Abstract
A novel and sensitive method based on nitrogen-doped carbon quantum dots as a fluorescence probe coupled with magnetic solid-phase extraction (MSPE) purification for analysis of folic acid (FA) in human serum samples has been established for the first time. In the developed system, magnetic nanoparticles coated with hexanoic acid (Fe3O4@C6) were synthesized by a one-step chemical co-precipitation method with good magnetic properties and dispersibility for sample purification, and it is better to be separated from the sample. High fluorescence nitrogen-doped carbon quantum dots (N-CQDs), simply prepared using a one-step hydrothermal method with nitrilotriacetic acid, could be selectively quenched by FA. Based on this phenomenon, a fluorescence assay was proposed for specific determination of FA. Various operational experiment parameters have been studied and optimized in detail. Under the optimum experimental conditions, the detection limit of the proposed method for FA was evaluated to be 0.5 nM (S/N = 3), while the relative standard deviation (RSD) was 1.2% (n = 6). Finally, the proposed method was applied for determination of trace levels of FA from human serum samples and quantitative recoveries were achieved within the range of 95.7–103.5%. All of the results showed that the proposed method had significant application in further research.

Schematic of synthesis of N-CQDs and schematic of suggested mode for analysis of folic acid (FA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Folic acid (FA) belongs to water-soluble B group vitamins, derived from its conjugates, folate polyglutamates, which exists widely in most foods, such as green leafy vegetables, organ meats, and some fresh fruits [1,2,3]. It is one of the significant components of hemotopoietic system and acts as substrates and coenzymes, which was involved in transport and enzymatic processing of one-carbon groups for the metabolism from amino acid and nucleic acid [4, 5]. In general, the normal level of FA in serum ranges from 7 to 42 nM [6]. Several chronic diseases (for example, macrocytic anemia associated with leucopenia, psychosis, heart attack, and stroke), particularly those concerned with malformation during pregnancy and carcinogenic processes, are in connection with folic acid deficiency [6, 7]. Taking the above considerations, developing a simple and rapid analytical method for determination of FA is of great significance.
Some analytical techniques have been reported for determination of FA, such as spectrophotometry [8], photochemical fluorimetry [9], chemiluminescence [10], voltammetry [2], and high-performance liquid chromatography methods [11]. Among the above methods, the fluorescence method has attracted extensive attention because of its fast, simple, and non-destructive operation analysis. Nevertheless, their application in the direct analysis of FA is limited due to its poor reproducibility and matrix interference, especially for trace analysis in biological samples, such as plasma samples [6, 7]. In plasma samples, relatively high plasma protein binding, low plasma concentrations, and occurrences of interfering lipids make accurate determination of FA a long-standing challenging task. In that case, it still needs developing selective purification and determination techniques to reduce or eliminate the influence of impurities in the matrix and gain high sensitivity, high recoveries, and good precision [12,13,14].
At present, as one of the most challenging classes of new materials, carbon quantum dots (CQDs) have been developed as luminescent probes for sensing events, which constituted an active field of research [15, 16]. In the merits of low toxicity, good biocompatibility, excellent chemical inertness, and high sensitivity and selectivity, they have popularized as an upstart of the nanocarbon family in fluorescence assay [12, 17] and have been widely used including metal ions [18, 19], inorganic anions [20], and organic compounds [21, 22] in the detection fields. Recently, to improve the fluorescence (FL) properties of CQDs, surface passivation and element doping (such as N, S, B, and P) have been widely explored. Compared with surface passivation, element doping is a powerful method because this method can effectively tune their intrinsic properties, like improving unique electronic and optical characteristics of CQDs and providing more active sites on CQD surface [12, 23, 24]. It has been reported that doping CQDs with electron-rich N atoms could own rich chemical group and offer more active sites, thus producing unique fluorescence phenomena and unexpected properties [23, 25]. However, the fluorescence assay is limited due to the poor fluorescent carbon quantum dots’ selectivity and strong matrix effects of complex samples. To overcome this drawback, the introduction of sample purification technique to the fluorescence assay is one of the effective ways [26].
Magnetic solid-phase extraction (MSPE) with magnetic nanoparticles as the adsorbents has elicited greatest interest in complex matrix sample preparations in chemical analysis [27]. In MSPE procedures, the magnetic adsorbents, containing Fe3O4 nanoparticles (NPs) with specific chemical functional group modifications on their surface, are extensively dispersed into the sample solution to achieve purification [28]. In such dispersive mode, the contact-specific surface area between the sorbents and the sample solution is sufficiently large to eliminate matrix components interfering with detection in the sample. Magnetic nanoparticles are super-paramagnetic at room temperature, which could be readily isolated from the sample matrix by an external magnetic field. Magnetic isolation is significantly more convenient, economic, and efficient, which avoid time-consuming operation such as filtration or centrifugation. Such advantages render MSPE as a promising alternative technique for sample purification and clean-up with high selectivity [29, 30].
The purpose of this work is to establish a simple, sensitive, and accurate technique for analysis of folic acid based on fluorescence probe detection coupled with MSPE purification (Scheme 1). A range of magnetic nanoparticles coated with fatty acids (C4–C11) were synthesized successfully by a one-step chemical co-precipitation method, and then applied as magnetic adsorbent for sample purification and clean-up. The novel nitrogen-doped CQDs (N-CQDs) was prepared by a one-step hydrothermal method of nitrilotriacetic acid and showed good selective and high sensitive fluorescence quenching for FA. Based on the property of sample purification and fluorescent probe detection, the proposed method was employed the first time for analysis of FA and was successfully further applied for determination of FA in human serum samples with a wide linear detection range, good selectivity, and low detection limit.
Experimental
Materials
All the chemicals and reagents were of analytical grade and employed without further purification. Ferric chloride (FeCl3·6H2O) and ammonia solution were supplied by Tianjin Zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China). Ammonium iron(II) sulfate hexahydrate ((NH4)2Fe(SO4)2·6H2O), carbamide, ethanediamine, tartaric acid, and thiourea were supplied by Guangzhou Jinhuada Chemical Reagent Co., Ltd. (Guangzhou, China). Zinc sulfate (ZnSO4), sodium hydrate (NaOH), citric acid, and sodium phosphate dibasic (Na2HPO4) were purchased from Fengchuan Chemical Reagent Co., Ltd. (Tianjin, China). Standard of folic acid (FA), butyric acid, valeric acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, and undecanoic acid were all purchased from Aladdin (Shanghai, China). Nitrilotriacetic acid was purchased from TCl (Shanghai) Development Co., Ltd. (Shanghai, China). Deionized water was provided by a Milli-Q system (USA).
Instruments
Agilent G9800A fluorescence spectrophotometer (Agilent Technologies, USA) was applied for fluorescence analysis. The ultraviolet–visible (UV-vis) spectra were obtained by a UV-2450 UV-vis spectrophotometer (Shimazu Co., Japan). Fourier transform infrared spectra (FT-IR) were recorded on a TENSOR27 FT-IR spectrometer (Bruker, Germany). Transmission electron microscope (TEM) images were collected from a FEI Tecnai G2F20 transmission electron microscope (FEI, Holland). Scanning X-ray photoelectron spectroscopy (XPS) data for N-CQDs was measured by a PHI5000 Versaprobe-II (Shimazu Co., Japan) for analysis structure information of N-CQDs. A pH-meter Sartorius PB10 (Gttingen, Germany) was used for the accurate pH adjustment. Vacuum drying oven BPZ-6033 (Shanghai, China) was used to dry synthesized nanomaterials. Other instruments including ultrasonic cleaner (Kunshan ultra-sonic instrument plant, Jiangsu, China), vortex mixer (Hanuo instrument Co.Itd, XH-B, Shanghai, China), centrifuge (Shanghai surgical instrument factory, 80-2, Shanghai, China), and vacuum drying oven BPZ-6033 (Shanghai, China) were applied in the whole experiments.
Preparation of standard solutions and human serum samples
The stock standard solution (100 μmol L−1) of FA was prepared by deionized triple-distilled water containing 100 μL of 10 mM NaOH. Working standard solutions were daily prepared by dilution of the stock solution with water. The stock solutions were stored in a refrigerator at ∼ 4 °C in the dark (stable for at least 2 month) and then brought to room temperature prior to use.
Human whole blood samples (5 mL) of ten volunteers were obtained from the First People’s Hospital of Yunnan Province (Kunming, China) at early morning time and stored in a refrigerator at ∼ 20 °C until analysis. Each whole blood sample was centrifuged at 5000 rpm for 10 min, and the upper plasma was collected into a 10-mL centrifuge tube. Then the protein precipitant and decolorizer of 400 μL NaOH (2.0 M) and 0.4 g ZnSO4 were added. The mixture was vortex blended approximately for 1 min and deposited by centrifugation at 5000 rpm for 10 min. The clear and colorless supernatant was transferred to a new tube and diluted to the volume of 5 mL with distilled water for further analysis.
Synthesis of nitrogen-doped carbon quantum dots
N-CQDs were synthesized via a one-step hydrothermal method [25]. In detail, 2.0 g of nitrilotriacetic acid was dissolved in 100 mL of deionized water. After ultrasonication in an ultrasonic bath for 5 min to form a white suspension, the solution was transferred to a 150 mL Teflon-lined stainless-steel autoclave kept at 200 °C for 5 h, and then naturally cooled to room temperature. Finally, the yellow aqueous solution was purified by filtration through 0.22 μm filter and additionally centrifuged at 10,000 rpm for 15 min to remove large particles, stored in a refrigerator at ∼ 4 °C until analysis.
Synthesis of fatty acid-coated magnetic nanoparticles (MNPs)
The fatty acid-coated magnetic nanoparticles (MNPs) were synthesized by a co-precipitation method with some modification [31]. Ammonium ferrosulfate ((NH4)2Fe(SO4)2·6H2O, 4.10 g) and ferric chloride (FeCl3·6H2O, 2.82 g) were dissolved in 100 mL deionized water. After vigorous stirring to 80 °C in a water bath under N2 atmosphere, another solution containing 200 μL or 200 mg of fatty acids (C4–C11) in 10 mL of acetone solution was added. After the mixtures were vigorously stirred for 5 min, 10 mL of 28% (w/v) ammonia solution was added. Subsequently, further fatty acids (2.0 mL or 2.0 g) were slowly added to the obtained suspension within 3 min. The reaction was maintained for 30 min at 80 °C with constant stirring under N2 atmosphere to produce a stable water-based suspension. Until the solution was cooled down slowly to room temperature, the suspension was precipitated with methanol and the precipitates were separated by an external super-magnet, which were washed alternately with deionized water-methanol (1:1, v/v) for several times. Finally, the obtained fatty acid (C4–C11)-coated MNPs were vacuum dried at 60 °C for 12 h.
Magnetic solid-phase extraction purification procedure
MSPE purification procedure was achieved as follows: first, 100 μL of the prepared Fe3O4-coated hexanoic acid (Fe3O4@C6) suspension were added into 3 mL of deionized water and sonicated for 5 min to ensure a good dispersion. And then 1 mL of human serum samples was added to the prepared sorbent suspension with pH 7.0, which was adjusted by citric acid-disodium hydrogen phosphate buffer solution. After the mixtures were vigorously stirred for 9 min, the adsorption equilibrium was achieved. The adsorbent was separated by an external super-magnet, and the supernatant was collected for further analysis. Namely, the magnetic purification procedure of human serum samples is completed.
Fluorescence detection procedure
All the fluorescence detection experiments were conducted by the same procedure. Eighty-microliter N-CQDs synthesized with nitrilotriacetic acid solutions were added to the purified human serum samples in a new 10 mL centrifuge tube. The mixtures were vortex-mixed approximately for 1 min and equilibrated for 4 min before the measurements of fluorescence emission spectral. All of the fluorescence emission spectra were determined at room temperature with excitation wavelength at 350 nm, emission wavelength at 440 nm, and the slit widths of the excitation and emission were both 5 nm.
Quenching data analysis
The quenching of fluorescence by FA was described using the Stern-Volmer equation:
where K sv is the Stern-Volmer quenching constant, C the concentration of FA, F 0 and F the fluorescence intensity of N-CQDs without FA and with different concentrations of FA, respectively. The detection limit used the equation 3σ/m, where σ is the relative standard deviation and m the slope of calibration curve as described in details [32].
Results and discussion
Physical characterization of N-CQDs
As-prepared N-CQDs, synthesized with nitrilotriacetic acid solutions, were characterized by TEM, FT-IR, XPS, and fluorescence spectroscopy, respectively. The surface morphology and microstructure of the as-prepared N-CQDs were investigated by TEM at Fig. 1a. It revealed that N-CQDs were well dispersed without obvious aggregation and had a lattice structure. The size distribution of N-CQDs fell within the range of 1–5 nm and an average diameter was about 3.7 nm. FT-IR was used to evaluate the characteristic peaks of the as-prepared N-CQDs. Amount of functional groups were observed from the FT-IR spectra in Fig. 1b. The peaks at about 1581 cm−1 could be ascribed to the characteristic absorption bands of the O–H stretching vibration mode. The peak at 1405 cm−1 could be attributed to the stretching vibrations of carboxylate (COO−). Additionally, the characteristic absorption band of CH2 stretching at 2809 cm−1 was also observed, and the typical absorption band at 1112 cm−1 implied the stretching vibrations of C–N groups. As a consequence, it could conclude that the surface of the water-soluble N-CQDs was occupied with hydrophilic groups (e.g., –COOH) and N-related groups (e.g., C–N). The above observations also confirmed that nitrogen was successfully doped into the CQDs.
To gain further insight into the surface functionality and elemental states of the as-prepared CDs, XPS was conducted. As shown in Fig. 1c, the XPS full-scan spectra show that four clear peaks around 283.2, 399.2, and 530.4 eV ascribe to carbon, nitrogen, and oxygen, respectively. As illustrated in Fig. 1d, The C1s peaks at 283.6, 285.6, 286.9, and 287.8 eV can be assigned to the form of C–C/C–H, C–N, C–O, and C=O, respectively. The O1s spectra (Fig. 1e) mainly were made of two peaks centered at 530.6 and 531.8 eV, suggesting that oxygen exists in two forms of C=O and C–O groups, respectively. The N1s peaks at 399 and 400.6 eV represented N–H and C–N groups, respectively (Fig. 1f). These results further indicated that nitrogen was successfully doped into the CQDs.
Spectroscopic properties of the N-CQDs
To investigate optical properties of the as-prepared N-CQDs, the UV-vis absorption spectra and fluorescence spectra of the as-prepared N-CQDs in aqueous solution were researched. The aqueous solution of N-CQDs showed strong bright blue fluorescence under UV lamp (365 nm) while showing almost colorless under white light (the inset of Fig. 2a). The UV-vis absorption spectrum (Fig. 2a) showed that the as-prepared N-CQDs had almost no absorption peaks in the wavelength range of 200–450 nm. Meanwhile, the as-prepared N-CQDs showed the maximum emission intensity at 350 nm with an excitation wavelength at 440 nm. Like other reported N-CDs, the as-prepared N-CQDs also exhibited excitation-dependent fluorescence behaviors, which might be related to less surface defects of the doped C-dots [33,34,35]. To further study the FL properties of the as-prepared N-CQDs, the FL spectra of N-CQDs were measured under different excitation wavelengths. As shown in Fig. 2b, the emission peaks did not shift under various excitation wavelengths that ranged from 270 to 450 nm, which indicated the uniformity of size and surface states. The constant emission wavelength further indicated that the as-prepared N-doped C-dots contained a unique single emitter, which could avoid auto-fluorescence interference and was favorable for the application of the water-soluble N-CQDs in quantitative analysis [36].
(a) UV-vis spectra and FL spectra of N-CQDs; inset, photographs of N-CQDs aqueous solution under daylight (left) and UV (365 nm) irradiation (right), (b) FL emission spectra of N-CQDs at different excitation wavelengths, and effects of various conditions on the fluorescence intensity of N-CQDs: (c) various NaCl concentrations and (d) storage time. (e) Variation of fluorescence intensity of N-CQDs under irradiation of 365 nm UV light
The fluorescent properties of the synthesized N-CQDs were further investigated under different conditions (storage time and ionic strengths). As displayed in Fig. 2c, the fluorescence intensity remained stable at concentrations of NaCl as high as 2 M, which verifies that N-CQDs have great stability under high ionic strength conditions. Furthermore, the fluorescence intensity of N-CQDs did not display significant change after 3 months of storage or even under 365 nm UV light for 180 min (Fig. 2d, e), showing their excellent photostability. All of these properties are indicative of its potential applications as an optical probe.
Optimization of magnetic purification procedure conditions
As we know, the human serum sample contains various plasma proteins, peptides, and fats, which may affect the accurate determination of target FA. Therefore, the main challenge was to remove these impurity substances under the premise of non-adsorption of target FA before analysis. For this purpose, it is necessary to optimize the proposed method based on MSPE purification procedure. In the developed system, the adsorption mechanism was physisorption based on hydrophobic interactions. When the surface of Fe3O4 magnetic nanoparticles coated with hexanoic acid, it could enhance its hydrophobicity. As a group of water-soluble B group vitamins, folic acid was hydrophilic, whereas other interference components were hydrophobic. Because of hydrophobic interactions, Fe3O4@C6 magnetic nanoparticles were more easily combined with interfering components, so as to achieve purification. The study and optimization of the experiment variables were performed using the one variable at a time method. And all the experiments were performed in triplicates, and their average was applied as analytical signal.
Selection of adsorbent in magnetic purification procedure
In order to select the optimum magnetic adsorbent to remove interference components as much as possible, eight different kinds of fatty acid (C4–C11)-coated MNPs were synthesized to investigate the adsorption ability. A comparative test was also carried out with pure MNPs to determine whether coatings could improve the purification efficiency (F 1–F 2)/F 1, where F 1 and F 2 represented fluorescence intensity of human serum containing target analyte FA before and after magnetic purification procedures, respectively. The obtained results of experiments were presented in Fig. 3a. The purification efficiency was improved obviously when fatty acid-coated MNPs were applied as adsorbents. The results also indicated that the purification efficiency improved remarkably with increasing the chain length of coatings and reached maximum with alkyl chains of six carbon atoms, and then reduced gradually with higher carbon atoms. It might suggest that a proper chain length provide enough Van der Waals interactions for the adsorption of interference components in human serum to achieve purification [31]. According to the obtained results, the synthesized Fe3O4@C6 was chosen as adsorbent to achieve human serum sample purification in the further study. A suitable amount usage of the adsorbent should be also carefully taken into account. Different amounts of prepared Fe3O4@C6 suspension ranging from 0 to 250 μL were investigated. Based on the results as shown in Fig. 3b, the purification efficiency increased with the increase of Fe3O4@C6 amount up to 100 μL which could effectively remove various matrixes and provided the highest recoveries, and then it almost kept constant. Consequently, 100 μL of the prepared Fe3O4@C6 suspension was chosen for efficient and robust purification in the following experiments.
Effect of purification time
The purification time should be sufficient to absorb interference components to achieve purification within a reasonable time without any carry over effect. The purification time profiles were examined in the range of 3–15 min. Due to the shorter diffusion route for Fe3O4@C6 NPs, purification of interference components could be achieved in shorter time. The obtained results, shown in Fig. 3c, revealed that the quantitative efficiency improved by enhancing the purification time from 3 to 9 min, and then remained almost constant. Based on the results obtained, MSPE purification procedure was almost complete when 9 min was chosen. Overall, a time value of 9 min was adopted as the optimum condition in the following experiments.
Optimization of fluorescence detection procedure conditions
In addition to purification, fluorescence detection is another important part for analysis of FA, it is also necessary to optimize the conditions of fluorescence detection procedure. Several analytical parameters were investigated and optimized to obtain the optimal fluorescence response of the N-CQDs system with FA.
Selection of N-CQDs in fluorescence detection procedure
In order to further extend the selectivity and enhance the determination sensitivity of the proposed method, several N-CQDs synthesized with different carbon and nitrogen sources, including citric acid, citric acid-urea, citric acid-ethylenediamine, tartaric acid-thiourea, and nitrilotriacetic acid were investigated. As seen in Fig. 4a, the highest quenching efficiency was obtained when using nitrilotriacetic acid as the carbon and nitrogen sources to synthesize N-CQDs. It might be because N-CQDs synthesized with nitrilotriacetic acid possessed a strong affinity and good biocompatibility towards FA. According to the satisfactory results, N-CQDs synthesized with nitrilotriacetic acid were selected as the optimum condition in the following experiments. The influence of N-CQDs volume on the fluorescence quenching intensity was also studied. The results (Fig. 4b) demonstrated that the quenching efficiency increased rapidly from 20 to 80 μL and obtained the highest when the volume of N-CQDs was 80 μL. Considering the obtained results, 80 μL N-CQDs was selected as the optimal condition in all of the following assays.
Effect of solution pH
In general, solution pH might play a role in the whole experimental procedure because it would affect the state of target analyte FA and interference components, as well as the surface group of N-CQDs [14]. The effect of different initial pH values was investigated from pH 4.0 to 9.0. As shown in Fig. 4c, it was found that the quenching efficiency increased with increasing solution pH until it reached 7.0. When the pH value was higher than 7.0, the quenching efficiency decreased gradually. Literature reported that FA could exist stably in neutral environment [3] and have the potential to slightly denature at lower or higher pH values [37]. There are two carboxyl groups in the molecular formula of folic acid (Scheme 1), which may be protonated in acidic solutions and decrease the solubility. On the other hand, FA molecular may be deprotonated at pH > 7.0, which decreased the association between the N-CQDs and FA. Hence, pH 7.0 was used to maintain the pH throughout the subsequent experiments.
Effect of fluorescence quenching response time
In order to obtain the optimal fluorescence response between of N-CQDs system with FA, the optimized fluorescence quenching response time was investigated ranging from 0 to 20 min, respectively. Figure 4d shows the response time between FA and N-CQDs. The results demonstrated that the maximum quenching of fluorescence was observed within 5 min, and no further distinct quenching of fluorescence were observed even after 5 min. It indicated that the equilibrium of the fluorescence quenching reaction was attained. Therefore, the duration of 5 min was chosen as the appropriate time for the fluorescence detection procedure in the proposed method.
Method validation
Under the optimum experimental conditions, the analytical performance of detection system for quantitative detection of FA was evaluated. As demonstrated in Fig. 5a, the FL intensity N-CQDs at 440 nm gradually decreases with increasing concentration of FA. The fluorescence-quenched efficiency showed linear increase with the increase of FA concentration in a range of 0.01–30 μM (Fig. 5b). The calibration curve could be expressed by the equation Y = F 0/F − 1 = 0.0112 × C FA + 0.0137 with a good correlation coefficient of 0.9994 (where F and F 0 represented the fluorescence intensities of the biosensor in presence and absence of FA, respectively). The limit of detection (LOD) was evaluated to be 0.5 nM (S/N = 3), while the relative standard deviation (RSD) was 1.2% (n = 6). The satisfactory results demonstrated that the proposed method could be employed as an excellent alternative for trace analysis of FA quantitatively.
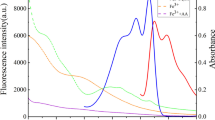
(a) Fluorescence emission spectra of N-CQDs with different concentrations of FA, (b) the linear relationship with respect to FL quenching ratio and the concentration of FA within the range of 0.01–30 μM, and (c) fluorescence emission spectra of N-CQDs (1), N-CQDs/serum samples without purification (2), and N-CQDs/serum samples after purification (3)
Analysis of human serum samples
To further demonstrate the feasibility of the practical application of this developed system, the proposed method was applied to detect FA levels in human serum samples (Fig. 5c). The satisfactory and acceptable results demonstrated great recoveries (95.7–103.5%) and relative standard deviation (RSD = 2.1–3.9%, n = 6) of the proposed method as listed in Table 1, clearly confirming the good precision and accuracy of the proposed method. As a contrast on the correctness of the results, the FA concentrations of the same samples were analyzed simultaneously by the HPLC method. The results summarized in Table 1 show that the FA concentrations found in human serum samples are fairly close to the results of HPLC. Moreover, the same samples were analyzed simultaneously by fluorescence detection technique without purification for comparison. As listed in Table 2, it could be concluded that the analytical performance of the developed technique was comparable better than fluorescence detection method without purification, such as low detection limit, high sensitivity, high efficiency, and good precision. It also indicated that the proposed assay had great potential in the practical biological sample analysis, which could be successfully applied for detecting FA levels in human serum samples.
The analytical results of this method were compared with other methods reported for the determination of FA in Table 3. The LOD of the method were comparable to or exceeded those of other methods. Fluorescence analysis has unique advantages, such as simplicity, speed, and low consumption compared with other methods. Fluorescence probes based on QDs for detecting FA have been developed by previous researchers. However, the LOD obtained was much higher than our study. It is important that the prepared Fe3O4@C6 magnetic nanoparticles possess good biological compatibility so that it can be directly applied to clean-up and purification of FA in a complex biological serum sample. Although these magnetic purification procedures make the experimental process more complicated, they also increase the detection sensitivity and reduce the detection limit.
Conclusion
In summary, a novel and sensitive method has been developed for analysis of folic acid in human serum samples for the first time based on fluorescence detection coupled with MSPE purification. Fe3O4@C6 was synthesized by a one-step chemical co-precipitation method for sample purification and clean-up, and high fluorescence N-CQDs were simply prepared using a one-step hydrothermal method with nitrilotriacetic acid for fluorescence detection, respectively. It had some remarkable advantages of high selectivity, high sensitivity, fast response, and wide response range. Under the optimum experimental conditions, quantitative recoveries were achieved. All of results showed that the proposed method could be a powerful alternative for determination of trace levels of FA from human serum samples, which had a significant application in further researches.
References
Quaglia M, Chenon K, Hall AJ, De LE, Sellergren B. Target analogue imprinted polymers with affinity for folic acid and related compounds. J Am Chem Soc. 2001;123(10):2146.
Kalimuthu P, John SA. Selective electrochemical sensor for folic acid at physiological pH using ultrathin electropolymerized film of functionalized thiadiazole modified glassy carbon electrode. Biosens Bioelectron. 2009;24(12):3575–80.
Dai H, Li Y, Zhang S, Gong L, Li X, Lin Y. Delicate photoelectrochemical sensor for folic acid based on carbon nanohorns supported interwoven titanate nanotubes. Sensor Actuat B Chem. 2016;222:120–6.
Xiao F, Ruan C, Liu L, Yan R, Zhao F, Zeng B. Single-walled carbon nanotube-ionic liquid paste electrode for the sensitive voltammetric determination of folic acid. Sensor Actuat B Chem. 2008;134(2):895–901.
Lermo A, Fabiano S, Hernandez S, Galve R, Marco M, Alegret S, et al. Immunoassay for folic acid detection in vitamin-fortified milk based on electrochemical magneto sensors. Biosens Bioelectron. 2009;24(7):2057–63.
Zhu Z, Wu H, Wu S, Huang Z, Zhu Y, Xi L. Determination of methotrexate and folic acid by ion chromatography with electrochemical detection on a functionalized multi-wall carbon nanotube modified electrode. J Chromatogr A. 2013;1283(6):62–7.
Prasad BB, Madhuri R, Tiwari MP, Sharma PS. Electrochemical sensor for folic acid based on a hyperbranched molecularly imprinted polymer-immobilized sol–gel-modified pencil graphite electrode. Sensor Actuat B Chem. 2010;146(1):321–30.
Rao GR, Kanjilal G, Mohan KR. Extended application of Folin-Ciocalteu reagent in the determination of drugs. Analyst. 1978;103(1230):993–4.
Rui ASL, Lima JFC, Reis BF, Santos JLM, Zagatto EAG. Photochemical-fluorimetric determination of folic acid in a multicommutated flow system. Anal Chim Acta. 1997;351(1):223–8.
Zhang BT, Zhao L, Lin JM. Determination of folic acid by chemiluminescence based on peroxomonosulfate-cobalt(II) system. Talanta. 2008;74(5):1154–9.
Lebiedzińska A, DąBrowska M, Szefer P, Marszałł M. High-performance liquid chromatography method for the determination of folic acid in fortified food products. Toxicol Mech Methods. 2008;18(6):463–7.
Wang H, Lu Q, Hou Y, Liu Y, Zhang Y. High fluorescence S,N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta. 2016;155:62–9.
Deng X, Guo Q, Chen X, Xue T, Wang H, Yao P. Rapid and effective sample clean-up based on magnetic multiwalled carbon nanotubes for the determination of pesticide residues in tea by gas chromatography–mass spectrometry. Food Chem. 2014;145(7):853.
Pan SD, Chen XH, Shen HY, Li XP, Cai MQ, Zhao YG, et al. Rapid and effective sample cleanup based on graphene oxide-encapsulated core-shell magnetic microspheres for determination of fifteen trace environmental phenols in seafood by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2016;919:34–46.
Shamsipur M, Rajabi HR. Pure zinc sulfide quantum dot as highly selective luminescent probe for determination of hazardous cyanide ion. Mat Sci Eng C-Mater. 2014;36(1):139–45.
Rajabi HR, Shamsipur M, Khosravi AA, Khani O, Yousefi MH. Selective spectrofluorimetric determination of sulfide ion using manganese doped ZnS quantum dots as luminescent probe. Spectrochim Acta A. 2013;107(7):256–62.
Mewada A, Pandey S, Thakur M, Jadhav D, Sharon M. Swarming carbon dots for folic acid mediated delivery of doxorubicin and biological imaging. J Mater Chem B. 2014;2(6):698–705.
Heinrichs RW, Ammari N. Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal Chem. 2012;84(14):6220.
Zhang YL, Wang L, Zhang HC, Liu Y, Wang HY, Kang ZH, et al. Graphitic carbon quantum dots as a fluorescent sensing platform for highly efficient detection of Fe3+ ions. RSC Adv. 2013;3(11):3733–8.
Simões EFC, Leitão JMM, Esteves da Silva JCG. Carbon dots prepared from citric acid and urea as fluorescent probes for hypochlorite and peroxynitrite. Microchim Acta. 2016;183(5):1769–77.
Chen H, Li W, Zhao P, Nie Z, Yao S. A CdTe/CdS quantum dots amplified graphene quantum dots anodic electrochemiluminescence platform and the application for ascorbic acid detection in fruits. Electrochim Acta. 2015;178:407–13.
Kim SRA, Jongsung. Selective detection of dopamine in the presence of ascorbic acid via fluorescence quenching of InP/ZnS quantum dots. Int J Nanomedicine. 2015;10(Spec Iss):113.
Zhang H, Chen Y, Liang M, Xu L, Qi S, Chen H, et al. Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal Chem. 2014;86(19):9846.
Shen LM, Liu J. New development in carbon quantum dots technical applications. Talanta. 2016;156-157:245.
Chen J, Liu J, Li J, Xu L, Qiao Y. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J Colloid Interf Sci. 2017;485:167–74.
Zhu L, Xu G, Song Q, Tang T, Wang X, Wei F, et al. Highly sensitive determination of dopamine by a turn-on fluorescent biosensor based on aptamer labeled carbon dots and nano-graphite. Sensor Actuat B Chem. 2016;231:506–12.
Cheng G, He M, Peng H, Hu B. Dithizone modified magnetic nanoparticles for fast and selective solid phase extraction of trace elements in environmental and biological samples prior to their determination by ICP-OES. Talanta. 2012;88(1):507.
Chen J, Zhu X. Magnetic solid phase extraction using ionic liquid-coated core-shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of Rhodamine B in food samples. Food Chem. 2016;200:10–5.
He Y, Li N, Ma JJ. Magnetic solid-phase extraction clean-up combined with solidified floating organic drop microextraction for determination of trace mercury (II) in tea samples. J Chem Soc Pakistan. 2014;36(6):1162–8.
Henry B. Preparation and characterization of graphene quantum dots-Fe3O4 nanocomposite as an efficient adsorbent in magnetic solid phase extraction: application to determination of bisphenol A in water samples. Anal Methods. 2014;6(20):8413–9.
Liao W, Ma Y, Chen A, Yang Y. Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo(a)pyrene in environmental water samples. Chem Eng J. 2015;271:232–9.
Liu Y, Zhao Y, Zhang Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sensor Actuat B Chem. 2014;196(2):647–52.
Li W, Zhang Z, Kong B, Feng S, Wang J, Wang L, et al. Simple and green synthesis of nitrogen-doped photoluminescent carbonaceous nanospheres for bioimaging. Angew Chem Int Ed Engl. 2013;52(31):8151–815.
Huang H, Li C, Zhu S, Wang H, Chen C, Wang Z, et al. Histidine-derived nontoxic nitrogen-doped carbon dots for sensing and bioimaging applications. Langmuir. 2014;30(45):13542–8.
Liao J, Cheng Z, Zhou L. Nitrogen-doping enhanced fluorescent carbon dots: green synthesis and their applications for bioimaging and label-free detection of Au3+ ions. ACS Sustain Chem Eng. 2016;4(6):3053–61.
Wang R, Wang X, Sun Y. One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sensor Actuat B Chem. 2016;241:73–9.
Li X, Chen L. Fluorescence probe based on amino-functionalized fluorescent magnetic nanocomposite for detection of folic acid in serum. ACS Appl Mater Inter. 2016;8:46.
Arvand M, Dehsaraei M. A simple and efficient electrochemical sensor for folic acid determination in human blood plasma based on gold nanoparticles-modified carbon paste electrode. Mat Sci Eng C. 2013;33(6):3474.
Aurora-Prado MS, Silva CA, Tavares MFM, Altria KD. Determination of folic acid in tablets by microemulsion electrokinetic chromatography. J Chromatogr A. 2004;1051(1–2):291–6.
Geszke-Moritz M, Clavier G, Lulek J, Schneider R. Copper- or manganese-doped ZnS quantum dots as fluorescent probes for detecting folic acid in aqueous media. J Lumin. 2012;132(4):987–91.
Funding information
The work was strongly supported by the Analysis and Testing Foundation of Kunming University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Informed consent was obtained from all individual participants serum samples were collected from. The studies have been approved by Kunming University of Science and Technology Ethics Committee and Carnegie Mellon University Ethics Committee and have been performed in accordance with the ethical standards.
Rights and permissions
About this article
Cite this article
Wang, M., Jiao, Y., Cheng, C. et al. Nitrogen-doped carbon quantum dots as a fluorescence probe combined with magnetic solid-phase extraction purification for analysis of folic acid in human serum. Anal Bioanal Chem 409, 7063–7075 (2017). https://doi.org/10.1007/s00216-017-0665-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0665-3