Abstract
We produced a prometryn-specific monoclonal antibody and propose a strategy for convenient on-site detection of prometryn residues in herbs for the first time. This strategy has perfect applicability in a complex herbal medicine matrix. The strategy combines a semiquantitative immunochromatographic strip assay with a heterologous indirect competitive ELISA. When there was no matrix interference, the ELISA had a half-maximal inhibitory concentration of 2.6 ng·mL-1 and a limit of detection of 0.2 ng·mL-1. The immunochromatographic strip assay can be completed within 5 min with a visual limit of detection of 1 ng·mL-1. Although the sample matrix had different effects on the sensitivity of the antibody, excellent repeatability and accuracy were achieved. The method was successfully applied for the screening and determination of prometryn residue in multiple complex herb samples for the first time, and the results were in good agreement with those obtained by liquid chromatography–tandem mass spectrometry. The proposed strategy is rapid, of high-throughput, and of low cost, and may be a promising choice for on-site detection of prometryn in different kinds of herbs.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prometryn [2,4-bis(isopropylamino)-6-methylthio-s-triazine], a triazine herbicide, is widely used for preemergence or postemergence control of broadleaf and grassy weeds in agriculture practices around the world [1]. Its widespread, excessive use and persistence in the environment (with a half-life of about 13 months [2]) have led to serious residue accumulation in soil and water [3]. According to reports, the rate of detection of prometryn in some waters was even up to 12.5%, and the concentrations were above the acceptable risk value [3]. These residues may be transferred to and accumulated in various biological organisms. For example, 77.2% of Apostichopus japonicus sea cucumbers in some areas have been found with prometryn residue [4]. Previous studies demonstrated that prometryn can cause abnormalities in the reproductive organs, nervous system, and immune system of humans [5, 6]. In addition, prometryn is also known to disrupt hormone stability, leading the European Union to put prometryn on the prioritization list as an endocrine disruptor [7]. Although prometryn has been banned in Europe since 2004 [8], it still exists in the environment [9, 10], and it is still widely used in China, the USA, Canada, Australia, and many other countries [5]. The environmental contamination of prometryn induces chronic toxic effects in humans via the food chain. Consequently, many countries have set strict maximum residue limits (MRLs) for a variety of agricultural products [11,12,13,14,15]. For example, China enforces an MRL for corn of 0.02 mg·kg-1, and the MRLs for food regulated by the USA, Japan, Australia, and Korea are 0.05–9 mg·kg-1, 0.05–0.2 mg·kg-1, 0.05–1 mg·kg-1, and 0.05–0.2 mg·kg-1, respectively. On the basis of the aforementioned details, the detection of prometryn residue in agricultural products is important for the worldwide export or import industry and quality control.
Herbs are special agricultural products, and numerous herbs are extensively used as functional foods for daily health protection, as well as for medicinal therapy, such as Chinese yam (Dioscorea opposita Thunb.), American ginseng (Panax quinquefolium L.), notoginseng [Panax notoginseng (Burk.) F. H. Chen], Astragali radix [Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao], and Achyranthis bidentatae radix (Achyranthes bidentata Bl.). The prometryn residue in water and soil may accumulate in herbs through root absorption, especially in perennial herbs. Moreover, prometryn is used for weed control during the cultivation of some herbs, which may lead to considerable contamination [16, 17]. Until now, there have been few specifications for the use of pesticides in the cultivation of herbs. Consequently, use of prometryn may be abused and this may result in residue in the edible parts, which poses potential harm to consumers’ health and affects the herbal trade worldwide. Therefore, there is an urgent need to establish a rapid, on-site, high-throughput assay method to screen and determine prometryn residue in herbal medicines.
The traditional, widely used, and well-developed methods for the detection of prometryn are gas chromatography, high-performance liquid chromatography (HPLC), and chromatographic methods in tandem with mass spectrometry (MS) [18,19,20,21]. However, because of high equipment costs, complex sample pretreatment procedures, and high organic solvent use, it is difficult to widely implement these techniques, especially in some remote areas. In recent years, immunoassays have become a convenient tool for rapid, low-cost, high-throughput, and on-site analysis of various analytes. Even though many immunoassays have been proposed for the detection of atrazine and other triazine herbicides [22,23,24], few immunoassay methods have been developed for the determination of prometryn. As far as we know, no sensitive prometryn-specified monoclonal antibody (mAb) has been reported, and no immunoassay has been developed for the screening of prometryn in complex matrices, such as herbs.
In our previous study, we proposed some hapten structures and studied the correlation between the structures of haptens and the sensitivity of antibodies against triazine herbicides [25]. On the basis of these haptens, a sensitive mAb against prometryn, called “MAb TZ5E4,” was generated, and a strategy for rapid on-site determination of prometryn in a variety of complex herbs was proposed. This detection strategy combined a rapid screening method based on a colloidal-gold-based lateral flow immunochromatographic (ICG) strip with a heterologous indirect competitive ELISA (icELISA). The accuracy and reliability of the proposed method were further confirmed by LC–MS/MS. To the best of our knowledge, this is the first report of an immunoassay method for the detection of prometryn residue in herbs.
Materials and methods
Reagents and materials
Triazine standards were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Ovalbumin (OVA), bovine serum albumin (BSA), N-hydroxysuccinimide, N,N′-dicyclohexylcarbodiimide, and dimethylformamide were obtained from Aladdin (Shanghai, China). The 3,3′,5,5′-tetramethylbenzidine substrate reagent set was purchased from BD Biosciences (USA). Goat anti-mouse IgG and horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (IgG–HRP) were obtained from Solarbio Science & Technology Co. (Beijing, China). Complete and incomplete Freund’s adjuvants, hypoxanthine aminopterin thymidine, hypoxanthine thymidine, poly(ethylene glycol) (PEG) 20000, and chloroauric acid (HAuCl4) were obtained from Sigma-Aldrich (St Louis, MO, USA). The mouse Sp2/0-Ag14 myeloma cell line was purchased from the Cell Resource Center of Peking Union Medical College (Beijing, China). Dulbecco’s modified Eagle’s medium and fetal bovine serum were obtained from Gibco BRL (Paisley, UK). Nitrocellulose (NC) membranes, glass fiber membranes, sample pads, absorbent paper, and poly(vinyl chloride) (PVC) sheet were purchased from Jieyi Biotech Shanghai Co. (Shanghai, China).
Synthesis of haptens
The synthetic route for the immunizing and coating haptens is shown in Fig. 1. For immunizing hapten (H1), equal amounts of 2,4,6-trichloro-1,3,5-triazine and propan-2-amine were dissolved in dry dichloromethane in a three-necked flask. The mixture was cooled to –30 °C under nitrogen. Then twice the amount of diisopropylethylamine (DIPEA) diluted with dichloromethane was slowly added to the flask, and the reaction was carried out at −20 to −10 °C. LC–MS was used to monitor the reaction. After 2 h, the raw material was completely converted, and a small amount of disubstituted by-product was formed. The reaction solution was concentrated to dryness. The residue was dispersed in water and filtered, and the filter cake was collected and recrystallized from ethanol. The intermediate 4,6-dichloro-N-isopropyl-1,3,5-triazin-2-amine was obtained, which was a white solid. The intermediate and 4-aminobutanoic acid, coupled with DIPEA, were dissolved in absolute ethanol and incubated at 60 °C for 12 h under nitrogen. The products were concentrated and purified. The intermediate 4-((4-chloro-6-(isopropylamino)-1,3,5-triazin-2-yl)amino)butanoic acid was added to an ethanol solution of sodium thiomethoxide, and the resulting mixture was refluxed under nitrogen for 4 h. The reaction solution was concentrated to dryness, and the product was purified by preparative HPLC (pHPLC; C18 column, 70:30 acetonitrile–water) to give the final product 4-((4-(isopropylamino)-6-(methylthio)-1,3,5-triazin-2-yl)amino)butanoic acid, which is a white solid after lyophilization.
For the coating hapten (H2), equal amounts of 2,4,6-trichloro-1,3,5-triazine and cyclopropanamine were dissolved in dry ethyl ether, and the mixture was cooled to -30 °C under nitrogen. Then twice the amount of DIPEA diluted with ethyl ether was slowly added to the flask, and the reaction was carried out at −30 to −20 °C for 2 h. The intermediate 4,6-dichloro-N-cyclopropyl-1,3,5-triazin-2-amine was redissolved in tetrahydrofuran and reacted with ammonia at 60 °C for 16 h. The intermediate 6-chloro-N2-cyclopropyl-1,3,5-triazine-2,4-diamine was obtained and redissolved in a mixture of acetonitrile and 3-mercaptopropanoic acid. Potassium hydroxide was added, and the reaction proceeded at 120 °C for 12 h. The final product was obtained after purification by pHPLC (C18 column, 52:48 acetonitrile–water) and lyophilization.
The structures of the haptens were confirmed by 1H NMR; the spectra are shown in Fig. S1.
H1.1H NMR (300 MHz, dimethyl-d6 sulfoxide) δ 12.02 (s, 1H), 6.91–7.34 (overlap, 2H), 3.94–4.12 (m, 1H), 3.05–3.39 (m, 2H), 2.36 (d, J = 4.8 Hz, 3H), 2.23 (t, J =7.5 Hz, 2H), 1.62–1.79 (m, 2H), 1.10 (t, J = 5.7 Hz, 6H).
H2. 1H NMR (300 MHz, dimethyl-d6 sulfoxide) δ 12.26 (s, 1H), 7.34 (m, 1H), 6.43–7.02 (overlap, 2H), 3.03–3.24 (m, 2H), 2.56–2.79 (overlap, 3H), 0.57–0.64 (m, 2H), 0.46 (s, 2H).
Preparation of mAb
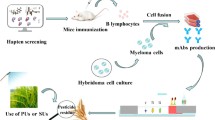
With use of the active ester method, the immunizing hapten was coupled with OVA, and the coating hapten was coupled with BSA to prepare the immunogen (H1–OVA) and coating antigen (H2–BSA), respectively [26, 27]. Female Balb/c mice, 6–8 weeks old, were immunized intraperitoneally and subcutaneously with the mixture of immunogen and Freund’s adjuvant in the form of an emulsion at 2-week intervals. After the third immunization, blood samples were drawn from the orbit to test the titers and specificity of serum. The mouse with the highest specificity and highest titer was selected. Splenocytes was isolated and fused with Sp2/0-Ag14 cells via the PEG method. Hybridomas positive for prometryn were cloned by the limiting dilution method. After further selection via icELISA, the clone was transplanted into the abdominal cavity of mice. The mAbs were obtained from the resulting ascites and purified according to the ammonium sulfate precipitation method. The immunoglobulin isotype was determined with a mouse mAb isotyping kit (Sigma), and the affinity of the mAb for the coating antigen was determined by indirect noncompetitive ELISA [28].
Indirect competitive ELISA
A heterologous icELISA was established to evaluate the sensitivity and specificity of the mAb. Each microplate well was coated with 100 μL H2–BSA, which was diluted in carbonate-buffered saline (0.05 M, pH 9.6). After incubation at 37 °C for 3 h, the unbound conjugates were removed by washing with PBST [0.01 M, pH 7.4 phosphate-buffered saline (PBS) containing 0.1% Tween 20] four times. Then 50 μL of prometryn sample and 50 μL of diluted mAb were successively pipetted into each well [29]. The prometryn samples were diluted with PBSTG (PBST with 0.5% gelatin) containing 10% (w/v) methanol [25]. After incubation at 37 °C for 30 min, the plate was washed four times with PBST. Afterwards, an aliquot of IgG–HRP diluted in PBSTG was added to each well. After incubation at 37 °C for 30 min, the plate was washed four times. Color development was achieved by addition of 100 μL 3,3′,5,5′-tetramethylbenzidine substrate reagent per well. Ultimately, the reaction was terminated by addition of 50 μL hydrochloric acid (1 mol·L-1) to each well, and the absorbance was measured at 450 nm with a microplate reader. The sensitivity of the mAb was assessed by the half-maximal inhibitory concentration (IC50), which was obtained via a sigmoidal logistical equation with use of OriginPro 9.0 (Origin Lab, Northampton, MA, USA). The specificity of the mAb was evaluated by the cross-reactivity (CR, %), which was calculated via the following formula:
ICG strip test
Preparation of colloidal gold–antibody conjugate
The colloidal gold particles, with an average diameter of 22 nm, were prepared by reduction of HAuCl4 with sodium citrate, as described by Zhou et al. [30]. The morphology and particle size of colloidal gold were characterized and determined by a UV–visible spectrometer and Zetasizer particle analyzer (Malvern Instruments), respectively. The pH of 12 mL colloidal gold suspension was adjusted to 8.5 with K2CO3 (0.2 mol·L-1), and 60 μg of the mAb was added dropwise. The mixture was incubated with stirring for 20 min, and 200 μL of 10% BSA and 200 μL of 1% PEG 20000 were added to block the residual surface of the colloidal gold. After another 20 min of incubation, the mixture was centrifuged at 14,000 rpm for 30 min at 4 °C. The supernatant was removed, and the precipitate was dissolved in resuspension buffer (PBS containing 1% BSA, 0.1% Tween 20, 5% sucrose, and 0.2% PEG 20000) [31].
Preparation of ICG strips and the assay procedure
The colloidal gold–antibody conjugate was sprayed on a glass fiber membrane (conjugate pad) at 6 μL·cm-1 and dried overnight at 37 °C. The test line (H2–BSA) and control line (goat anti-mouse IgG) were separately immobilized on NC membrane at 1 μL·cm-1 and dried overnight at 37 °C. The ICG strips were assembled in the following order: sample pad, conjugate pad, NC membrane, absorbent paper (Fig. 2). The NC membrane was stuck on the center of the PVC sheet. The conjugate pad and absorbent paper overlapped with the edges of the NC membrane by 1 mm and were immobilized on the PVC sheet. The sample pad overlapped with the conjugate pad by 1 mm and was also pasted on the PVC sheet. Finally, the assembled ICG strips were cut into 3-mm-wide strips and stored in a desiccator for later use.
For the ICG strip test, 80 μL of the sample solution was added dropwise on the sample pad of the strips. A blank sample was used as a negative control. The color change of the test line was observed and compared by the naked eye. Because of the competitive inhibition of prometryn in the samples with the gold–antibody conjugate, the intensity of the signal generated at the test line decreased with increasing prometryn concentration. By comparison with the images of the strips of standard solutions, the concentration of prometryn residue in the samples was estimated.
Sample preparation and LC–MS/MS verification
Chinese yam, American ginseng, notoginseng, Astragali radix, and Achyranthis bidentatae radix were finely ground and homogenized. An aliquot of 2.0 g sample powder was combined with 5 mL methanol, and the mixture was vortexed for 1 min. The samples were extracted by ultrasonic-assisted extraction for 10 min. After centrifugation, 0.5 mL of the supernatant was transferred to a clean tube and diluted with 2 mL PBS for the ELISA and 1.5 mL of PBS for the ICG strip test, respectively.
The LC–MS/MS method used for validation studies was modified according to a previous report [32]. Briefly, 2.0 g sample powder was extracted with 10 mL acetonitrile via ultrasonication. After centrifugation, the sample solution was passed through a 0.22-μm filter. Chromatographic separation was performed on an AQUITY UPLC BEH Shield RP18 column (50 mm × 2.1 mm, 1.7 μm) from Waters (Milford, MA, USA), with a gradient elution at a flow rate of 0.20 mL·min−1. The limit of detection (LOD) and limit of quantification for prometryn in the selected herbs were 0.5 and 1 μg·kg-1, respectively.
Results and discussion
Production and characterization of mAbs
The H1–OVA and H2–BSA conjugates were estimated from the UV–visible spectrum in the range from 200 to 400 nm. The peak shape and the absorption peak wavelength of the conjugates varied compared with proteins (Fig. 3), indicating that the haptens were successfully coupled with the carrier proteins. After immunization and screening, the mAb with the greatest sensitivity and selectivity, MAb TZ5E4, was expanded for ascites production. The titer of the ascites (number of dilutions that gave an absorbance of 1.0 in the noncompetitive ELISA) was 6.4 × 104 [33]. The affinity constant Kaff for MAb TZ5E4 and H2–BSA was 3.13 × 108 L·mol-1. What is more, MAb TZ5E4 is an IgG1 isotype.
Optimization of icELISA conditions
The concentrations of coating antigen, MAb TZ5E4, and IgG–HRP were optimized by a two-dimensional checkerboard titration. After optimization, H2–BSA at 62.5 ng·mL-1, MAb TZ5E4 at 62.5 ng·mL-1, and IgG–HRP at 100 ng·mL-1 were used throughout the remainder of this work. Under the optimal conditions, a representative inhibition curve was plotted with a series of logarithmic concentrations of prometryn (0.1, 0.5, 1, 2, 5, 10, 20, 50, and 100 ng·mL-1) diluted with PBSTG as the lateral coordinates and the corresponding OD/OD0 values (where OD and OD0 are the absorbance at 450 nm in the presence and the absence of prometryn, respectively) as the longitudinal coordinates. As shown in Fig. 4, the IC50 was 2.6 ng·mL-1; the LOD, which was calculated by D/D0 = (OD0 − 3 × SD)/OD0, where SD is the standard deviation [34,35,36], corresponds to 0.2 ng·mL-1 on the inhibition curve. The linearity range was 0.7−9.1 ng·mL-1, which was based on the 20% inhibitory concentration and the 80% inhibitory concentration of the inhibition curve.
Specificity of the mAb
Because of the similar structures of triazine herbicides, 21 triazine herbicides and three metabolites of atrazine were used to evaluate cross-reactivity. As shown in Table 1, MAb TZ5E4 has the greatest cross-reactivity with prometon (43.3%), which is most similar to prometryn in structure. The triazines with a sulfur or oxygen had greater cross-reactivity than those with chlorine, and this result was in accordance with our previous finding [25]. The mAb exhibited excellent specificity and largely avoided interference from most triazine herbicides.
Development of the ICG strips
Characterization of colloidal gold
The optimal colloidal gold suspension is clear, is transparent, and has uniform particle size. Figure 5a shows the UV–visible spectrum of colloidal gold. The absorption peak at 520 nm is consistent with literature reports. The peak is smooth and narrow, indicating relatively homogeneous colloidal gold particles. Figure 5b shows the particle size distribution of colloidal gold, where there is only one distribution area and the colloidal gold particles have a relatively uniform size of about 22 nm. The colloidal gold exhibited good quality and was used for the preparation of gold-labeled antibodies.
Sensitivity of the ICG strips
The color intensity of the test lines decreased with increasing concentration of prometryn. As shown in Fig. 6a, when the prometryn concentration was 1 ng·mL-1, the color of the test line became obviously lighter than that of the blank control, and the test line almost disappeared when it was 5 ng·mL-1. No test line was visible when the sample had a prometryn concentration up to 10 ng·mL-1. In addition, as the color of the test line becomes lighter, the color of the control line gradually deepens. When the prometryn concentration was 1 ng·mL-1, the color of the test line was slightly lighter than that of the blank control, but the color of the control line was significantly more intense. Therefore, the change in color intensity of the control line could be used as an auxiliary judgment. The LOD of the ICG strips was as low as 1 ng·mL-1 when no matrix interfered. The ICG strip test can be completed within 5 min.
Immunoassay for prometryn detection in herb samples
Sample pretreatment
To avoid interference from the complicated ingredients in herbs, the type and amount of solvent used for pretreatment was investigated. Prometryn is easily dissolved in organic solvent; therefore, a high proportion of organic solvent would facilitate prometryn extraction. The presence of water might increase dissolution of water-soluble components in herbs, which may magnify the complexity of the extract and influence the recognition of the antibody to prometryn. Moreover, some herbs, such as Astragali radix, can absorb large amounts of solvents with water, making the extraction difficult. Therefore, organic solvent was chosen for prometryn extraction in herbs. Acetonitrile can greatly reduce the extraction of lipophilic pigment from herb matrices, and the extraction solution was much clearer than that extracted by an equal volume of methanol. However, the presence of acetonitrile may decrease the activity of the antibody, leading to low absorbance of the ELISA. As a result, methanol was used as the extraction solvent, and the matrix effect was evaluated. Considering the sensitivity of the mAb and the influence of organic solvent, 2.5 mL methanol per gram of herb sample was used for extraction, and then the solution was diluted five and four times with PBS before the ELISA and the ICG strip test, respectively.
Matrix effect
Since the complex components of herbs may interfere with efficient antigen–antibody interaction, the sensitivity of MAb TZ5E4 for prometryn in different herb matrices was evaluated. A blank matrix extract was obtained by our treating the blank herbs according to the sample preparation method described earlier. Then matrix-matched standards were prepared by our diluting prometryn standard with the blank matrix extract. The inhibition curves for prometryn in different herb matrices are shown in Fig. 7. Achyranthis bidentatae radix and Astragali radix presented a suppression effect on the antigen–antibody interaction, with IC50 values larger than IC50 obtained from blank solvent. Notoginseng, American ginseng, and Chinese yam had smaller IC50 values, and they showed a slightly enhanced effect. The IC50 values and the linear range of each matrix are shown in Table 2. The LODs for prometryn in all herb matrices were 0.1–1.3 ng·mL-1, which were equivalent to concentrations ranging from 1.25 to 16.25 μg·kg-1 in herbs. The LODs were far below the strictest MRL of prometryn set by most countries for food (50 μg·kg-1); thus, this method can be used for the detection of prometryn residue in herbs. The matrix effects of the five herbs on the ICG strips test are displayed in Fig. 6b–f. The matrices of the herbs have a minimal effect on the efficacy of the ICG strips.
Accuracy and precision
Prometryn recovery from the five herb matrices was performed by our analyzing fortified samples in five replicates at three different concentrations [37]. With use of the strictest MRL regulated by most countries for prometryn in food (50 μg·kg-1), the spiking levels for each herb matrix were 25, 50, and 100 μg·kg-1. The fortified samples were prepared and processed as previously described. Because of the narrow linear ranges of notoginseng, American ginseng, and Chinese yam, the sample solution of these samples was additionally onefold diluted with 20% methanol–PBS before analysis. The recovery results are listed in Table 3. It can be seen that both acceptable accuracy and excellent reproducibility were obtained for the ELISA method. The precision and recovery results for the ICG strips are shown in Figs. S3 and S4.
Screening and determination of prometryn residues in herb samples
Three batches of Chinese yam, American ginseng, notoginseng, Astragali radix, and Achyranthis bidentatae radix were purchased from different medicinal markets in China. Two batches of verified blank samples of these herbs were randomly spiked with prometryn. A total of 25 batches of samples were analyzed for prometryn residue screening by ICG strips, followed by quantification and verification by ELISA and LC–MS/MS. Sample preparation was performed according to the method described earlier. Each sample was analyzed in triplicate. The results are shown in Table 4 (the screening results for the ICG strips are shown in Fig. S5).
No prometryn residues were found in real samples, as determined by the ICG strips and the ELISA method. No prometryn residue was detected in real samples by LC–MS/MS, except for a batch of American ginseng and Astragali radix samples, with concentrations below the limit of quantification (1 μg·kg-1). For the fortified blank samples, the results of the ICG strip tests were consistent with those determined by ELISA, and the LC–MS/MS results further confirmed the efficacy of the immunoassay in accurately quantifying prometryn residues in herb samples.
Although the ICG strip test is less accurate than ELISA in terms of quantification, its simple operation and rapid detection are particularly suitable for residue screening of on-site samples. Once the concentration of prometryn residues approaches or exceeds the MRL (50 μg·kg-1), the change in color of the test line is easily interpreted and compared with the negative blank samples. The ICG strip test is convenient for fast MRL screening. The positive samples screened by ICG strips can be further verified and quantified by the ELISA method, and the results of the ELISA method were statistically similar to those obtained by LC–MS/MS. Thus, when LC–MS/MS is not available, the ICG strips and ELISA are good choices for the determination of prometryn residue in herbs.
Many similar immunoassay reports (ELISA and dipstick strip) were collected for comparative analysis. The comparison results (LODs, IC50, and applicable matrix) are shown in Table 5. It can be seen that the ELISA work was designed to analyze herbs, which are much more complicated than cereals and water matrices [23, 38,39,40,41,42]. For this reason, the LOD and IC50 results in this work were slightly higher than those for other matrices, but they are sufficient for the control of triazine herbicides. With regard to the ICG strip, some other dipstick strip and ELISA experiments are reported, and the applicable matrices are compared [23, 43, 44]. Almost no related dipstick strip has been used for the analysis of triazine herbicides in complex herb samples. Comparison of the LODs and IC50 values was not conducted for different detection means. Furthermore, some novel designs for the analysis of triazine herbicides, such as fluorescence detection [45], electrochemical analysis [46], and sensor immunoassay [47], also have some inevitable problems. On the one hand, the cost of the technology is high; on the other hand, current research is applicable only to simple matrices, for example, water and fruit samples. On the basis of the above comparison, the method proposed in this study is more suitable for detection of residual prometryn in herbs.
In this study, a sensitive mAb against prometryn, with high affinity, was prepared. On the basis of the mAb, a heterologous icELISA and a rapid ICG strip test were proposed to detect prometryn residue in herbs. Despite the various complex components in herbs, excellent repeatability and accuracy were achieved. Compared with the polyclonal antibody produced in our previous study [25], the mAb prepared in this study exhibited greater specificity and sensitivity, allowing a streamlined pretreatment procedure. The simple one-step extraction and dilution pretreatment method used in this study is more suitable for rapid detection technology, which can satisfy the intensive testing requirement of trace residue. Comparison between the ICG strip, ELISA, and LC–MS/MS methods revealed consistent results for herb samples. Therefore, the semiquantitative ICG strip assay coupled with ELISA offers a promising alternative for the rapid on-site screening and high-throughput determination of prometryn in complex herb matrices.
References
Heri W, Pfister F, Carroll B, Parshley T, Nabors JB. Production, development, and registration of triazine herbicides. In: LeBaron HM, McFarland JE, Burnside OC, editors. The Triazine Herbicides. Elsevier; 2008. 50:31–43. https://doi.org/10.1016/B978-044451167-6.50006-4.
Guo LJ, Qu JR, Miao SS, Geng HR, Yang H. Development of a molecularly imprinted polymer for prometryne clean-up in the environment. J Sep Sci. 2014;36(24):3911–7.
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E. A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf. 2015;116:1–9.
Ren C, Tian X, Sun Y, Deng X, Liu H, Xue J, et al. Residues and risk assessment of 13 triazine herbicides in Apostichopus japonicus. Modern Food Science and Technology. 2014;30(3):244–9.
Velisek J, Stara A, Koutnik D, Machova J. Effects of prometryne on early life stages of common carp (Cyprinus carpio L.). Pestic Biochem Physiol. 2015;118:58–63.
Wang Y, Zhang G, Wang L. Interaction of prometryn to human serum albumin: insights from spectroscopic and molecular docking studies. Pestic Biochem Physiol. 2014;108:66–73.
PAN Pesticides Database. Pesticide Action Network North America http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC34259.
Zhou J, Hu F, Jiao J, Liu M, Li H. Effects of bacterial-feeding nematodes and prometryne-degrading bacteria on the dissipation of prometryne in contaminated soil. J Soil Sediment. 2012;12(4):576–85.
Vryzas Z, Alexoudis C, Vassiliou G, Galanis K, Papadopoulou-Mourkidou E. Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern Greece. Ecotoxicol Environ Saf. 2011;74(2):174–81.
Caquet T, Roucaute M, Mazzella N, Delmas F, Madigou C, Farcy E, et al. Risk assessment of herbicides and booster biocides along estuarine continuums in the Bay of Vilaine area (Brittany, France). Environ Sci Pollut Res. 2013;20(2):651–66.
Ministry of Health, Labour and Welfate, Japan (Enforcement on May 29, 2006) Positive list system for agricultural chemical residues in foods. http://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228.
G/SPS/N/CAN/671 (2013) Proposed maximum residue limit: prometryn (PMRL2013-16).
G/SPS/N/USA/2585 (2013) Prometryn; pesticide tolerances. World Trade Organization, Committee on Sanitary and Phytosanitary Measures.
GB2763-2016 National food safety standard - maximum residue limits for pesticides in food.
Code of Federal Regulations, Part 180 - tolerances and exemptions for pesticide chemical residues in food. US Environmental Protection Agency.
Wang L, Wang L, Wu M. The screening of suitable herbicide for Angelica. Journal of Agricultural Science Yanbian University. 2008;30(1):46–51.
Wang L, Wang L, Wu M. Analysis of residual prometryme in Angelica. Journal of Agricultural Science Yanbian University. 2007;29(4):262–6.
Zhou J, Chen J, Cheng Y, Li D, Hu F, Li H. Determination of prometryne in water and soil by HPLC–UV using cloud-point extraction. Talanta. 2009;79(2):189–93.
Wu M, Li T, Zheng C. Analysis of prometryn residue in roots of Codonopsis lanceolata. Plant Prot. 2010;36(6):100–2.
Chae Y, Cho Y, Jang K, Kim J, Lee S, Chang M. Establishment of an analytical method for prometryn residues in clam using GC-MS. Korean J Food Sci Technol. 2013;45(5):531–6.
Sun S, Li Y, Lv P, Punamiya P, Sarkar D, Dan Y, et al. Determination of prometryn in vetiver grass and water using gas chromatography–nitrogen chemiluminescence detection. J Chromatogr Sci. 2016;54(2):97–102.
Kramer K. Synthesis of a group-selective antibody library against haptens. J Immunol Methods. 2002;266(1):209–20.
Na Y, Sheng W, Yuan M, Li L, Liu B, Zhang Y, et al. Enzyme-linked immunosorbent assay and immunochromatographic strip for rapid detection of atrazine in water samples. Microchim Acta. 2012;177(1-2):177–84.
Delaunay-Bertoncini N, Pichon V, Hennion M-C. Experimental comparison of three monoclonal antibodies for the class-selective immunoextraction of triazines. J Chromatogr A. 2003;999(1-2):3–15.
Liu C, Dou X, Lei Z, Kong W, Liu W, Duan Y, et al. Development of a broad-specificity antibody-based immunoassay for triazines in ginger and the quantitative structure-activity relationship study of cross-reactive molecules by molecular modeling. Anal Chim Acta. 2018;1012:90–9.
Wang Y, Wei D, Yang H, Yang Y, Xing W, Li Y, et al. Development of a highly sensitive and specific monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of Sudan I in food samples. Talanta. 2009;77(5):1783–9.
Cui Y, Nan T, Tan G, Li QX, Wang B, Liu S. Production of monoclonal antibody to herbicide fenoxaprop-ethyl. Hybridoma. 2011;30(5):463–7.
Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987;100(1-2):173–9.
Cui Y, Liu K, Xu C, Liu F, Li QX, Liu S, et al. Development of a sensitive monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for analysing chlorantraniliprole residues. Food Chem. 2014;143:293–9.
Zhou W, Kong W, Dou X, Zhao M, Ouyang Z, Yang M. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J Chromatogr B. 2016;1022:102–8.
Chen X, Liu L, Kuang H, Song S, Xu C. A strip-based immunoassay for rapid determination of fenpropathrin. Anal Methods. 2013;5(21):6234–9.
Liu C, Dou X, Zhang L, Li Q, Qin J, Duan Y, et al. Determination of triazine herbicides and their metabolites in multiple medicinal parts of traditional Chinese medicines using streamlined pretreatment and UFLC-ESI-MS/MS. Chemosphere. 2017;190:103–13.
Zhang B, Nan T, Zhan Z, Kang L, Yang J, Yuan Y, et al. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for luteoloside detection in Flos Lonicerae Japonicae. Anal Bioanal Chem. 2016;408(22):6053–61.
Sapozhnikova Y, Simons T, Lehotay SJ. Evaluation of a fast and simple sample preparation method for polybrominated diphenyl ether (PBDE) flame retardants and dichlorodiphenyltrichloroethane (DDT) pesticides in fish for analysis by ELISA compared with GC-MS/MS. J Agric Food Chem. 2015;63(18):4429–34.
Qian G, Wang L, Wu Y, Zhang Q, Sun Q, Liu Y, et al. A monoclonal antibody-based sensitive enzyme-linked immunosorbent assay (ELISA) for the analysis of the organophosphorous pesticides chlorpyrifos-methyl in real samples. Food chem. 2009;117(2):364–70.
Geis-Asteggiante L, Lehotay SJ, Fortis LL, et al. Development and validation of a rapid method for microcystins in fish and comparing LC-MS/MS results with ELISA. Anal Bioanal Chem. 2011;401(8):2617–30.
European Commission. Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. 2015. SANTE/11945/2015.
Gascón J, Oubina A, Ballesteros B, Barceló D, Camps F, Marco M-P, et al. Development of a highly sensitive enzyme-linked immunosorbent assay for atrazine Performance evaluation by flow injection immunoassay. Anal Chim Acta. 1997;347:149–62.
Sai N, Sun W, Wu Y, Sun Z, Yu G, Huang G. A highly sensitive immunoassay for atrazine based on covalently linking the small molecule hapten to a urea–glutaraldehyde network on a polystyrene surface. Int Immunopharmacol. 2016;40:480–6.
Bruun L, Koch C, Jakobsen MH, Aamand J. New monoclonal antibody for the sensitive detection of hydroxy-s-triazines in water by enzyme-linked immunosorbent assay. Anal Chim Acta. 2000;423(2):205–13.
Maqbool U, Haq A-U, Qureshi MJ, Iqbal MZ, Hock B, Kramer K. Development of ELISA technique for analysis of atrazine residues in water. J Environ Sci Health B. 2002;37(4):307–22.
Wortberg M, Goodrow MH, Gee SJ, Hammock BD. Immunoassay for simazine and atrazine with low cross-reactivity for propazine. J Agric Food Chem. 1996;44(8):2210–9.
Gabaldón JA, Maquieira A, Puchades R. Determination of atrazine in vegetable samples using a dipstick immunoassay format. Int J Environ Anal Chem. 2002;82(3):145–55.
Wittmann C, Bilitewski U, Giersch T, Kettling U, Schmid RD. Development and evaluation of a dipstick immunoassay format for the determination of atrazine residues on-site. Analyst. 1996;121(6):863–9.
Turiel E, Fernández P, Pérez-Conde C, Gutiérrez AM, Cámara C. Flow-through fluorescence immunosensor for atrazine determination. Talanta. 1998;47(5):1255–61.
Gonzalez-Techera A, Zon MA, Molina PG, Fernandez H, Gonzalez-Sapienza G, Arevalo FJ. Development of a highly sensitive noncompetitive electrochemical immunosensor for the detection of atrazine by phage anti-immunocomplex assay. Biosens Bioelectron. 2015;64:650–6.
Ciumasu IM, Krämer PM, Weber CM, Kolb G, Tiemann D, Windisch S, et al. A new, versatile field immunosensor for environmental pollutants: Development and proof of principle with TNT, diuron, and atrazine. Biosens Bioelectron. 2006;21(2):354–64.
Acknowledgements
We thank Baomin Wang from the College of Agronomy and Biotechnology, China Agricultural University, China, for providing technical guidance.
Funding
This work was supported by the National Natural Science Foundation of China (no. 81573595 and 81703699), the CAMS Innovation Fund for Medical Sciences (no. 2017-I2M-1-013) and the Biology Key Construction Discipline Fund project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The use of laboratory animals (mice) was in accordance with the relevant Chinese laws and according to the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College regulations concerning protection of animals used for scientific purposes. The mice used in this study were approved by the Ethics Committee on Experimental Animals and Animal Tests of Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College. The review number is SLXD-20170301058.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 753 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Wang, Y., Zhang, L. et al. An integrated strategy for rapid on-site screening and determination of prometryn residues in herbs. Anal Bioanal Chem 412, 621–633 (2020). https://doi.org/10.1007/s00216-019-02224-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02224-z