Abstract
With more than a thousand constituents at trace level concentrations, exhaled breath analysis (EBA) allows for non-invasive point-of-care (POC) disease diagnostics and metabolic status monitoring in or close to real-time. A number of biomarkers in breath may be used to not only identify diseases and disease progression but also to monitor therapeutic interventions. Although the relationship of selected breath components/biomarkers with certain disease pathologies is well established, diagnosing the exhaled breath composition remains an analytical and practical challenge due to the concentration levels of molecules of interest, i.e., low parts-per-billion (ppb) regime and below. Besides the analytical assessment of breath components via conventional methods such as gas chromatography coupled to mass spectrometry and related techniques, the application of cascade laser spectroscopy (CLS) is relatively new and exhibits several advantages when aiming for compact and user-friendly trace gas sensors with high molecular selectivity, the required sensitivity, and potentially reasonable instrumental costs. This trend article highlights the current status and potential of CLS in breath diagnostics with a focus on recent advancements in instrumentation and application along with future prospects and challenges.

Cascade laser technology in the mid-infrared spectral range enables sensitive and molecularly selective exhaled breath analysis with near real-time response, label-free detection, and point-of-care feasibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last two decades, increase interest in the field of exhaled breath analysis is noticeable. As a non-invasive diagnostic window, the exhaled breath matrix is perfectly suited, as its chemical composition directly reflects metabolic processes and related gas exchange in the lungs, and may be collected at almost any volume with minimal sampling efforts. Hence, the breath matrix ideally serves for biomonitoring purposes, as well as in medical diagnostics. Since the molecular components entailed in the exhaled breath matrix are of both endogenous and exogenous origin, the detailed analysis of their composition may provide a distinctive signature of the physiological processes occurring in the organism (a.k.a. “breath fingerprint”) and along the pathways of ingestion or resorption. One may thus summarize that “we exhale who we are, and how we are.” Hence, if this “breath fingerprint” is recorded and correctly analyzed, it may serve as a valuable source of information of the current health status of any breathing organism with the potential to even predict future metabolic of therapeutic developments.
Physicians have always taken advantage of the fact that a person’s breath may contain a wealth of information on their general state of health, and more specifically even identify or diagnose some diseases. Evidently, it is of substantial interest identifying molecular indicators of pathological processes within a breathing organism such as human body via the detailed analysis of the exhaled breath matrix and its composition. Modern exhaled breath analysis has been pioneered by Linus Pauling et al. [1]; using gas chromatographic analysis, well over 250 volatile organic compounds (VOCs) have immediately been identified in the exhaled breath matrix (EBM), and within the headspace of urine. Since then, numerous publications using a variety of instrumental analytical approaches have reported on the composition of EBM in ever increasing molecular detail.
In general, EBM is mainly composed of nitrogen (~ 78%), oxygen (~ 17%), carbon dioxide (~ 4%), water vapor, and several hundreds of VOCs including but not limited to, e.g., isoprene, ethane, pentane, and acetone at levels usually below the ppmv range. Due to their relatively low concentrations and variety in chemical nature, various preconcentration/enrichment approaches have been tested to enhance their detectability. Besides using solid-phase microextraction (SPME) and membrane extraction via a sorbent interface, preconcentration onto solid sorbents with subsequent thermal desorption has been applied [2]. The “gold standard” analytical method for the detection of breath biomarkers is gas chromatography in combination with mass spectrometry (GC-MS) allowing for multi-analyte detection at ppb to ppt sensitivity. Besides its comparatively high instrumental cost, GC-MS usually requires manual sampling, preconcentration from large breath volumes onto chemical traps, a considerable level of consumables and maintenance, and well-trained personnel to operate. Alternative methods providing on-line and in real-time analytical access such as selected ion flow tube mass spectrometry (SIFT-MS) or proton-transfer-reaction mass spectrometry (PTR-MS) have successfully been tested for advanced breath profiling, however, have to be considered laboratory-bound techniques. For the development of handheld breath sensing/monitoring devices, vacuum-free ion mobility spectrometry (IMS) combined with multi-capillary columns has demonstrated its potential in routine analysis. Furthermore, electrical sensors (i.e., electronic nose systems in a variety of configurations) are increasingly popular as a low-cost alternative in breath analysis, however, are limited in molecular selectivity and discriminatory power [3].
Given the information content of EBM, there is interest in developing breath analyzers for on-line monitoring and analysis with possibly high accuracy and precision at reasonable costs. Compared with the conventional analytical methods, laser spectroscopic techniques and specifically devices operating in the infrared spectral regime have the potential to establish compact and robust optical sensing devices enabling rapid detection, time-resolved monitoring, and—in the case of mid-infrared devices—inherent molecular selectivity. Laser-based spectroscopic (LAS) methods have been widely adopted in a range of sensing scenarios such as general molecular spectroscopy, industrial process analysis, and environmental atmospheric monitoring. The fundamental vibrational, roto-vibrational, and rotational transitions especially in the mid-infrared (MIR; 2.5–20 μm) range are characterized by high absorption coefficients, which—combined with a high spectral power density of the laser source—promise not only sufficient sensitivity, but also high discriminatory power at a molecular level. Consequently, LAS methods are increasingly adapted for biomedical applications requiring detectivities at ppm to ppb levels, potentially in low-volume samples. In addition, LAS offers a suitable temporal resolution (< 1 s), usually requires little to no sample preparation, and thus enables close to real-time in situ measurements. With the advent of cascade laser technology (CLS), the field of LAS is continuously revolutionized since the mid-1990s, as almost any wavelength—and via appropriate tuning techniques even wavelength windows of up to 300 cm−1—in the analytically relevant regime of the MIR band is accessible with room-temperature operated on-chip light source technology facilitating trace gas analysis of a wide range of (bio)medically and clinically relevant molecules with characteristic absorption features in the MIR. Given the tailorability of these laser light sources, the laser emission may be readily matched to a selective absorption line or band of a particular analyte and may in addition be selected such that matrix compounds at much higher concentrations do not overlap with analyte signature.
As the most prominent representative, quantum cascade lasers (QCL) operating in continuous wave or pulsed mode have received significant attention since their introduction. They offer single mode operation, comparatively high optical output power at room temperature, and precise tunability due to their narrow linewidths. The first experimental realization was shown by Faist et al. in 1994 [4], way after their theoretical introduction in 1971 by Kazarinov and Suris, who presented a theoretical study on the stimulated emission of photons via electron transitions in the conduction band thus created the theoretical basis for cascade laser technology [5]. Since many molecules exhibit strong roto-vibrational signatures in the MIR spectral window, their utility in biomedical applications was immediately evident. Based on the use of interband transitions in so-called type II structures or “broken-gap” heterostructures, interband cascade lasers (ICL) based on InAs/(In, Ga)Sb/AlSb on GaSb material systems have been presented as alternative concept [6]. Using interband transitions allows reducing the power consumption of ICLs by an order of magnitude vs. QCLs. Thus, significant advances towards handheld or battery-operated cascade laser-based sensors are evident, as the performance of ICLs has improved significantly since their introduction, and continuous wave operation has been demonstrated [7]. Yet, their wavelength coverage is currently significantly limited vs. QCLs to < 5 μm.
A major disadvantage of CLS in comparison to conventional, i.e., broadband infrared spectroscopy and sensing (e.g., using compact Fourier-transform infrared (FT-IR) spectrometers) is the restriction to selected analytes. Hence, in general, CLS appears more suitable for target analyte detection rather than broad constituent screening. However, broadly tunable external cavity (EC)-QCLs provide sizeable tuning ranges (> 300 cm−1) and therefore enable the detection of at least several analytes within that spectral window. In addition to the choice of the laser source, appropriate detection schemes are central to maximizing the utility of LAS; however, their discussion goes beyond the scope of this article.
Based on the general concept of LAS, various techniques have been reported and applied including tunable diode laser absorption spectroscopy (TDLAS), wavelength modulated spectroscopy (WMS), photoacoustic spectroscopy (PAS), and cavity-enhanced absorption spectroscopy (CEAS) just to name a few. TDLAS utilizes tunable diode lasers for the in situ determination of absolute gas species concentrations and gas temperatures without the need of sampling and frequent calibration. The emission of the MIR source is tuned across a certain absorption line of the analyte gas varying either the operation current or temperature of the laser diode. Using in addition a sinusoidal frequency modulation of the laser emission, the achievable signal-to-noise ratio (SNR) can be increased significantly, a.k.a., WMS. Another approach to enhance the sensitivity is achieved by increasing the absorption path length via so-called multi-pass cells (MPCs). Conventional MPCs such as White and Herriott cells allow an increase of the optical path length up to several hundreds of meters [8, 9]. Another promising approach is PAS, which is based on the conversion of light-induced molecular vibrations into acoustic waves that can then be detected via a microphone within a photoacoustic cell. With this technology, it is possible to realize cost-effective and sensitive sensing systems for in situ studies. CEAS techniques are highly sensitive optical detection methods based on the application of an optical resonator (e.g., established between two concave mirrors). The mirror surfaces are coated with highly reflective dielectric layers (i.e., usually reflectivity of > 99.99%), and thus, the light-matter interaction is enhanced considerably, as the radiation may reflect through the high-finesse resonator volume several hundred times—a concept utilized in cavity ring-down systems and the like.
Cascade laser spectroscopy for exhaled breath analysis: state-of-the-art
Using MIR LAS, a variety of gas sensors for the analysis of exhaled breath based on concepts such as TDLAS [10], WMS [11], PAS [12], and CEAS [13] have been developed. These methods allow for time-resolved and almost real-time EBA with high sensitivity and selectivity. In the following, novel instrumental approaches in combination with cascade lasers applied in EBA will be highlighted.
13C breath tests are innovative and—to the patient—nearly stress-free detection methods, which contribute significantly to the clarification of complex disorders and various clinical symptoms without the need of the administration of radioactive substances. Quantification of exhaled 13CO2 may be utilized for the direct detection of Helicobacter pylori infections. The bacterium resides within the gastric mucosa and is associated with the occurrence of gastric ulcers (ulcers), gastric cancer (gastric carcinoma), and MALT lymphoma in the stomach. The basic principle of 13C-labeled breath tests is the enzymatic conversion of a 13C-labeled substrate to its metabolite 13CO2. Isotope ratio mass spectrometers (IRMS) and non-dispersive isotope-selective infrared spectroscopy (NDIR) have been applied for 13C breath testing [14]. However, these detection methods are either complex or require sample volume of several hundreds of milliliters (mL). In recent years, cascade laser-based sensors have attracted attention, because they may overcome limitations of existing methods. For example, these sensors combine distributed feedback (DFB)-QCLs emitting at ~ 4.3 μm covering the asymmetric stretching modes of 12CO2 and 13CO2 with dual multi-pass absorption gas cells for breath isotope analysis [15], and take advantage of balanced detection techniques [16]. To minimize the required sample volume and demonstrate the feasibility of breath analysis for small animal models, hollow waveguides (HWG) acting both as radiation propagation conduit and low-volume gas cells have been used to analyze sample volumes of few hundreds of microliters, thereby significantly increasing the achievable temporal resolution [17]. For direct detection of exhaled mice breath without the need of sampling, an exhaled breath sensor system based on a DFB-ICL and a miniaturized dual-channel substrate-integrated hollow waveguide (iHWG) using balanced ratiometric detection (see Fig. 1) was developed. The sensor demonstrated a measurement precision of 1.6‰ during a 480-s integration time, as confirmed via Allan variance analysis. Routine quantification and continuous monitoring of 13CO2/12CO2 isotopic ratio changes with comparable results to GC-MS could be achieved during a range of mouse experiments [18].
Dual-channel substrate-integrated hollow waveguide (iWHG) enables the analysis of ~ 300 μL sample volume within a compact and robust gas cell/waveguide configuration (94 mm × 50 mm × 22 mm, L × W × H). The achievable sensitivity was further improved via balanced ratiometric detection (BRD) implementing a reference channel (reprinted from [18] with permission)
Acetone, which is frequently associated with the smell of rotten apples, is produced intracellularly by the decarboxylation of acetoacetate, which in turn results from lipolytic or proteoloytic processes. An increase in the concentration of acetone in exhaled breath usually correlates closely with the plasma concentration and occurs not only with deregulated diabetes mellitus but also with energy deficiencies once the body metabolizes fat instead of glucose as an energy source. Exploiting the C-C stretching vibration with a broad absorbance feature between 850 and 1250 cm−1, QCLs emitting in the MIR have been employed for breath acetone analysis. Using a pulsed EC-QCL and a multi-pass cell with an optical path length of approx. 55 m (internal volume 600 mL), an acetone concentration detection limit (LOD) of 0.05 ppmv (1σ) was obtained by applying multiline fitting [19]. Another approach for breath acetone detection was reported employing CEAS achieving a LOD of 0.51 ppmv for acetone. Breath acetone sensing has significant potential during more complex medical examinations where glucose monitoring via blood tests is limited [20].
The clinical measurement of exhaled NO (eNO) has received attention as a potential biomarker during the diagnosis of airway inflammation and oxidative stress in the lung. In addition, NO is involved in the defense against infections; accordingly, elevated NO values are found during or after respiratory tract infections. Using chemiluminescence or electrochemical transduction techniques, a variety of sensors have been introduced for eNO monitoring [21]. Advancements in MIR technology with emphasis on QCL and ICL technology are a major advancement in the development of laser-based eNO gas sensors. Various approaches have been proposed utilizing CRDS techniques [22], integrated cavity output spectroscopy [23], or multi-pass gas cells based on TDLAS [24]. NO measurements by optical feedback CEAS via an ICL emitting at 5.3 μm was demonstrated by Richard et al. with a 50 ppt NO detection limit, and a short response time of only 180 ms demonstrating the capability for on-line breath analysis resolving the details of respiratory phases [25].
An interesting approach to monitor ammonia as a biomarker for Helicobacter pylori infection, and for determining disorders of the liver and kidney was demonstrated by calibration-free ammonia breath sensor using a QCL emitting at 9 μm in combination with WMS, as shown in Fig. 2 [26]. Using a multi-pass gas cell with approx. 77 m, the sensor was tested for the analysis of exhaled ammonia in the breath of healthy patients, and of patients diagnosed with chronic kidney disease (CKD). With a detection limit of 7 ppbv and 5% total uncertainty for breath measurements, the determination of elevated levels of ammonia in the breath of patients with CKD and monitoring of ammonia decrease during dialysis could be demonstrated. Healthy individuals had a 100-times lower ammonia concentration in exhaled breath compared to CKD patients.
Experimental setup used for measurement of ammonia in breath as samples flow through a multi-pass cell with 238 passed totalling 76.45 m of absorption path length, and a DFB-QCL emitting in the range 1100.4–1108.2 cm−1 (reprinted from [26] with permission)
Oxidative metabolic processes of carbon disulfide and deficient methionine metabolism lead to the production of exhaled carbonyl sulfide (OCS). Increased levels of OCS have been associated to liver-related diseases. For the analysis of exhaled OCS, Wysocki et al. [27] used an Herriott cell with an optical path length of 36 m in combination with a pulsed QCL emitting between 4.85 and 4.87 μm (2.054.5–2.060.5 cm−1). This compact breath sensor was used to determine trace levels of OCS and CO2 in exhaled human breath with a detection limit of 1.2 ppb for OCS, and at an acquisition time of 0.4 s. In order to resolve transition lines of both analytes, the cell pressure of the Herriott cell was maintained at 60 Torr. Hence, analyzing OCS in breath has substantial potential as diagnostic for patients suffering from chronic liver disease.
In a remarkable instrumental approach, multiple components including NO, CO2, CO, and N2O were determined by Shorter et al. combining two QCLs into an advanced breath analyzer system [28]. The simultaneous quantification of multiple breath components offers new perspectives for researchers when studying and diagnosing lung diseases. A pulsed DFB-QCL emitting at 2190 cm−1 for CO and N2O, and another one emitting at 1900 cm−1 for CO2 and NO analysis was combined with a multi-pass gas sampling cell providing a volume of 0.5 l with 76 m of effective absorption pathlength at an acquisition rate of 10 Hz enabling real-time breath monitoring. The system was tested for patients with asthma and COPD. It has been identified that NO and CO are related to inflammatory diseases and COPD, whereas CO2 analysis may be used for dead space volume determination.
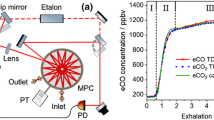
ICL-based CO monitoring was also demonstrated using a room temperature ICL emitting at 4.69 μm in combination with WMS utilizing a low-volume circular multi-pass cell. The CO breath sensor and breath sampler are shown in Fig. 3a and b. The applied ICL allows real-time detection of stable isotopes (i.e., 13C16O and 12C18O) for recording CO expirograms. This compact CO sensor by Ghorbani et al. with a detection limit of 9 ± 5 ppbv at 0.07 s of data acquisition time was tested to record exhalation profiles before and after smoking from non-smokers and an occasional smoker [29]. Increased levels of eCO were clearly indicated after smoking as shown in Fig. 4(a and b).
Scheme of an ICL-based TDLAS setup (a) and the utilized breath sampler (b) (reprinted from [29] with permission)
Exhaled CO profiles for 12CO (a) and 13CO (b) from a healthy occasional smoker before smoking (19 h after the last cigarette), and 15 s after smoking. Gray—raw data, colored lines—smoothed raw data (reprinted from [29] with permission)
As a non-invasive tool for monitoring and diagnosing diseases by their related biomarkers in exhaled breath, these types of mid-infrared laser-based gas sensors may readily be adapted for research and clinical study purposes, thus paving the way towards rapid and low-cost point-of-care testing within the exhaled breath matrix.
Outlook
Breath analysis using cascade laser sensing techniques for the determination of exhaled biomarkers represent a cutting-edge solution for monitoring the metabolic status, and for disease diagnostics. Considerable progress has been made with regard to the development of highly selective and sensitive spectroscopic techniques taking advantage of the continuous evolution in mid-infrared light source technology, i.e., QCLs and ICLs. Compared to the rather bulky and expensive alternatives including difference frequency generation (DFG) systems or optical parametric oscillators (OPOs), cascade lasers nowadays facilitate high optical output power at room-temperature operation, which renders them ideally suited for POC sensor/system development.
Exhaled breath sensors based on CLS lasers are already commercially available, e.g., for nitric oxide monitoring (Ekips Technologies, Inc), and ammonia detection (Pranalytica, Inc). Nevertheless, personalized breath sensors for everyday usage based on CLS techniques are still limited by the associated costs of the MIR light source. Next to the light source, costs, compactness, and robustness of POC breath analysers also require appropriate signal transduction interfaces, which are nowadays predominantly based upon adequate waveguide technologies. Recent progress in hollow waveguide and planar semiconductor waveguide technology demonstrates significant advancements towards the miniaturization and cost-reduction of the sensing interface, which promises a bright perspective for compact and robust sensor/system design. On the detection side, quantum cascade detectors (QCD) using quantum heterostructures similar to cascade lasers have readily been demonstrated, and show great potential for further system miniaturization, and for the development of clinical breath sensors [30, 31].
As volatile organics (VOCs) in exhaled breath are present at concentrations ranging from the ppm to the ppt level, the achievable sensitivity levels are crucial to the success of any sensing scheme applied for the analysis of breath VOCs. Although a variety of mid-infrared gas sensors have already been established for breath analysis in the ppb range, ultra-trace analysis at ppt concentration levels remains limited utilizing CLS-based systems. Although combined gas chromatography-mass spectrometry techniques are still considered the current “golden standard” for the qualitative and quantitative detection of multiple breath components at ppt levels, innovative approaches have been reported using amplification strategies for advancing infrared spectroscopy to the required sensitivity levels [32].
The measurement of many VOCs at low concentrations in exhaled breath is challenging, as their weak absorption peaks are overlapped by spectral bands of water vapor of higher intensity. In addition, reproducible detection of VOCs is affected by varying humidity in breath samples leading to problems in interpretation and falsification of measured data. In order to dehumidify breath samples, several techniques have been tested. As an alternative to adsorption, nafion membranes, and drying Maiti et al. used water condensation to reduce water vapor by passing the breath sample through a 12 m long copper tube at an operating temperature of − 60 °C [33]. Water reduction by a factor of approximately 2500 was achieved, and thus, allowed the spectroscopic analysis of several trace gases with previously obscured characteristic spectral features in breath, i.e., methane, isoprene, acetone, aldehyde, and carbon monoxide.
Likewise, the simultaneous detection of several biomarkers via optical methods has only been demonstrated for few species. Due to the to date limited tunability across rather narrow spectral bands, laser-based gas sensors provide only single molecule coverage or access to a reduced selection of biomarkers that absorb within a narrow spectral window. Hence, progress to that end focuses on broadening the addressable spectral window either via external cavity feedback or the integration of a multiple (DFB) lasers (i.e., laser arrays) combined with on-chip beam combiners [34]. An alternative concept emerges via frequency comb technologies facilitating QCLs emitting equidistant wavelengths across a wide spectral window by combining DFB technology with mode-hop-free tuning and external cavities [35]. Exhaled breath analysis of larger molecules such as octane, acetaldehyde, and 3-methylheptane, which have been identified as volatile biomarkers of acute respiratory distress syndrome (ARDS), presents a formidable challenge for laser-based diagnostics [36]. High-resolution infrared spectroscopy of complex molecules is restricted, as manifold rotational/vibrational states are excited at room temperature, and thus, the assignment of absorption lines is difficult. Conventional infrared spectroscopy based on QCLs and ICLs that have intrinsically narrow emission linewidths is therefore limited to the analysis of relatively small molecules. In order to resolve the rovibrational structure of large molecular species, physicists at JILA and Harvard University combined cavity-enhanced direct frequency comb spectroscopy (CE-DFCS) with a helium buffer gas cooling method [37]. The resulting absorption spectrum is drastically simplified, as molecules are cooled down to only a few degrees above absolute zero, and the Doppler-broadened linewidths are narrowed. Although the system was tested so far only for nitromethane, naphthalene, and other large molecules, the developed approach represents a significant advancement and certainly has potential in the analysis of breath addressing complex molecules via MIR spectroscopy.
However, several challenges in exhaled breath analysis are not associated with laser-based instrumental limitations. Exhaled breath analysis itself is only of diagnostic value, if the correlation between a certain disease and an individual or a panel of biomarkers is firmly established and understood. For that reason, the clinical application of breath analysis remains somewhat in its infancy, as only a few compounds among the hundreds of exhaled VOCs have been clinically approved. The remaining high variability of thus obtained results within healthy individuals (i.e., within-patient-variance), and in comparison to diseased patients (i.e., in-between-patient-variance) renders the determination of precise correlations between biomarker concentrations and disease—or therapy—progress rather difficult. For example, depending on an individual’s environment, as well as dietary and life style conditions relevant biomarkers may be elevated without a metabolic disorder. Via inhalation or skin absorption, background air VOCs may in addition affect the reliability of the obtained results. Hence, not only the reliability and precision of the applied analytical and/or sensing technique is crucial, but standardized and normalized sample collection, preparation, storage, and analysis protocols are essential to establish the applicability of breath sensing in routine clinical practice.
In summary, significant progress in mid-infrared light source technology, and specifically cascade lasers including QCLs and ICLs along with advancements in waveguide and detection technologies during the last decades have established the potential of molecularly discriminatory optical sensing in exhaled breath analysis. Next to the demonstration of real-time and non-invasive analysis, first clinical studies using cascade laser technologies have been conducted highlighting that laboratory-based systems are increasingly evolved into POC breath analysis systems. Yet, for nurturing a more widespread acceptance and adoption of breath diagnostics based on CLS, interdisciplinary research of physicians, optical engineers, chemists, and physicists is required to overcome still pending technical and scientific challenges. Last but not least, extended clinical testing on larger patient populations and validation via established analytical techniques is essential to verify the diagnostic accuracy and validity of mid-infrared sensing technologies in exhaled breath analysis.
References
Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci U S A. 1971;68:2374–6.
Kokoric V, Wissel PA, Wilk A, Mizaikoff B. muciPRECON: multichannel preconcentrators for portable mid-infrared hydrocarbon gas sensors. Anal Methods. 2016;8:6645–50. https://doi.org/10.1039/C6AY01447J.
Lourenço C, Turner C. Breath analysis in disease diagnosis: methodological considerations and applications. Metabolites. 2014;4:465–98. https://doi.org/10.3390/metabo4020465.
Faist J, Capasso F, Sivco DL, Sirtori C, Hutchinson AL, Cho AY. Quantum cascade laser. Science. 1994;264:553–6. https://doi.org/10.1126/science.264.5158.553.
Kazarinov RF, Suris RA. Possibility of amplification of electromagnetic waves in a semicon- ductor superlattice. Sov Phys Semicond. 1971;5:707–8.
Vurgaftman I, Geiser P, Bewley WW, Merritt CD, Canedy CL, Warren MV, et al. Sensitive chemical detection with distributed feedback interband cascade lasers. In: Meyers RA, editor. Encyclopedia of analytical chemistry. Chichester: John Wiley & Sons, Ltd; 2016. p. 1–19.
Dallner M, Scheuermann J, Nähle L, Fischer M, Koeth J, Höfling S, et al. InAs-based distributed feedback interband cascade lasers. Appl Phys Lett. 2015;107:181105. https://doi.org/10.1063/1.4935076.
White JU. Long optical paths of large aperture. J Opt Soc Am. 1942;32:285–8. https://doi.org/10.1364/JOSA.32.000285.
Herriott DR, Schulte HJ. Folded optical delay lines. Appl Opt. 1965;4:883–9. https://doi.org/10.1364/AO.4.000883.
Stacewicz T, Bielecki Z, Wojtas J, Magryta P, Mikolajczyk J, Szabra D. Detection of disease markers in human breath with laser absorption spectroscopy. Opto-Electron Rev. 2016;24. https://doi.org/10.1515/oere-2016-0011.
Pakmanesh N, Cristescu SM, Ghorbanzadeh A, Harren FJM, Mandon J. Quantum cascade laser-based sensors for the detection of exhaled carbon monoxide. Appl Phys B. 2016;122. https://doi.org/10.1007/s00340-015-6294-7.
Lewicki R, Kosterev AA, Thomazy DM, Risby TH, Solga S, Schwartz TB, et al. Real time ammonia detection in exhaled human breath using a distributed feedback quantum cascade laser based sensor. In: Quantum Sensing and Nanophotonic Devices VIII: International Society for Optics and Photonics; 2011. p. 79450K.
Thorpe MJ, Balslev-Clausen D, Kirchner MS, Ye J. Cavity-enhanced optical frequency comb spectroscopy: application to human breath analysis. Opt Express. 2008;16:2387. https://doi.org/10.1364/OE.16.002387.
Goddard AF, Logan RPH. Review article: urea breath tests for detecting Helicobacter pylori. Aliment Pharmacol Ther. 1997;11:641–9. https://doi.org/10.1046/j.1365-2036.1997.00206.x.
Weidmann D, Wysocki G, Oppenheimer C, Tittel FK. Development of a compact quantum cascade laser spectrometer for field measurements of CO2 isotopes. Applied Physics B. 2005;80:255–60. https://doi.org/10.1007/s00340-004-1639-7.
Kasyutich VL, Martin PA. 13CO2/12CO2 isotopic ratio measurements with a continuous-wave quantum cascade laser in exhaled breath. Infrared Phys Technol. 2012;55:60–6. https://doi.org/10.1016/j.infrared.2011.09.003.
Wörle K, Seichter F, Wilk A, Armacost C, Day T, Godejohann M, et al. Breath analysis with broadly tunable quantum cascade lasers. Anal Chem. 2013;85:2697–702. https://doi.org/10.1021/ac3030703.
Tütüncü E, Naegele M, Becker S, Fischer M, Koeth J, Wolf C, et al. Advanced photonic sensors based on interband cascade lasers for real-time mouse breath analysis. ACS Sensors. 2018. https://doi.org/10.1021/acssensors.8b00477.
Reyes-Reyes A, Horsten RC, Urbach HP, Bhattacharya N. Study of the exhaled acetone in type 1 diabetes using quantum cascade laser spectroscopy. Anal Chem. 2015;87:507–12. https://doi.org/10.1021/ac504235e.
Ciaffoni L, Hancock G, Harrison JJ, van Helden J-PH, Langley CE, Peverall R, et al. Demonstration of a mid-infrared cavity enhanced absorption spectrometer for breath acetone detection. Anal Chem. 2013;85:846–50. https://doi.org/10.1021/ac3031465.
Marchenko D, Mandon J, Cristescu SM, Merkus PJFM, Harren FJM. Quantum cascade laser-based sensor for detection of exhaled and biogenic nitric oxide. Appl Phys B. 2013;111:359–65. https://doi.org/10.1007/s00340-013-5341-5.
De A, Banik GD, Maity A, Pal M, Pradhan M. Continuous wave external-cavity quantum cascade laser-based high-resolution cavity ring-down spectrometer for ultrasensitive trace gas detection. Opt Lett. 2016;41:1949–52. https://doi.org/10.1364/OL.41.001949.
Bakhirkin YA, Kosterev AA, Curl RF, Tittel FK, Yarekha DA, Hvozdara L, et al. Sub-ppbv nitric oxide concentration measurements using cw thermoelectrically cooled quantum cascade laser-based integrated cavity output spectroscopy. Appl Phys B. 2006;82:149–54. https://doi.org/10.1007/s00340-005-2058-0.
Menzel L, Kosterev AA, Curl RF, Tittel FK, Gmachl C, Capasso F, et al. Spectroscopic detection of biological NO with a quantum cascade laser. Appl Phys B Lasers Opt. 2001;72:859–63.
Richard L, Romanini D, Ventrillard I. Nitric oxide analysis down to ppt levels by optical-feedback cavity-enhanced absorption spectroscopy. Sensors. 2018;18:1997. https://doi.org/10.3390/s18071997.
Owen K, Farooq A. A calibration-free ammonia breath sensor using a quantum cascade laser with WMS 2f/1f. Appl Phys B. 2014;116:371–83. https://doi.org/10.1007/s00340-013-5701-1.
Wysocki G, McCurdy M, So S, Weidmann D, Roller C, Curl RF, et al. Pulsed quantum-cascade laser-based sensor for trace-gas detection of carbonyl sulfide. Appl Opt. 2004;43:6040–6. https://doi.org/10.1364/AO.43.006040.
Shorter JH, Nelson DD, McManus JB, Zahniser MS, Milton DK. Multicomponent breath analysis with infrared absorption using room-temperature quantum cascade lasers. IEEE Sensors J. 2010;10:76–84. https://doi.org/10.1109/JSEN.2009.2035764.
Ghorbani R, Schmidt FM. ICL-based TDLAS sensor for real-time breath gas analysis of carbon monoxide isotopes. Opt Express. 2017;25:12743. https://doi.org/10.1364/OE.25.012743.
Szedlak R, Harrer A, Holzbauer M, Schwarz B, Waclawek JP, MacFarland D, Zederbauer T, Detz H, Andrews AM, Schrenk W, Lendl B, Strasser G (2016) Remote sensing with commutable monolithic laser and detector. https://pubs.acs.org/doi/full/10.1021/acsphotonics.6b00603. Accessed 24 Aug 2018.
Harrer A, Szedlak R, Schwarz B, Moser H, Zederbauer T, MacFarland D, et al. Mid-infrared surface transmitting and detecting quantum cascade device for gas-sensing. Sci Rep. 2016;6:21795. https://doi.org/10.1038/srep21795.
Perez-Guaita D, Kokoric V, Wilk A, Garrigues S, Mizaikoff B. Towards the determination of isoprene in human breath using substrate-integrated hollow waveguide mid-infrared sensors. J Breath Res. 2014;8:026003. https://doi.org/10.1088/1752-7155/8/2/026003.
Maiti KS, Lewton M, Fill E, Apolonski A. Sensitive spectroscopic breath analysis by water condensation. J Breath Res. 2018;12:046003. https://doi.org/10.1088/1752-7163/aad207.
Zhou W, Bandyopadhyay N, Wu D, McClintock R, Razeghi M. Monolithically, widely tunable quantum cascade lasers based on a heterogeneous active region design. Sci Rep. 2016;6:25213. https://doi.org/10.1038/srep25213.
Faist J, Villares G, Scalari G, Rösch M, Bonzon C, Hugi A, et al. Quantum cascade laser frequency combs. Nanophotonics. 2016;5. https://doi.org/10.1515/nanoph-2016-0015.
Bos LDJ, Weda H, Wang Y, Knobel HH, Nijsen TME, Vink TJ, et al. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur Respir J. 2014;44:188–97. https://doi.org/10.1183/09031936.00005614.
Spaun B, Changala PB, Patterson D, Bjork BJ, Heckl OH, Doyle JM, et al. Continuous probing of cold complex molecules with infrared frequency comb spectroscopy. Nature. 2016;533:517–20. https://doi.org/10.1038/nature17440.
Acknowledgements
The authors acknowledge the outstanding collaboration with the team of P. Radermacher at the Institute of Anesthesiologic Pathophysiology and Method Development at the Ulm University Medical Center. Partial support of their research by the project APOSEMA (German BMBF within the M-Era.net program), the EC H2020 Program H2020-MSCA-RISE-2014, Grant Agreement No. 645758 (“TROPSENSE”), and the Graduate School PULMOSENS at Ulm University (GRK 2203) by the Deutsche Forschungsgemeinschaft (DFG) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ABC Highlights: authored by Rising Stars and Top Experts.
Rights and permissions
About this article
Cite this article
Tütüncü, E., Mizaikoff, B. Cascade laser sensing concepts for advanced breath diagnostics. Anal Bioanal Chem 411, 1679–1686 (2019). https://doi.org/10.1007/s00216-018-1509-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1509-5