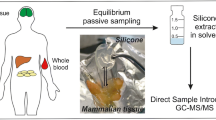
Abstract
Food contact materials (FCM) polyethylene terephthalate (PET) and polybutylene terephthalate (PBT) used extensively in food packaging may contain cyclic oligomers which may migrate into food and thus cause toxic effects on human health. A simple, fast, and sensitive ultra-high-performance liquid chromatography method quadrupole time-of-flight mass spectrometer was developed for the analysis of 7 cyclic oligomers in post-mortem blood samples. The targeted analytes were separated on a Waters BEH C18 (150 × 2.1 mm, 1.7 µm) analytical column by gradient elution. Calibration curves were constructed based on standard solutions and blood samples and Student’s t-test was applied to evaluate the matrix effect. The LODs ranged from 1.7 to 16.7 μg mL−1, while the method accuracy was assessed by recovery experiments and resulting within the range 84.2–114.6%. Such an analytical method for the determination of PET and PBT cyclic oligomers in biological samples is reported for the first time. The developed methodology allows the determination of these oligomers in blood providing a useful analytical tool to assess the exposure and thus the potential hazard and health risks associated with these non-intentionally added substances (NIAS) from PET and PBT FCM through food consumption. The method was validated and successfully applied to the analysis of 34 post-mortem whole blood samples. Polyethylene terephthalate trimer was detected in four of them, for the first time in literature.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyesters (PES) are a group of polymers used extensively in many fields of applications. They are among the plastics of preference used in the production of food contact materials (FCMs), due to properties like impact resistance, strength, flexibility, and resistance to high temperatures [1, 2]. Among the most relevant FCMs of PES, polyethylene terephthalate (PET) and polybutylene terephthalate (PBT) are prevailing [3]. PET is the most used PES in beverage packaging applications due to its excellent properties like light-weight and low carbon dioxide permeability [1, 4,5,6].
However, during the synthesis of almost all polymers, low molecular weight circular oligomers are formed as a result of uncontrolled polymerization reactions that take place. These cyclic oligomers are characterized as “non-intentionally added substances” (NIAS). Undesired and unregulated, NIAS apart from cyclic oligomers encompass several different types of chemical families, and their presence in the FCMs may originate from impurities in the raw materials, by-products from reaction processes, and even degradation products of the used additives or the polymers [7].
Among the most well-known NIAS in plastics are a group of substances called oligomers. Normally formed during polymerization, oligomers are side-reaction products ranging from simple dimers up to decamers, in either cyclic or linear forms. Their formation seems to be unavoidable and even thermodynamically favored, with some works also pointing at their potential formation due to degradation of the plastic packaging itself [1, 5, 8]. Oligomers have been shown to migrate from the plastic material into the food, which has led in recent years to an increase of published works on this topic [1, 8,9,10]. There is also a growing interest on oligomers at EU level, with the European Union Reference Laboratory for Food Contact Materials (EURL-FCM) having organized a couple of proficiency tests (PTs) on the topic [5, 11, 12]. These PTs evaluated the capacity of EU National Reference Laboratories and Official Control Laboratories in the determination and quantification of both PET and PBT cyclic oligomers in food simulants, substances which have not been analyzed before by any of the FCM network laboratories.
It has been long known that FCMs can be a medium of human exposure to hazardous substances present in these materials. There is a wide range of toxicologically uncharacterized chemicals, both intentionally added substances (IAS) and NIAS, that can end up in food [13]. That is also the case of the PES plastic packaging oligomers, which unavailable toxicological data leaves their risk for human health largely unclear [5]. However, in a recent study, some PET cyclic oligomers have been classified via an in silico assessment as having a Cramer III toxicity, the highest level apart from genotoxicity [11, 14, 15]. This toxicity is expected only for the cyclic oligomers, as their linear counterparts are Cramer I, thus having a reduced toxicity when consumed. In another work, the endocrine activity of cyclic PES oligomers has been studied with in vitro assays, where it was indicated a weak androgen receptor antagonism for these substances, with no arylhydrocarbon receptor activity or binding to the thyroid hormone transport protein being reported [16].
Despite the potential toxicity of the cyclic PES oligomers, existing works claim that upon ingestion their amounts are considerably decreased via hydrolysis reactions that occur during digestion [16]. These reactions originate from their corresponding non-toxic linear counterparts, thus decreasing the overall toxicity concerns. In another work, Eckardt et al. studied the in vitro intestinal digestion of individual cyclic PET and PBT oligomers. The results showed a cleavage of the ester group of these cyclic oligomers after 4 h of simulated intestinal incubation, pointing to an expected similar occurrence in real human intestinal conditions [17]. However, in order to evaluate human exposure and hazard assessment, accurate analytical tools are required.
In the literature, few analytical methodologies have been described for oligomers’ determination probably due to the difficulties that may occur from the lack of the available analytical standards [15]. Most studies use semi-quantitative approaches, quantifying all PET linear and cyclic oligomers using the PET cyclic trimer as an analytical standard [4, 18,19,20,21]. However, these semi-quantitative methods may suffer from limitations related to the accurate quantification of oligomers with longer chain lengths and higher molecular masses. Recently, some studies [15, 16, 22,23,24,25] reported their synthesis and the development and optimization of analytical methods used for the quantification of PET and PBT oligomers presenting satisfactory results. However, none of these methodologies has been applied in biological samples.
Here, an analytical methodology was developed and validated for the quantification of seven PET and PBT cyclic oligomers in blood plasma with the aim to investigate the potential exposure through the detection and quantification of these substances in blood. The method included a liquid–liquid (LLE) extraction step followed by a targeted UHPLC-qTOF-MS analysis achieving low detection limits. The optimized method was successfully applied in post-mortem blood samples, where the identification and quantification of one of the oligomers were found for the first time. The obtained results indicate that these compounds are not fully hydrolyzed during digestion as previously expected, giving rise to the possibility of accumulation in the human body in their cyclic and toxic conformation. This is the first method reported for determining cyclic PES oligomers in biological samples that can provide critical data enabling the assessment of exposure to these NIAS.
Materials and methods
Reagents and materials
Acetonitrile, methanol, dichloromethane, n-hexane, and formic acid (≥ 99%) were all LC–MS analytical grade (HiPerSolv CHROMANORM) and were supplied from VWR Chemicals (Darmstadt, Germany). Butyl acetate (LC–MS grade) was purchased by Sigma-Aldrich (Merck Darmstadt, Germany), while glacial acetic acid (99–100%) and ethyl acetate (LC–MS grade) were acquired from Chem-Lab (Zedelgem, Belgium). 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) was supplied from Honeywell Fluka™ (Buchs, Switzerland). Ultrapure water by a Milli-Q purification system (Merck Darmstadt, Germany) at 18 MΩ-cm (at 25 °C). The first series PET cyclic oligomer standards up to pentamer and the internal standard PET cyclic trimer-d12 were obtained from TRC chemicals (Toronto, Canada), with stated purities ranging from 95 to 97%. PBT cyclic dimer, trimer, and tetramer were obtained as a standard mixture (20 µg mL−1) from the European Union Reference Laboratory for Food Contact Materials (EURL-FCM).
Calibration
Calibration and preparation of solutions in standard mixtures
Stock solutions of the PET dimer and the isotope internal standard were prepared at a concentration of 5000 mg L−1 in HFIP whereas PET trimer, tetramer, and pentamer stock solutions were at 2500 mg L−1. PBT mix solution in HFIP was at a concentration of 20 mg L−1, in HFIP as solvent. A multi-component solution (2.5 mg L−1) containing equal concentrations of every analyte was prepared weekly by mixing the appropriate volumes of the stock solutions. Serial dilutions of this mix solution followed in amber vials with ethanol:H2O 50:50 (v/v), to prepare working standard mixtures at ten concentrations (5, 10, 25, 50 100, 250, 500, 750, 1000, 2500 µg L−1). Stock solutions and working standards were both kept at − 20 °C. Calibration curves were constructed by plotting the means of ratios of the compound peak areas to the internal standard peak areas (PET cyclic trimer-d12) against concentrations of the analytes.
Preparation of solutions for calibration
A post-mortem blood sample in which the analytes were not detected was selected to constitute the “blank blood sample” providing the matrix background. For the construction of the calibration curves in blood, the blank blood sample was fortified with known standard mixtures of the 7 cyclic oligomers to a final concentration of 12.5, 25, 50, 125, 250, 500, 750, 1000, and 1500 µg L−1. A blank sample was also prepared with the same procedure. The calibration curves were constructed as mentioned above, in the “Calibration and preparation of solutions in standard mixtures” section.
Sample preparation
A total of 34 post-mortem blood samples were collected at the Forensic Service of Greek Ministry of Justice in Thessaloniki during autopsy and stored in glass tubes containing NaF from cases aged 65 years old or older and kept at − 20 °C. A 250 μL aliquot of blood sample was fortified with 12.5 µL of IS and 600 µL of ethyl acetate was added as the extraction solvent. The mixture was vortexed for 10 min and then centrifuged at 12,000 rpm for 10 min. The upper organic phase was transferred into a 1.5 mL Eppendorf tube and the extract was evaporated to dryness, using an eppendorf concentrator. Finally, the samples were reconstituted with 100 µL ethanol:H2O 50:50 (v/v) and were transferred in LC vials and 5 µL was injected into the chromatographic system. Prior to the main analytical run, 5 injections (5 µL) of blank blood samples were performed to ensure the absence of the cyclic oligomers’ peaks in the chromatographic run and adequate system conditioning. In order to avoid any potential source of contamination, the samples came in contact only with containers (e.g., Eppendorf tubes) and syringes made of other plastic materials, such as polypropylene and polyethylene with metal needles.
UHPLC-TOF–MS analysis
An UHPLC system (Bruker, Germany) was used for chromatographic separations using a Waters BEH C18 (150 × 2.1 mm, 1.7 µm) analytical column, protected by a UPLC BEH C18 (5 × 2.1 mm, 1.8 μm) VanGuard pre-column. The mobile phase consisted of solvent A: H2O with 0.1% formic acid and solvent B: ACN with 0.1% formic acid at a flow rate of 0.3 mL/min. The elution was performed in a 12 min gradient as follows: 0–1 min: 50–70% B; 1–12 min: held to 90% B. The composition was returned to the initial (50% B) in 0.1 min. An equilibration time of 4 min was set for the column before the next injection. The analytical column was temperature-controlled at 60 °C and the injection volume was 5 µL. The system was operated by Compass HyStar 5.1 software (Bruker, Germany) and was hyphenated to a timsTOF mass spectrometer (Bruker, Germany) operating in positive ionization mode (ESI) at a 3.5 kV capillary voltage. The source operated at 300 °C and nitrogen was used as drying (12.0 L/min) and nebulizing gas (2.0 Bar). The Funnel 1 RF, Multipole RF, Deflection Delta, and Collision RF were set at 200 V, 200 Vpp, 50 V, and 700 Vpp, respectively. The acquisition mode was set at full scan MS acquiring data over the range of 300 to 1000 m/z at a rate of 3 spectra/s. For individual recalibration of the chromatograms, sodium formate solution was injected before every analysis from 0.1 to 0.3 min. Data Analysis 5.3 software was used for data handling (Bruker, Germany).
Extraction recovery
The extraction recovery of the method was determined by adding different concentrations of the analytes to the blank blood sample in two replicates. Solvents tested for the extraction of the seven oligomers included (a) ethyl acetate, (b) butyl acetate, (c) hexane, and (d) dichloromethane. The samples were fortified to obtain a nominal concentration of 1000 μg L−1 for each analyte and were extracted and analyzed according to the method described in the “Sample preparation” section. Finally, the extract was transferred into a LC–MS vial prior to LC–MS analysis.
The recovery was expressed as a percentage and calculated by using the following equation:
where spiked (b) is the calculated concentration in the blood sample before the extraction process and spiked (a) is the nominal concentration of the extracts.
Matrix effect
The effect of the matrix can be variable and unpredictable in the occurrence of coeluting endogenous components [26] and it is important to evaluate the matrix-induced signal suppression or enhancement of the analytes. Thus, the matrix effect (ME) was determined by comparing the slopes of the solvent and SAM calibration curves, using Student’s t-test [27], according to the equation:
where b1 and b2 are the slopes of the regression lines. The standard error of the difference between the regression slopes is calculated as:
where (s2y·x)p is the pooled residual mean square and the subscripts 1 and 2 refer to the two regression lines (SAM and solvent) when compared. The critical values of t-test were calculated taking into account (n1 − 2) + (n2 − 2) degrees of freedom.
Method validation
Linearity and limits of detection and quantification
The sensitivity and linearity of the proposed method were assessed by analyzing both standard solution mixtures and spiked samples over the range of 5–2500 μg L−1 and 12.5–2500 μg L−1, respectively. The slopes, intercepts, and correlation coefficients were calculated using linear least squares regression. The limits of detection (LOD) were calculated as the lowest concentrations of standard solutions or fortified samples with a 3 times signal to noise (S/N) ratio, while the limits of quantification (LOQ) were defined as three times the LODs.
Precision and accuracy in standard solutions
Precision and accuracy were assessed with standard solutions in short term (repeatability and intra-day accuracy) and for a longer period (intermediate precision and inter-day accuracy), by evaluating relative standard deviation at four concentration levels. Both short-term repeatability and accuracy were calculated by six replicate injections of each of the four multi-component standards on the same day while inter-day precision and accuracy were determined by performing triplicate measurements for three consecutive days. The measurement accuracy of the analytical method was expressed as relative error and precision as relative standard deviation Sr (%).
Results and discussion
Method development
Chromatographic parameters
PET and PBT cyclic oligomers exhibit limited solubility in most common organic solvents; hence, HFIP was selected as the solvent of choice for the solubilization of the 1st series of the target compounds based on previous studies [2, 18]. For further dilutions, a mixture of ethanol:H2O 50:50 (v/v) was used, in order to facilitate their chromatographic analysis.
Chromatographic conditions, such as the mobile phase composition, affect apart from the retention times also the ionization efficiency of the compounds and their respective analysis sensitivity. In ESI mode in particular, volatility and solvent’s ability to donate a proton are of high importance. According to previous reports, a mobile phase solvent composed of ACN and H2O was the most effective [5, 18]. Hence, a mobile phase consisting of solvent A: H2O with either 0.1% acetic acid or formic acid and solvent B: ACN with 0.1% acetic acid or formic acid was tested in gradient mode. The formic acid was finally chosen as it gave better chromatographic peak shapes and ion intensities. The gradient program was optimized to obtain the elution of the 7 analytes within an analytical run of 12 min, and with satisfactory separation of the oligomers. Figure 1 illustrates the extracted ion chromatograms of the analytical standard mixture and the representative spiked sample at 1000 μg L−1.
UHPLC-QTOF-MS extracted ion chromatogram (XIC) of the analytes (1) PET dimer, (2) PET trimer d12, (3) PET trimer, (4) PBT dimer, (5) PET tetramer, (6) PET pentamer, (7) PBT trimer, (8) PBT tetramer, at the optimal chromatographic conditions. (A) Standard multi-component mixture (1000 µg L−1) in ethanol:H2O 50:50 (v/v) solution. (B) Example chromatogram from a representative blood sample spiked with a standard multi-component mixture (1000 µg L−1) of the analytes
TOF–MS parameters
In full scan MS mode, the source (capillary voltage, temperature) and tune general parameters (Funnel 1 RF, Funnel 2 RF, Multipole RF, Collision RF, and transfer time) were optimized based on the target compounds’ precursors, in order to maximize the ion signals. Full scan data acquisition from 300 to 1000 m/z was performed for all oligomers, after direct injection of a multi-component standard mix solution at a concentration of 1000 µg L−1. All target compounds showed higher response in positive ESI mode, and these findings were in agreement with those previously reported [5, 18]. Every analyte was identified based on its precursor ion and its retention time.
Method validation
Linearity and sensitivity
The method was validated in terms of linearity, LODs, and LOQs and the results along with molecular formulas, molecular ions, and retention times are presented in Table 1. In all cases, the analytes displayed excellent linearity with correlation coefficients (r2) > 0.99. For solvent calibration, the LODs ranged from 1.7 to 6.7 µg L−1, while LOQs from 5 to 20 µg L−1. For the SAM calibration, the LODs ranged between 4.2 and 16.7 µg L−1 and the LOQs between 12.5 and 50 µg L−1. To our knowledge, this is the first method that determines cyclic oligomers in blood samples. Hence, no relevant previous analytical work exists for these groups of analytes in this matrix and a comparison of the figures of merit of the method presented here can only be evaluated per se (Table 2).
Extraction recovery and matrix effect
With the aim to investigate the optimum extraction recovery, different extraction solvents were tested, including (a) ethyl acetate, (b) hexane, (c) butyl acetate, and (d) dichloromethane. The blank blood sample was fortified with the compounds of interest to obtain a nominal concentration of 1000 µg L−1 for each analyte and the results obtained from the 4 different extractions solvents are illustrated in Fig. 2. Ethyl acetate was able to extract all seven analytes and showed the most satisfactory results, with good extraction efficiency and acceptable extraction repeatability. Among all four solvents, hexane provided the least satisfactory results with the lowest peak areas, while butyl acetate was not able to extract the PBT tetramer and PET pentamer compounds. Dichloromethane showed high extraction efficiency in five out of the seven compounds of interest; however, PBT dimer was not extracted when the specific solvent was used. Thus, ethyl acetate was selected for the extraction of the blood samples. Extraction recovery was calculated for the selected protocol based on Eq. 1 for all the analytes, which showed R% ranging from 84.2 to 114.6% at the studied concentrations (50, 250, and 500 µg L−1) levels as it is shown in Table 3.
Student’s t-test was performed in order to evaluate the matrix effect, as described in the “Matrix effect” section. The results are summarized in Table 4. Clearly, a significant difference (at 95% confidence level) existed between the calibration curves in blood and solvent calibration curves for almost all oligomers (texperimental = 3.292–27.849 > tcritical = 2.262–2.447), except for PBT tetramer (texperimental = 2.229 < tcritical = 2.306); therefore, the SAM calibration curves were used for quantification to compensate for the bias due to matrix effect.
Precision and accuracy in standard solutions
Intra-day precision of the method, calculated as relative standard deviation (Sr), ranged between 0.6 and 21.6%. Similarly, intermediate precision of all studied compounds was expressed with Sr values below 17.4% at low concentration standards. The method accuracy was expressed as relative error (percentage difference of the nominal value, Er%) and ranged for intra-day from − 12.5 to 16.9% and for inter-day between − 11.2 and 20.5%. Therefore, the results ranged within the acceptable values of ± 0.1 to ± 20.5% for the low concentrations, while higher concentrations exhibited lower relative error values. The data of the precision and accuracy study are provided in Table 2.
Analysis of blood samples
The blood samples were analyzed under the optimal conditions, and as it is shown in Table 5, PET cyclic trimer was detected in four out of the 34 examined samples. Furthermore, in sample 6, apart from the ion 577.1341, which derives from the cyclic PET trimer, a molecular ion with m/z 576.7249 was detected. A comparison of the different proposed structures using the ACD Lab MS workbook (Toronto, Canada) revealed a match of 78% for an open trimer structure, which indicates the possibility of the presence of the PET linear compounds in human body.
In the past, in vitro experiments provided evidence for the cleavage of cyclic polyester oligomers under human intestinal conditions [20]. Thus, the relatively more toxic cyclic oligomers (Cramer III) are transformed to the respective linear molecules (Cramer I toxicity). However, in this paper, the presence of cyclic PET trimer is reported for the first time in human biological samples indicating that a significant portion escapes hydrolysis at intestinal conditions during digestion and may result in the human blood at concentration levels of ca. 6–25 μg L−1. Since the fate of the more potent cyclic PET trimer in the human body has not been investigated yet, nor experimental toxicological studies have been performed, it is unknown whether this concentration is limited by hydrolysis or other metabolic activities or if cyclic PET trimer accumulates in adipose, liver, or other fatty tissues. The latter could certainly constitute significant risk that needs to be assessed with regard to public safety and exposure.
The developed methodology allows us for the determination of these oligomers in blood and thus provides a useful analytical tool to assess the exposure to these NIAS from PET and PBT FCM through food consumption.
Conclusions
The presence of oligomers in real human samples is reported for the first time using an efficient liquid–liquid extraction protocol followed by UHPLC-TOF–MS analysis. The proposed analytical method reports the quantification of 7 of the most common cyclic oligomers of PET and PBT in less than 12 min. The acquisition mode offered high sensitivity with sufficient low LOQs, capable of making quantitative statements at target levels of interest. Precision and accuracy were within the acceptable ranges for all target analytes. Finally, the method was applied for the quantification of the substances in post-mortem blood samples where only one of the examined oligomers was detected and highlighted the need for toxicological data, in order to evaluate the human health risk assessment.
References
Lopes JA, Tsochatzis ED, Karasek L, Hoekstra EJ, Emons H. Analysis of PBT and PET cyclic oligomers in extracts of coffee capsules and food simulants by a HPLC-UV / FLD method. Food Chem. 2021;345:128739. https://doi.org/10.1016/j.foodchem.2020.128739.
Tsochatzis ED, Lopes JA, Holland MV, Reniero F, Emons H, Guillou C. Isolation, characterization and structural elucidation of polybutylene terephthalate cyclic oligomers and purity assessment using a 1H qNMR method. Polymers (Basel). 2019;11:1–13. https://doi.org/10.3390/polym11030464.
Piccinini P, Senaldi C, Lopes JFA (2013) Fibre labelling polytrimethylene terephthalate - PTT- DuPonthttps://doi.org/10.2788/34915
Hoppe M, Fornari R, de Voogt P, Franz R. Migration of oligomers from PET: determination of diffusion coefficients and comparison of experimental versus modelled migration. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34:1251–60. https://doi.org/10.1080/19440049.2017.1322222.
Tsochatzis E, Dehouck P, Lopes JA, Emteborg H, Robouch E, Hoekstra E. Determination of PBT cyclic oligomers in and migrated from food contact materials-FCM-19/01 Proficiency Testing Report. JRC Technical Reports. 2019; JRC118572, Ispra, European Commission.
Ubeda S, Aznar M, Nerín C. Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC-MS-QTOF. Anal Bioanal Chem. 2018;410:2377–84. https://doi.org/10.1007/s00216-018-0902-4.
Muncke J, Backhaus T, Geueke B, Maffini MV, Martin OV, Myers JP, Soto AM, Trasande L, Trier X, Scheringer M. Scientific challenges in the risk assessment of food contact materials. Environ Health Perspect. 2017;125:1–9. https://doi.org/10.1289/EHP644.
Brenz F, Linke S, Simat T. Linear and cyclic oligomers in polybutylene terephthalate for food contact materials. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35:583–98. https://doi.org/10.1080/19440049.2017.1414958.
Kubicova M, Eckardt M, Simat TJ. Polybutylenterephthalat (PBT) im Lebensmittelkontakt: Migrationspotential von oligomeren Verbindungen. Lebensmittelchemie. 2019;73:1–2. https://doi.org/10.1002/lemi.201951018.
Tsochatzis ED, Alberto Lopes J, Dehouck P, Robouch P, Hoekstra E. Proficiency test on the determination of polyethylene and polybutylene terephthalate cyclic oligomers in a food simulant. Food Packag Shelf Life. 2020;23:100441. https://doi.org/10.1016/j.fpsl.2019.100441.
Alberto Lopes J, Tsochatzis ED, Robouch P, Hoekstra E. Influence of pre-heating of food contact polypropylene cups on its physical structure and on the migration of additives. Food Packag Shelf Life. 2019;20:100305. https://doi.org/10.1016/j.fpsl.2019.100305.
Dehouck P, Tsochatzis ED, Lopes JA, Cizek-Stroh A, Robouch P, Hoekstra EJ. Determination of the mass fractions of PBT and PET oligomers in food simulant D1-FCM-18-01 Proficiency Test Report. JRC Validated methods, Reference Methods and Measurements Report. 2018; JRC113664, Ispra, European Commission.
Muncke J, Andersson AM, Backhaus T, Boucher JM, Carney Almroth B, Castillo Castillo A, Chevrier J, Demeneix BA, Emmanuel JA, Fini JB, Gee D, Geueke B, Groh K, Heindel JJ, Houlihan J, Kassotis CD, Kwiatkowski CF, Lefferts LY, Maffini MV, Martin OV, Myers JP, Nadal A, Nerin C, Pelch KE, Fernández SR, Sargis RM, Soto AM, Trasande L, Vandenberg LN, Wagner M, Wu C, Zoeller RT, Scheringer M. Impacts of food contact chemicals on human health: a consensus statement. Environ Heal A Glob Access Sci Source. 2020;19:1–12. https://doi.org/10.1186/s12940-020-0572-5.
Cramer GM, Ford RA, Hall RL. Estimation of toxic hazard-a decision tree approach. Food Cosmet Toxicol. 1976;16:255–76. https://doi.org/10.1016/S0015-6264(76)80522-6.
Tsochatzis ED, Alberto Lopes J, Kappenstein O, Tietz T, Hoekstra EJ. Quantification of PET cyclic and linear oligomers in teabags by a validated LC-MS method – in silico toxicity assessment and consumer’s exposure. Food Chem. 2020;317:126427. https://doi.org/10.1016/j.foodchem.2020.126427.
Ubeda S, Aznar M, Alfaro P, Nerín C. Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal Bioanal Chem. 2019;411:3521–32. https://doi.org/10.1007/s00216-019-01831-0.
Eckardt M, Schneider J, Simat TJ. In vitro intestinal digestibility of cyclic aromatic polyester oligomers from polyethylene terephthalate (PET) and polybutylene terephthalate (PBT). Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019;36:1882–94. https://doi.org/10.1080/19440049.2019.1658903.
Begley TH, Dennison JL, Hollifield HC. Migration into food of polyethylene terephthalate (Pet) cyclic oligomers from pet microwave susceptor packaging. Food Addit Contam. 1990;7:797–803. https://doi.org/10.1080/02652039009373941.
Isella F, Canellas E, Bosetti O, Nerin C. Migration of non intentionally added substances from adhesives by UPLC-Q-TOF/MS and the role of EVOH to avoid migration in multilayer packaging materials. J Mass Spectrom. 2013;48:430–7. https://doi.org/10.1002/jms.3165.
Kim DJ, Lee KT. Determination of monomers and oligomers in polyethylene terephthalate trays and bottles for food use by using high performance liquid chromatography- electrospray ionization-mass spectrometry. Polym Test. 2012;31:490–9. https://doi.org/10.1016/j.polymertesting.2012.02.001.
Lim BH, Kwon SH, Kang EC, Park H, Lee HW, Kim WG. Isolation and identification of cyclic oligomers of the poly(ethylene terephthalate)-poly(ethylene isophthalate) copolymer. J Polym Sci Part A Polym Chem. 2003;41:881–9. https://doi.org/10.1002/pola.10637.
Tsochatzis ED, Alberto Lopes J, Gika H, Kastrup Dalsgaard T, Theodoridis G. Development and validation of an UHPLC-qTOF-MS method for the quantification of cyclic polyesters oligomers in pasta by applying a modified QuEChERS clean-up. Food Chem. 2021;347:129040. https://doi.org/10.1016/j.foodchem.2021.129040.
Ubeda S, Aznar M, Rosenmai AK, Vinggaard AM, Nerín C. Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives. Food Chem. 2020;311:125918. https://doi.org/10.1016/j.foodchem.2019.125918.
Úbeda S, Aznar M, Vera P, Nerín C, Henríquez L, Taborda L, Restrepo C. Overall and specific migration from multilayer high barrier food contact materials–kinetic study of cyclic polyester oligomers migration. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34:1784–94. https://doi.org/10.1080/19440049.2017.1346390.
Úbeda S, Aznar M, Alfaro P, Nerín C. Migration of oligomersfrom a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal Bioanal Chem. 2019;411:3521–32. https://doi.org/10.1007/s00216-019-01831-0.
Diamantidou D, Begou O, Theodoridis G, Gika H, Tsochatzis E, Kalogiannis S, Kataiftsi N, Soufleros E, Zotou A. Development and validation of an ultra high performance liquid chromatography-tandem mass spectrometry method for the determination of phthalate esters in Greek grape marc spirits. J Chromatogr A. 2019;1603:165–78. https://doi.org/10.1016/j.chroma.2019.06.034.
Likas DT, Tsiropoulos NG, Miliadis GE. Rapid gas chromatographic method for the determination of famoxadone, trifloxystrobin and fenhexamid residues in tomato, grape and wine samples. J Chromatogr A. 2007;1150:208–14. https://doi.org/10.1016/j.chroma.2006.08.041.
Acknowledgements
The authors would like to thank Dr. João Alberto Lopes (JRC-Geel) for his support.
Funding
This research is carried out/funded in the context of the project “Method development for the determination of cyclic oligomers from food contact materials and exposure assessment.” (MIS 5048286) under the call for proposals “Supporting researchers with emphasis on new researchers” (EDULLL 103). The project is co-financed by Greece and the European Union (European Social Fund-ESF) by the Operational Programme Human Resources Development, Education and Lifelong Learning 2014–2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The collection and analysis of post-mortem blood samples used in this study were approved by Forensic Service of Thessaloniki.
Conflict of interest
The authors declare no competing interests.
Disclaimer
This manuscript does not represent European Food Safety Authority (EFSA) position.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diamantidou, D., Mastrogianni, O., Tsochatzis, E. et al. Liquid chromatography-mass spectrometry method for the determination of polyethylene terephthalate and polybutylene terephthalate cyclic oligomers in blood samples. Anal Bioanal Chem 414, 1503–1512 (2022). https://doi.org/10.1007/s00216-021-03741-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03741-6