and of the center of mass are very small and cannot be assessed with sufficient accuracy. On the other hand, we wanted to limit our analysis to a small time window that has typically been used in APA studies (Massion 1992). This is a limitation to be overcome in future.
Using the UCM approach to identify postural synergies
In our previous experiment (Krishnamoorthy et al., in press), we showed that indices of muscle activity (integrated EMG) associated with an early shift of the COP could be described with a few principal components. This approach is somewhat similar to attempts at identifying motor synergies using PCA applied to kinematic or kinetic data (Alexandrov et al. 1998a, 1998b; Vernazza-Martin et al. 1999; Sabatini 2002). We interpreted the presence of a few reproducible PCs as evidence that the CNS uses a few central variables (“muscle modes” or M-modes) to adjust activity of the many postural muscles contributing to the production of a desired COP shift. Further, the directions of vectors of PCs in the muscle space were similar across subjects and across tasks. This was not a trivial finding, since the tasks varied not only in the direction of required COP shift (anterior or posterior) and magnitude of perturbation (releasing a heavy or light load), but the tasks also involved either an explicit (voluntary sway, VS) COP shift or implicit COP shift associated with anticipatory postural adjustments (APAs, see Massion 1992) prior to releasing a load held in the hands of extended arms.
We did not interpret M-modes as multi-muscle synergies. This contrasts with other recent work that employed statistical procedures similar to PCA to identify groups of muscles employed in the performance of functional tasks (Bizzi et al. 2002; Tresch et al. 1999). Those investigators have characterized the identified muscle groupings as synergies that are used as basic building blocks in the construction of functional postures or movements. Instead, we prefer to characterize such muscle groupings as control modes and view identification of M-modes as only the first step along the road to identification of multi-muscle synergies, namely the step of identification of a set of independent central variables that are organized into synergies by the CNS. Another important result from that study has been the identification of a task associated with most reproducible M-modes across subjects, namely load release in the back of the body. We used this result to select the first series to identify M-modes in the present study. Figure 4 illustrates the idea of control using a set of M-modes. The controller is assumed to define magnitudes of the three M-modes ultimately resulting in changed levels of activation of all the postural muscles. According to our control hypothesis, changes in magnitudes of the M-modes co-varied to preserve a particular value of COP shift.
A scheme illustrating idea of control using a set of M-modes. The controller defines magnitudes of the three M-modes resulting in changed levels of activation of all the postural muscles, which preserve a particular value of COP shift. Abbreviations for muscles are the same as in Fig. 2
Further analysis in the M-mode space was directed at computing a null-space (UCM) of the Jacobian linking variations of magnitudes of M-modes to COP shifts. This null-space provides a linear estimate of a manifold in the space of M-modes, the values of which stabilize a particular value of COP shift. Variance in the M-mode space obtained in Step 3 experiments was then projected onto this UCM and orthogonal to it. We found that the variance along the UCM was significantly higher than orthogonal to it for the tasks LRF, AMF and AMB when analysis was based on the Jacobians computed using series associated with COP shifts in the same direction. Thus, we can conclude that there is a multi-M-mode synergy, which selectively stabilizes the desired magnitude of the COP shift in these conditions. In other words, the gains at the M-modes co-varied across trials in such a way that the effects of their variation on the COP shift compensated (partly) for each other.
Two separate postural synergies
We purposefully used different tasks at different steps of the study. The differences among the tasks were two-fold. First, COP shift could be an explicit (voluntary sway) or implicit (APA) task component. Second, it could be directed forward or backwards. Because of the natural limitation on the number of trials that could be performed by a subject within one session, we could not do a complete crossover design. However, one result suggests that two different M-mode synergies are used to stabilize the displacement of the COP in different directions. The use of different Jacobians defined in the three different series at Step 2 (see the Introduction) yielded different results when the UCM analysis was performed. Note that the tasks LRBV and VSF required an anterior early shift of the COP, while VSB required an early posterior COP shift. In the main experiment, the tasks LRF and AMF were accompanied by an early posterior COP shift, whereas AMB was accompanied by an anterior COP shift (see Table 3).
When the UCM analysis (Step 3) was done using the Jacobian from a task (Step 2) requiring COP shift in the same direction at both Steps, VUCM was significantly higher than VORT (except when the Jacobian from the LRBV task was used, which never showed significant results). However, there were no differences in the two variance components when early COP shifts at Steps 2 and 3 were in opposite directions. One may conclude, therefore, that even though the same M-modes were being used, their gains were adjusted differently to perform COP shifts in the anterior and in the posterior directions.
We would like to note that such tasks as voluntary sway forward and backwards and fast arm movements forward and backwards started from similar postures and, as such, may be assumed to be associated with similar levels of the background postural muscle activity. Hence, we have assumed that the control system acted about the same initial operating point and the differences in the Jacobians and outcomes of the UCM analysis were related to the required different directions of the COP shift. Note that the load release tasks are associated with significantly different background levels of postural muscle activity. This may be a reason why using the Jacobian defined in the load release task did not show significant UCM effects. This observation suggests that our assumption of linear relations between COP shifts and magnitudes of the M-modes may be correct only locally, about a given operating point in the M-mode space, but may be violated significantly if the operating point changes.
During quiet comfortable standing, the COP falls just in front of the ankle joint (Winter et al. 1998). As a result, there is a larger ‘safe’ area for COP shift within the base of support in the anterior direction as compared to its shift backwards. Therefore, a posterior COP shift may be perceived as potentially more destabilizing and a different strategy of muscular interactions may be used to produce it as compared to a forward COP shift. Note that different postural strategies have been described for tasks that differed in conditions of postural stability (Horak and Nashner 1986; Szturm and Fallang 1998). These observations are compatible with our conclusion on the two synergies used to produce anterior and posterior COP shifts.
M-modes and postural synergies
As was mentioned in the Introduction, the term postural synergy has been used by a number of researchers, usually to mean co-variation of EMGs or kinematic indices over several trials of postural tasks. In particular, terms such as ankle strategy, hip strategy (Horak and Nashner 1986; Horak et al. 1990), or multi-link strategies (Allum et al. 1989) have been used implying different postural synergies. We have now shown that even though patterns of activity of peripheral elements (muscles) may co-vary, making it possible to identify M-modes, this co-variation falls short of proving stabilization of an important performance variable. Indeed, the M-modes may then be manipulated differently in a task-specific fashion, resulting in at least two different synergies. This adds support to our general view that muscle synergies are not merely a set of muscles that ‘work together’, but a set of central variables that show task-specific co-variations to stabilize significant performance variables.
References
Alexandrov A, Aurenty R, Massion J, Mesure S, Viallet F (1998a) Axial synergies in parkinsonian patients during voluntary trunk bending. Gait Posture 8:124–135
Alexandrov A, Frolov A, Massion J (1998b) Axial synergies during human upper trunk bending. Exp Brain Res 118:210–220
Allum JH, Honegger F (1993) Synergies and strategies underlying normal and vestibulary deficient control of balance: implication for neuroprosthetic control. Prog Brain Res 97:331–348
Allum JH, Honegger F, Pfaltz CR (1989) The role of stretch and vestibulo-spinal reflexes in the generation of human equilibrating reactions. Prog Brain Res 80:399–409
Aruin AS, Latash ML (1995) Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res 103:323–332
Aruin AS, Latash ML (1996) Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol 101:497–503
Baratto L, Morasso PG, Re C, Spada G (2002) A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Motor Control 6:246–270
Belen’kii V, Gurfinkel VS, Pal’tsev YI (1967) Elements of control of voluntary movements. Biofizika 10:135–141
Bernstein N (1967) The coordination and regulation of movements. Pergamon, London
Bizzi E, D’Vella A, Saltiel P, Tresch M (2002) Modular organization of spinal motor systems. Neuroscientist 8:437–442
Bouisset S, Lestienne F, Maton B (1977) The stability of synergy in agonists during the execution of a simple voluntary movement. Electroencephalogr Clin Neurophysiol 42:543–551
Collins JJ, De Luca CJ (1993) Open-loop and closed-loop control of posture: a random-walk analysis of center-of-pressure trajectories. Exp Brain Res. 95:308–318
Crenna P, Frigo C, Massion J, Pedotti A (1987) Forward and backward axial synergies in man. Exp Brain Res 65:538–548
Danion F, Duarte M, Grosjean M (1999) Fitts’ law in human standing: the effect of scaling. Neurosci Lett 277:131–133
Gelfand IM, Tsetlin ML (1966) On mathematical modeling of the mechanisms of the central nervous system. In: Gelfand IM, Gurfinkel VS, Fomin SV, Tsetlin ML (eds) Models of the structural-functional organization of certain biological systems. Nauka, Moscow, pp 9–26
Gollhofer A, Horstmann GA, Berger W, Dietz V (1989) Compensation of translational and rotational perturbations in human posture: stabilization of the centre of gravity. Neurosci Lett 105:73–78
Hair JF, Anderson RE, Tatham RL, Black WC (1995) Factor analysis. In: Borkowsky D (ed) Multivariate data analysis. Prentice Hall, Englewood Cliffs, NJ, pp 364–404
Holdefer RN, Miller LE (2002) Primary motor cortical neurons encode functional muscle synergies. Exp Brain Res 146:233–243
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55:1369–1381
Horak FB, Nashner LM, Diener HC (1990) Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82:167–177
Hughlings Jackson J (1889) On the comparative study of disease of the nervous system. Br Med J 17:355–362
Kilbreath SL, Gandevia SC (1994) Limited independent flexion of the thumb and fingers in human subjects. J Physiol 479:487–497
Krishnamoorthy V, Goodman S, Zatsiorsky VM, Latash ML (in press) Muscle synergies during shifts of the center of pressure by standing persons: identification of steadfast muscle groups. Biol Cybern
Latash ML, Scholz JF, Danion F, Schöner G (2001) Structure of motor variability in marginally redundant multifinger force production tasks. Exp Brain Res 141:153–165
Latash ML, Scholz JF, Danion F, Schöner G (2002a) Finger coordination during discrete and oscillatory force production tasks. Exp Brain Res 146:419–432
Latash ML, Scholz JP, Schöner G (2002b) Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 30:26–31
Ledebt A, Bril B, Wiener-Vacher S (1995) Trunk and head stabilization during the first months of independent walking. Neuroreport 6:1737–1740
Massion J (1992) Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 38:35–56
Massion J, Gurfinkel V, Lipshits M, Obadia A, Popov K (1992) [Strategy and synergy: two levels of equilibrium control during movement. Effects of the microgravity]. C R Acad Sci III 314:87–92
Pozzo T, Berthoz A, Lefort L (1990) Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp Brain Res 82:97–106
Sabatini AM (2002) Identification of neuromuscular synergies in natural upper-arm movements. Biol Cybern 86:253–262
Scholz JP, Schöner G (1999) The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126:289–306
Scholz JP, Schöner G, Latash ML (2000) Identifying the control structure of multijoint coordination during pistol shooting. Exp Brain Res 135:382–404
Scholz JP, Danion F, Latash ML, Schöner G (2002) Understanding finger coordination through analysis of the structure of force variability. Biol Cybern 86:29–39
Schöner G (1995) Recent developments and problems in human movement science and their conceptual implications. Ecol Psychol 8:291–314
Shiratori T, Latash ML (2000) The roles of proximal and distal muscles in anticipatory postural adjustments under asymmetrical perturbations and during standing on rollerskates. Clin Neurophysiol 111:613–623
Simoneau GG, Leibowitz HW, Ulbrecht JS, Tyrrell RA, Cavanagh PR (1992) The effects of visual factors and head orientation on postural steadiness in women 55 to 70 years of age. J Gerontol 47:M151–158
Szturm T, Fallang B (1998) Effects of varying acceleration of platform translation and toes-up rotations on the pattern and magnitude of balance reactions in humans. J Vestib Res 8:381–397
Tresch MC, Saltiel P, Bizzi E (1999) The construction of movement by the spinal cord. Nat Neurosci 2:162–167
Vernazza S, Alexandrov A, Massion J (1996) Is the center of gravity controlled during upper trunk movements? Neurosci Lett 206:77–80
Vernazza-Martin S, Martin N, Massion J (1999) Kinematic synergies and equilibrium control during trunk movement under loaded and unloaded conditions. Exp Brain Res 128:517–526
Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K (1998) Stiffness control of balance in quiet standing. J Neurophysiol 80:1211–1221
Zatsiorsky VM, Duarte M (2000) Rambling and trembling in Quiet standing. Motor Control 4:185–200
Zatsiorsky VM, Li ZM, Latash ML (1998) Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biol Cybern 79:139–150
Zatsiorsky VM, Li ZM, Latash ML (2000) Enslaving effects in multi-finger force production. Exp Brain Res 131:187–195
Acknowledgements
Supported in part by grants AG-018751 and NS-35032 from the National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00221-003-1779-8
Rights and permissions
About this article
Cite this article
Krishnamoorthy, V., Latash, M.L., Scholz, J.P. et al. Muscle synergies during shifts of the center of pressure by standing persons. Exp Brain Res 152, 281–292 (2003). https://doi.org/10.1007/s00221-003-1574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1574-6