Abstract
The recent development of a homogeneous time-resolved Förster resonance energy transfer (TR–FRET) immunoassay enables one-step, rapid (minutes), and direct detection compared to the multistep, time-consuming (hours), heterogeneous ELISA-type immunoassays. The use of the time-resolved effect of a donor lanthanide complex with a delay time of microseconds and large Stokes shift enables the separation of positive signals from the background autofluorescence of the sample. However, this study shows that the sample matrices directly interfere with donor fluorescence and that interference cannot be eliminated by time-resolved settings alone. Moreover, the reduction in donor emission did not appear to be equivalent to the reduction in acceptor emission, resulting in incorrect FRET signal measurements. To overcome this limitation, an internal standard approach was developed using an isotype control antibody. This new approach was used to develop TR–FRET assays for rapid detection (15–30 min) of Bacillus anthracis spores and botulinum toxin (type E) in beverages, which are major concerns in bioterrorism involving deliberate food contamination. Additionally, we demonstrate the detection of B. anthracis-secreted protective antigen (PA) and the Yersinia pestis-secreted markers F1 and LcrV in blood cultures, which are early markers of bacteremia in infected hosts following a possible bioterror attack. The use of an internal standard enables the calculation of correct ΔF values without the need for an external standard. Thus, the use of the internal standard approach in homogeneous immunoassays facilitates the examination of any sample regardless of its origin, and therefore expands the applicability of TR–FRET assays for complex matrices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing interest in rapid diagnostic tools for medical use (e.g., clinical diagnosis and point-of-care) has encouraged the development of simple-to-operate immunoassays. However, most immunoassays such as the enzyme-linked immunosorbent assay (ELISA) are based on heterogeneous approaches, with several steps of washing and reagent incubations that are labor and time consuming. To simplify and shorten the assay setup, different approaches for homogeneous assays have been designed on the basis of the Förster resonance energy transfer (FRET) process using materials with a high energy transfer capability [1–6]. FRET is a distance-dependent energy transfer process between donor and acceptor fluorophores, inversely proportional to the sixth power of the distance between them. Accordingly, donors and acceptors must be in close spatial proximity (<10 nm) to accomplish efficient FRET. In the homogeneous FRET assay, the tested sample is briefly incubated with donor and acceptor fluorophores conjugated to two specific antibodies and then the FRET signal is directly measured in a fluorimeter without the requirement for washing steps (Fig. 1, “test”). A positive FRET signal can only be achieved by mutual interactions of the pair of antibodies with the target molecule in the sample. Hence, one-step homogeneous immunoassays are easier and quicker than heterogeneous ELISA. A recent approach has used lanthanides (e.g., europium and terbium) in complexes with chelate or cryptate, which were incorporated as donors in homogeneous FRET immunoassays [7–10]. Lanthanide complexes characterized by a high fluorescence quantum yield, a very long emission lifetime (400–800 μs) and a very large Stokes shift (approx. 300 nm) were achieved using a time-resolved fluorescence (TRF) fluorimeter. These properties help to eliminate the autofluorescence noise of biomolecules and debris (impurities) in samples characterized by short luminescent lifetimes (nanoseconds) and small Stokes shifts (10–20 nm). Thus, the combination of TRF and FRET (TR–FRET) has facilitated the development of homogeneous immunoassays with high sensitivity and specificity [11–13].
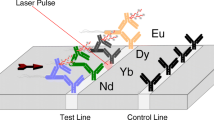
Illustration of control settings in the TR–FRET homogenous assay. The assay settings include co-incubation of the tested sample with pair antibodies (black Y), one conjugated to the EuK donor (blue circle) and the other conjugated to the AlexaFluor 647 acceptor (red circle), referred to as “assay antibodies” in the text. The amount of energy transfer (FRET) was determined by calculating ΔF values (see “Materials and methods”) using three different control standards: (I) buffer control, the incubation of the assay antibodies in buffer (PBS) without the antigen (no Ag); (II) external standard, the incubation of the assay antibodies in a matrix sample without the antigen (unspiked control); (III) internal standard, the incubation of the tested sample (including the antigen) with the donor antibody and an irrelevant antibody (purple Y), but with the same isotype, conjugated to the AlexaFluor 647
However, in addition to the autofluorescence issue, the impurities in the sample may induce a quenching effect on the donor signals by directly interrupting interactions or competing with the donor for the input excitation energy, leading to a reduction in the amount of energy transferred to the acceptor and eventually to reduced output signals. Complex matrices, such as biological fluids (blood, urine, and mucosal specimens) or food products (beverages and milk products), contain different biomolecules and chemicals that may directly interfere with the donor signal in the homogeneous assay format. Therefore, the use of an assay buffer as a background standard control (as illustrated in Fig. 1I) would not be sufficient. Previously it was shown that impurities in urine samples interfere with the excitation energy of the donor in TR–FRET-based immunoassays leading to an overestimation of the results. To correct the positive signal values, normal human urine was used as an external standard instead of a buffer [14]. However, the use of an external standard as a reference background may not be accurate in all cases because of the large diversity in the composition of clinical samples (e.g., hemolytic serum and blood in the urine). Moreover, in most cases, an adequate reference standard would not be available to the operator in the clinical laboratory.
In this manuscript, we present a novel method for overcoming the surrounding interference in the assay by using a generic internal standard control regardless of the availability of an external standard. Figure 1 illustrates the different control standards that can be used in the TR–FRET assay to measure background FRET signals: (I) buffer control, using a clear assay buffer; (II) external “unspiked” control using the same matrix composition as the tested sample without the target antigen; and (III) internal control. The internal control involves the use of the tested sample itself as a control in the assay by incubating it with the same donor antibody, but replacing the acceptor with a non-relevant antibody with the same isotype and amount of acceptor fluorophore. Using the internal control setup, each sample is split and tested twice, first with the correct donor/acceptor assay antibodies and then with the donor antibody mixed with the isotype control. We used this new internal standard approach to develop homogeneous TR–FRET assays for the detection of virulence biomarkers of Yersinia pestis (Y. pestis), the etiological agent of plague, and Bacillus anthracis (B. anthracis), the etiological agent of anthrax disease. These acute diseases induce overwhelming inflammation, leading to death in untreated cases. Both bacteria are included in the list of group A infectious agents from the Centers for Disease Control and Prevention (CDC), and their possible use as bioterror agents is a potential threat to public health. To monitor the progression of plague and anthrax diseases, blood samples of infected patients are usually examined for the presence of bacterial biomarkers. F1 antigen, the bacterial capsule protein, and LcrV antigen, the bacterial major virulence factor, are reliable specific markers used to diagnose plague [15, 16]. Exotoxin protective antigen (PA) is the major biomarker of B. anthracis that is responsible (among other toxins) for the induction of tissue damage and even death in untreated patients. The amount of PA in serum fractions or in whole-blood cultures of infected patients is highly correlated with bacteremia [17]. Thus, the rapid detection of these bacterial biomarkers in blood samples from infected patients at early stages is essential for effective treatment. In addition to the diagnosis of bacterial markers in blood cultures, we demonstrate the detection of botulinum toxin type E, an extremely potent neurotoxin of Clostridium botulinum, and whole B. anthracis spores spiked in beverages and fresh milk. The bioterror anthrax incidents of 2001 [18] and the possible use of botulinum toxin for bioterrorism purposes [19] have increased the concern regarding the possible deliberate contamination of food products and water. Thus, the development of rapid and simple-to-operate assays for early detection of contaminated food and beverages is essential to prevent a public catastrophe and to allow better treatment of infected patients.
Materials and methods
Bacterial strains
Bacillus anthracis Δ14185, a nontoxinogenic, nonencapsulated (Tox−Cap−) derivative of ATCC 14185 [20] (Bacillus Genetic Stock Center), and EV76 [21] were obtained from the Israel Institute for Biological Research collection.
Reagents and antibodies
Protective antigen protein, 83 kDa (PA), was purified by Q-Sepharose chromatography as previously described [22]. Botulinum toxin E was prepared from concentrated supernatants of C. botulinum culture [23]. Recombinant Y. pestis LcrV and F1 were prepared as previously described [24]. Mouse anti-PA monoclonal IgG antibodies (Mab3, Mab5) were used as previously described [11]. Anti-B. anthracis spore antibodies were raised against a soluble exosporium fraction [25], and anti-LcrV and anti-F1 were prepared as previously described [26]. Polyclonal antibodies were purified from hyperimmune serum with Amino-link columns according to the manufacturer’s protocol (Pierce, Rockford, IL). The antibodies were labeled with the europium cryptate (EuK) labeling kit (Cisbio Bioassays, Codolet Cedex, France) and with an AlexaFluor 647 (A647) carboxyl acid succinimidyl ester labeling kit (Invitrogen, Eugene, Oregon, USA), according to the manufacturers’ instructions. Beverages and milk were obtained from a local grocery. The Bacec and Bact/Alert blood culture bottles were obtained from Becton Dickinson, BD, New Jersey, USA. Fresh horse blood (10 ml) was injected into culture bottles and the culture was then inoculated with 105 Y. pestis bacteria (EV). The culture bottles were incubated overnight at 37 °C and samples were taken for the assays at different time points.
TR–FRET assay
The TR–FRET assays were performed out in a white (non-Maxisorp) micro-plate (Nunc, Roskilde, Denmark) in triplicate using europiumIII tris-bipyridine cryptate (EuK) (CisBio, Cat# 62EUSPEA) as the donor, and AlexaFluor 647 (A647) (Invitrogen) as the acceptor conjugated to the specific antibodies indicated in the text. Excitation of the EuK donor at 340 nm leads to energy emission at 612 nm or to resonance energy transfer in close proximity (d < 10 nm) to the acceptor, resulting in emission at 665 nm. The final assay volume was 60 μl, comprised of 40 μl of the mixture of the donor/acceptor antibodies diluted in assay buffer containing 50 mM phosphate buffer (pH 7), 400 mM potassium fluoride (KF) (Sigma, Rehovot, Israel), 0.1 % BSA (Israeli Biological Industries, Israel), 0.01 % sodium azide (Sigma), and 20 μl of the analyte. The optimal ratio of the antibodies in the assay was determined in a preliminary experiment, in which a fixed amount of analyte was examined with increasing concentrations of donor and acceptor antibodies, as indicated in the text. The assay was incubated at 37 °C with shaking for 1 h and the results were read every 15 min with an Infinite F200 reader (Tecan, Switzerland) using the following settings: excitation 340 nm (±35 nm), emission 612 nm (±10 nm) or 665 nm (±8 nm), lag time 100 μs, integration time 400 μs. To eliminate the formation of bacterial clumps during the assay, we added Tween to the assay buffer and added the bacterial samples after vigorous vortexing and pipetting.
Calculation of the HTRF signals
The HTRF signals (ΔF) were calculated as a normalized fluorescence transfer signal [27] using the following formula:
The average fluorescence intensity of the acceptor (A647) in the sample at 665 nm (F665 nm) was divided by the average fluorescence intensity of the donor (EuK) in the sample at 612 nm (F612 nm). The same ratio in the control setup was subtracted from the resulting value. Then the resulting value from this calculation was divided by the control ratio. The limit of detection (LOD) was determined as three standard deviations above the control background. The control background was calculated as the average fluorescence of the matrix without the antigen (e.g., beverage or blood culture) minus that of the control (buffer or unspiked or internal control; Fig. 1), as indicated in the text, and divided by the same control.
Statistics
The InStat 3 program (GraphPad) was used for the statistical analysis. Statistical analysis was performed using Welch’s corrected T test. Differences were considered statistically significant at p < 0.05.
Results
Sample matrices affect donor signal measurements
The goal of this study was to develop a homogeneous TR–FRET assay methodology that would be applicable for the detection of biomarkers in complex matrices such as biological fluids, e.g., blood cultures and food products. The TR–FRET assays were developed using the donor EuK and the acceptor A647 conjugated to specific antibodies that recognize the analyte, which are referred to as “assay antibodies” in the text. The FRET signal was achieved by donor excitation at 340 nm and energy transfer to the acceptor leading to a final emission of the acceptor at 665 nm. As a result of the homogeneous format of the assay, impurities in the sample matrix were not washed out and might also have emitted autofluorescence at 612 nm, resulting in undesirable FRET signals (noise). The time-resolved effect of the lanthanide donor allowed us to measure the results with a lag time; therefore the short-lived noise of the impurities in the sample could be eliminated. To determine the optimal lag time for use in our experimental system, we measured the emissions of different beverages that would be used in this study using increased lag times in an attempt to determine the optimal time required to reduce the noise signal. The matrices, namely orange and apple juices, cola, mineral and tap waters, and fresh milk, as well as hemoglobin extracted from bovine blood (5 μg/ml), were excited at 340 nm and fluorescence emission was measured at 612 nm after increasing elevated delay times (0–600 μs). The measurement settings in this experiment were adjusted to the calibrated assay settings of the donor in buffer (data not shown) using a gain value of 175 and an integration time of 400 μs.
Figure 2 presents the ratio of the intensity of the different tested matrices compared with the assay buffer using increasing delay time settings (0–600 μs). It can be observed that the emission intensity without a delay is 30- to 40-fold higher for the different beverages and 150-fold higher for milk compared with the PBS buffer. After a 100-μs delay (or longer), the intensity of the beverages declined to background levels, which is indicated in the graph as a ratio of 1. However, the emission intensity of the milk was reduced by only 4-fold. A longer delay of up to 600 μs was unable to reduce the signal to background intensity. Mineral and tap waters, as well as hemoglobin, exhibited minimal autofluorescence with or without delay. These results demonstrate that the use of a delay time of at least 100 μs is essential to reduce autofluorescence noise in the homogeneous TR–FRET assay. Because the use of a delay time longer than 100 μs reduced the total gain signal, a lag time of 100 μs was set for the subsequent experiments.
Fluorescence intensity of matrices at 612 nm in delay time measurements. Different beverages, namely apple juice (black circle), orange juice (black square), cola (black triangle), fresh milk (black inverted triangle), mineral water (black diamond), tap water (open circle), and 5 μg/ml hemoglobin (open square), were excited at 340 nm, and the fluorescence emissions were measured at 612 nm in different delay times of 0–600 μs using a gain value of 175 and an integration time of 400 μs in the reader machine. The values presented in the graph are ratio intensities of each matrix compared to buffer
In addition to the autofluorescence effects on the homogeneous assay, we examined the possible direct interference effect of the different sample matrices on donor emission. To that end, EuK (5 nM) was diluted in different beverages representing drinking samples. To eliminate the possible effect of low pH on the donor integrity and further on the assay performance, acidic beverages were neutralized to pH 7 using sodium hydroxide (5 N). Then, each sample was excited at 340 nm and the emission intensities were measured at 612 nm using a 100-μs delay time. Figure 3a presents the donor emission in each of the beverages compared with PBS. The emission intensities of the donor in apple and orange juices and in cola were reduced by 2-fold lower compared with PBS. In contrast, the donor emission in milk was increased by 2.5-fold compared with PBS, probably as a result of the background autofluorescence of milk that was consistent even after the 100-μs delay time (Fig. 2). Mineral and tap waters had no significant effects on the donor signal.
EuK donor emission in the presence of different matrices. EuK (5 nM) was examined for fluorescence emission at 612 nm using a delay time of 100 μs when incubated in a different beverages or b increased concentrations of hemoglobin (0.156–150 μg/ml) or Bactec and Bact/Alert supplemented with fresh horse blood compared to PBS. The donor fluorescence signal values presented in the graph are in arbitrary units (a.u.). p < 0.05 by comparing milk, cola, orange and apple juices with PBS, and by comparing blood cultures (Bactec and Bact/Alert) and hemoglobin above 1.25 μg/ml with PBS using the T test
Accordingly, we tested the effect of blood culture extracts and hemoglobin on donor emission intensity. Blood samples taken from patients in hospital are regularly tested in Bactec or Bact/Alert culture bottles. Here we used these bottles supplemented with fresh horse blood (10 ml) instead of human blood. After a short incubation of the blood in the bottles, 1-ml samples were centrifuged and the supernatants were incubated with the donor EuK (5 nM). Blood samples can be hemolytic; therefore high levels of hemoglobin can be observed in the supernatants after centrifugation. To examine the possible effect of hemoglobin on donor emission, we also incubated the donor EuK with increasing concentration of purified hemoglobin protein (0–150 μg/ml). As shown in Fig. 3b, the donor emission intensity in the Bactec and Bact/Alert plus blood culture supernatants was reduced by 2-fold compared with PBS. The supernatant of Bactec and Bact/Alert alone did not have an effect on the donor signal (data not shown). In accordance with the blood cultures, purified hemoglobin in concentrations higher than 1.25 μg/ml significantly reduced donor signal. These results collectively suggest that complex matrices such as beverages and blood have a direct quenching effect on the donor signal. Although the autofluorescence noise could largely be resolved using a lanthanide donor in assay settings with a lag time readout, the sample substances still directly interfere with the donor. The subsequent experiments were conducted to overcome the direct interference effect of the matrix on donor fluorescence using an internal standard approach (illustrated in Fig. 1III).
Development of a TR–FRET assay for detection of botulinum toxin and anthrax spores spiked in beverages and milk
Food contamination with botulinum toxins or B. anthracis spores is a major national security concern in many countries seeking to prevent bioterror attacks. Thus, the need for a rapid and simple assay for the diagnosis of contaminated food is of great interest. Our aim was to develop rapid TR–FRET assays for the detection of botulinum type E toxin (BotE) (as a representative of the botulinum toxins) and B. anthracis spores in different beverages and fresh milk. To that end, we first optimized the assay settings for the detection of these agents in a buffer (PBS) by determining the optimal concentration ratio of the assay antibodies conjugated to a donor (EuK) and acceptor (A647). The assay antibodies used in the study were polyclonal serotypes that were specifically derived from rabbits immunized with botulinum E toxin or anthrax spores and later isolated and purified (see “Materials and methods”). For assay optimization, increasing concentrations of the donor and acceptor antibodies (1–50 nM) were examined in elevated concentration one against the other, using a fixed concentration of target antigens (data not shown). A donor–acceptor ratio of 1:1 (5 nM each) was selected for the botulinum E test, whereas a 1:10 ratio (2.5 nM and 20 nM) was selected for the anthrax test. To determine assay performance, dose–response assays were developed using increasing amounts of botulinum E (0–825 ng/ml) or anthrax spores (0–5 × 108/ml) diluted in PBS. The ΔF values were calculated (see “Material and methods” for the formula), where the control background referred to the test in PBS without the antigens. Figures 4a and 5a present the standard curves (in black dots) of botulinum E and anthrax spores, respectively, showing a linear range of approx. two orders of magnitude with the LODs of 2 ng/ml for botulinum E and 5 × 106/ml for the spores. The LOD was determined as three standard deviations above the control background and is represented by the dashed line in the graph. The minimal incubation time required for optimal assay performance was 30 min (data not shown).
TR–FRET assays for the detection of botulinum E toxin in beverages. Botulinum E toxin (BotE) was spiked in increased concentrations (0–825 ng/ml) in different beverages, namely apple juice (red), orange juice (blue), milk (green), cola (purple), tap water (orange), mineral water (gray), PBS (black). Then the samples were examined in TR–FRET assay using polyclonal anti-BotE antibodies conjugated to the donor EuK and the acceptor AlexaFluor 647. The energy transfer values (ΔF) were calculated using three different control standards: a PBS buffer, b unspiked sample, and c internal control. The LOD levels were determined as three SDs above the PBS background and are indicated in the graph as dashed line
TR–FRET assay for the detection of B. anthracis spores in beverages. B. anthracis spores were spiked in increased concentrations (0–5 × 108/ml) in different beverages, namely apple juice (red), orange juice (blue), milk (green), cola (purple), tap water (orange), mineral water (gray), PBS (black). Then the samples were examined in the TR–FRET assay using polyclonal anti-spore antibodies conjugated to the donor EuK and the acceptor AlexaFluor 647. The energy transfer values (ΔF) were calculated using three different control standards: a PBS buffer, b unspiked sample, and c internal control. The LOD levels were determined as three SDs above the PBS background and are indicated in the graph as a dashed line
Next, we examined the detection of botulinum E and anthrax spores spiked in different beverages and fresh milk. As shown above (Fig. 3), the emission intensity of the donor in the spiked beverages was decreased compared with that of the donor in PBS. This quenching effect was anticipated to induce a corresponding reduction in the amount of acceptor emission, as the FRET efficacy correlates with the amount of donor intensity. However, the reduction in donor emission at 612 nm was not equal to the reduction in acceptor emission at 665 nm. Instead, the donor emission decreased by approx. 2-fold compared with an only approx. 1.5-fold reduction in acceptor emission (data not shown). When considering the formula for the energy transfer (ΔF) calculation, one can see that a reduction in F(612 nm) that is greater than F(665 nm) would result in a higher F(665 nm)/(F612 nm) ratio, leading to a higher ΔF value. This observation led us to search for alternative control standards to normalize the quenching effect on the donor in the calculation of ΔF. The use of an external control, such as an unspiked sample comprised of exactly the same matrix composition as the tested sample (Fig. 1II), might facilitate the calculation of the normalized ΔF values. However, an external standard with a precisely identical matrix composition would probably not be available to the investigator in all cases. Therefore, we aimed to design an internal standard (Fig. 1III) using a non-relevant antibody with the same isotype as the internal control. To this end, the tested sample itself was used as the control for the assay, without the need for an external standard such as an unspiked control. To examine this internal standard approach in our system, sets of experiments were designed to examine the detection of BotE (0–825 ng/ml) and anthrax spores (0–5 × 108/ml) in spiked beverages and fresh milk using the pair of specific donor/acceptor antibodies. The assays were read at 612 nm and 665 nm, and the ΔF values were calculated using the following standards: PBS buffer, unspiked or internal controls. Figures 4 and 5 present the dose–response curves for the detection of botulinum E and anthrax spores in spiked beverages compared with PBS respectively. Using the PBS buffer standard (Figs. 4a and 5a) it can be seen that the background ΔF values of beverages without the antigen (marked as 0.1 for BotE and 100 for the spores in the logarithmic scale graph) were above the LOD limit, showing false positive results. Conversely, the ΔF values of the botulinum E or anthrax spores spiked in fresh milk were less than in PBS and even below the LOD, showing false negative results at low concentrations (2.5 ng/ml BotE and 5 × 106/ml spores). These results agreed with the observation that a reduction in emission measurements at 612 nm that was greater than at 665 nm resulted in higher ΔF values, whereas the opposite trend resulted in lower ΔF values. In contrast, the use of an unspiked control (Figs. 4b and 5b) or internal control (Figs. 4c and 5c) for the calculation of ΔF values normalized the results in accord with the PBS dose–response curves (black dots), giving rise to ΔF values that were below the LOD, with no false positive results. These results indicate that the PBS standard that is currently used as a reference in TR–FRET assays to examine clean samples can no longer be used for complex matrices. However, the use of internal standard allowed us to normalize the ΔF values and to correct the dose–response curves to be similar to those produced using unspiked controls. In the subsequent experiments we extended the use of the internal standard approach in the TR–FRET assay for the detection of analytes in blood cultures.
Development of a TR–FRET assay for detection of LcrV, F1, and PA in blood cultures
Bacteremia, the dissemination of bacteria into the bloodstream, is frequently associated with the secretion of bacterial virulence factors into the bloodstream. To monitor bacteremia in infected patients arriving at hospital, the medical staff routinely collect blood samples and grow them in culture bottles for 18–24 h. Then, the bacterial count and the presence of specific bacterial markers are determined. Patients infected with Y. pestis or B. anthracis are anticipated to have detectable amounts of bacterial factors in their blood in early stages, before the possible detection of bacteria [17, 26]. Thus, the rapid detection of the Y. pestis biomarkers (LcrV and F1) and B. anthracis protective antigen (PA) in the blood samples of infected patients at early stages is essential for effective medical treatment. We aimed to develop TR–FRET assays to directly detect these bacterial biomarkers in blood cultures. To that end, we first determined the optimal concentration ratio of the assay antibodies conjugated to donor and acceptor in a clear assay buffer. The assay antibodies used in the study were polyclonal rabbit antibodies purified from hyperimmune sera raised against recombinant LcrV and F1 and monoclonal mouse anti-PA antibodies (Mab3 and Mab5) [11]. For assay optimization, increasing concentrations of the donor and acceptor antibodies (1–50 nM) were examined one against the other, using a fixed concentration of target antigens. A donor–acceptor ratio of 1:1 (5 nM each) was determined as the optimal ratio for all assays (data not shown) and was chosen for further experiments. To determine the performance of the assays in detecting a wide range of concentrations in PBS buffer, a dose–response assay was performed using increasing amounts of recombinant LcrV, F1, and PA (0–200 ng/ml). Figure 6a–c present the standard dose–response curve of each assay (black dots) showing an increase in FRET signal (ΔF) as a function of the amount of antigens in the mixture. The assay curves had wide linear ranges, with LODs of 5 ng/ml for LcrV and PA and 8 ng/ml for F1, and the optimal incubation times were 15 min for the PA assay and 30 min for the LcrV and F1 assays. Next, the detection of the biomarkers in blood culture was examined. Bactec bottles supplemented with 10 ml of fresh horse blood were centrifuged, and the supernatants were spiked with elevated concentrations of LcrV, F1, or PA (0–200 ng/ml) and then examined in the TR–FRET assays. Similar to the results of the beverage samples, blood cultures had quenching effects on the donor, resulting in an approx. 2-fold reduction in the emission intensity, but only an approx. 1.5-fold reduction in acceptor emission (data not shown), leading to incorrect ΔF value calculations when a PBS standard was used. In order to correct these values, the implementation of the internal control approach was investigated here. To that end, ΔF values were calculated using a PBS buffer, unspiked and internal controls, and the dose–response curves of each were produced. As shown in Fig. 6 the dose–response curves of the ΔF values calculated using the PBS standard (red dots) for each of the tested biomarkers tested were higher than the dose–response curves of the markers spiked in PBS (black dots), and the zero levels were above the LOD, showing false-positive results. In contrast, the dose–response curves of the ΔF values calculated using the internal control (blue triangle) were aligned with the black dots, with no false-positive values, and were similar to those calculated using an unspiked standard (green triangle). These results demonstrate again that the use of a buffer control standard for the calculation of ΔF values leads to incorrect dose–response curves. In contrast, the internal standard normalizes the quenching effects of the sample matrix, giving rise to correct ΔF values, demonstrating again that this standard should be used instead of an unspiked control.
TR–FRET assays for the detection of LcrV (a), F1 (b), and PA (c) in blood cultures using different control standards. LcrV, F1, and PA were spiked in increased concentrations (0–200 ng/ml) in Bactec supplemented with fresh horse blood. Then the samples were examined in the TR–FRET assay using monoclonal anti-PA antibodies and polyclonal anti-LcrV and F1 antibodies conjugated to the donor EuK and the acceptor AlexaFluor 647. The energy transfer values (ΔF) were calculated using three different control standards, namely PBS buffer (red square), unspiked blood culture (green inverted triangle), and internal control (blue triangle). The ΔF values of LcrV, F1, and PA spiked in PBS are presented in the graph as a comparison (black). The LOD levels were determined as three SDs above the PBS background and are indicated in the graph as a dashed line
Next, the TR–FRET assays that were developed for the detection of LcrV and F1 were validated in growing bacterial cultures. To this end, we inoculated Bactec and Bact/Alert bottles supplemented with 10 ml of horse blood with 105 EV95 strain of Y. pestis bacteria and cultured them at 37 °C for 10, 18, and 22 h. At each time point, 1-ml samples were collected from the bottles, centrifuged, and examined for the presence of LcrV and F1 in the TR–FRET assay. The ΔF values were calculated using the internal standard approach, as shown above; each sample was examined twice, first using the pair of assay antibodies and second using the donor antibody mixed with the internal isotype control antibody. The calculated ΔF values were converted into quantitative amounts of LcrV and F1 using the linear equation from the standard curves that were produced by measuring increasing concentrations of recombinant LcrV and F1 (1–200 ng/ml) in PBS (data not shown). The tested samples were serially diluted to fit into the linear range of the standard curves. To evaluate the validity of the TR–FRET assay, we also examined the blood culture samples using the previously established heterogeneous TRF sandwich method [28], and the same reporting antibody conjugated to the Eu–N1 chelate complex. Table 1 presents the quantitative amounts of LcrV and F1 in the Bactec and Bact/Alert cultures that were calculated using the homogeneous TR–FRET assay compared with those calculated using the heterogeneous TRF assay. Detectable amounts of LcrV and F1 (tens to hundreds of nanograms per milliliter) were observed after 18–22 h in culture using both methods, and no significant difference were observed using the T test (p > 0.01), with the exception of the amount of F1 in Bactec after 22 h. However there was only a 1.5-fold difference. Thus, the TR–FRET method produced similar results with the same sensitivity as the gold standard heterogeneous TRF method. Nevertheless, the TR–FRET assay was more rapid, producing results in 30 min compared to 3 h using the TRF homogeneous assay.
Discussion
This study is the first to address the use of the TR–FRET immunoassay for the examination of complex matrices. Using the TR–FRET technology, we showed that a delay time of 100 μs or longer facilitated the reduction of background noise in hemolytic blood cultures or beverages (Fig. 2). However, incubation of the donor in these matrices resulted in a significant reduction in donor emissions at 612 nm (Fig. 3), suggesting that the matrices directly interfered with donor fluorescence. It was also observed that the reduction in donor emission is not equal to the reduction in acceptor emission, resulting in an artificial increase in the ΔF values that were above the LOD. A possible explanation for this observation is that the impurities in the sample might have different blocking effects on donor and acceptor fluorescence wavelengths [29], or the donors and acceptors may exhibit differences in absorption efficiency in such turbid solutions. This result clearly suggests that the donor time-resolved effect would not be sufficient when testing complex matrices, and a different approach is needed. Therefore we searched for an alternative standard control that would normalize these blocking effects and could be used for any type of matrix. The approach we adopted was to use an internal standard of a control irrelevant antibody labeled with the same amount of AlexaFluor 647. Thus, using this approach, any given sample can be used as internal control, regardless of its origin, and without the need for an external standard. Because the composition of any external standard would be different from the tested sample to some extent, it would not be an accurate control for the assay. For instance, the use of “normal” clinical fluids (e.g., serum, urine, and blood) of an uninfected donor as an external standard of the assay would not be precise because of variations among patients. Moreover, the use of an internal standard approach allows one to apply the TR–FRET assay for the examination of samples for which the matrix composition is unknown, in cases for which an adequate external standard would not be available to the laboratory.
To demonstrate this new approach, we developed TR–FRET assays for the detection of 106/ml of B. anthracis spores and nanograms per milliliter of botulinum toxin E in beverages and fresh milk as a possible scenario of bioterrorism involving deliberate food contamination. The use of real-time PCR has demonstrated higher sensitivity for the detection of pathogens, such as anthrax in food matrices [30]. However this method requires at least 1000 DNA copies for specificity, together with an extended sample preparation procedure. In contrast the homogeneous TR–FRET assay is based on a direct fluorescence measurement rather than enzyme amplification and it is therefore faster and easier. To examine the applicability of the new approach in the examination of clinical samples, we developed TR–FRET assays for the diagnosis of the Y. pestis markers LcrV and F1 and the B. anthracis marker PA in blood. The use of the PBS background as an external standard for the calculation of ΔF values in each of the tested matrices gave incorrect results that were higher than the PBS standard curve and led to false-positive results in unspiked samples (above the expected LOD). However, the use of an internal control as a standard shifted the dose–response curves down and aligned them with the PBS standard curve. Finally, we validated our new TR–FRET approach using the internal standard to detect bacterial markers in blood cultures by comparing the performance of the TR–FRET method to the previously established TRF heterogeneous assay for the detection of LcrV and F1 in growing blood cultures inoculated with Y. pestis. The one-step TR–FRET assay gave rapid results without the need for any washing steps. However, the high analyte concentrations in the sample might compete for binding to the assay antibodies, leading to a reduction in FRET signals (“pro-zone” in the dose–response curve). This result is in contrast to heterogeneous assays such as ELISA, paper strips (lateral flow), and microarrays in which the excess analyte is washed away. To overcome this limitation, highly concentrated samples were serially diluted.
In conclusion, this study presents the development of TR–FRET assays for the examination of complex matrices, namely beverages and blood cultures. The developed one-step and simple-to-operate TR–FRET assays allowed the detection of both whole bacteria and bacterial-secreted proteins with a comparable sensitivity to that of heterogeneous TRF assays; however, the TR–FRET assays were faster (15–30 min) and easier to operate. The internal standard approach was undertaken to overcome the direct interference of the matrices with donor fluorescence and to serve as the correct control for the assay. Thus, the use of an internal standard allows one to examine any type of sample, regardless of its origin, without the requirement of an available external standard.
References
Lee JS, Joung HA, Kim MG, Park CB. Graphene-based chemiluminescence resonance energy transfer for homogeneous immunoassay. ACS Nano. 2012;6(4):2978–83. doi:10.1021/nn300684d.
Watanabea J, Ishihara K. Single step diagnosis system using the FRET phenomenon induced by antibody-immobilized phosphorylcholine group-covered polymer nanoparticles. Sensors Actuators B Chem. 2008;129(1):87–93. doi:10.1016/j.snb.2007.07.100.
Wei Q, Lee M, Yu X, Lee EK, Seong GH, Choo J, et al. Development of an open sandwich fluoroimmunoassay based on fluorescence resonance energy transfer. Anal Biochem. 2006;358(1):31–7. doi:10.1016/j.ab.2006.08.019.
Boeneman K, Delehanty JB, Susumu K, Stewart MH, Deschamps JR, Medintz IL. Quantum dots and fluorescenct protein FRET-based biosensors. In: Zahavy E, Ordentlich A, Yitzhaki S, Shafferman A, editors. Nano-biotechnology for biomedical and diagnostic research. Advances in experimental medicine and biology. Dordrecht: Springer; 2012.
Hildebrandt N, Geissler D. Semiconductor Quntum dots as FRET acceptors for multiplexed diagnostic ruler application. In: Zahavy E, Ordentlich A, Yitzhaki S, Shafferman A, editors. Nano-biotechnology for biomedical and diagnostic research. Advances in experimental medicine and biology. Dordrecht: Springer; 2012.
Spindel S, Granek J, Sapsford KE. In vitro FRET sensing, diagnostic and personlized medicine. In: Medintz IL, Hildebrandt N, editors. FRET - from theory to application. Weinheim: Wiley-VCH; 2014.
Harma H, Dahne L, Pihlasalo S, Suojanen J, Peltonen J, Hanninen P. Sensitive quantitative protein concentration method using luminescent resonance energy transfer on a layer-by-layer europium(III) chelate particle sensor. Anal Chem. 2008;80(24):9781–6. doi:10.1021/ac801960c.
Kokko T, Liljenback T, Peltola MT, Kokko L, Soukka T. Homogeneous dual-parameter assay for prostate-specific antigen based on fluorescence resonance energy transfer. Anal Chem. 2008;80(24):9763–8. doi:10.1021/ac801875a.
Kupstat A, Kumke MU, Hildebrandt N. Toward sensitive, quantitative point-of-care testing (POCT) of protein markers: miniaturization of a homogeneous time-resolved fluoroimmunoassay for prostate-specific antigen detection. Analyst. 2011;136(5):1029–35. doi:10.1039/c0an00684j.
Medintz I, Hildebrandt N, editors. FRET - Förster resonance energy transfer: from theory to applications. Wiley Online Library; 2013.
Cohen N, Mechaly A, Mazor O, Fisher M, Zahavy E. Rapid homogenous time-resolved fluorescence (HTRF) immunoassay for anthrax detection. J Fluoresc. 2014;24(3):795–801. doi:10.1007/s10895-014-1354-7.
Geissler D, Stufler S, Lohmannsroben HG, Hildebrandt N. Six-color time-resolved Forster resonance energy transfer for ultrasensitive multiplexed biosensing. J Am Chem Soc. 2013;135(3):1102–9. doi:10.1021/ja310317n.
Mechaly A, Cohen N, Weiss S, Zahavy E. A novel homogeneous immunoassay for anthrax detection based on the AlphaLISA method: detection of B. anthracis spores and protective antigen (PA) in complex samples. Anal Bioanal Chem. 2013;405(12):3965–72. doi:10.1007/s00216-013-6752-1.
Qin QP, Peltola O, Pettersson K. Time-resolved fluorescence resonance energy transfer assay for point-of-care testing of urinary albumin. Clin Chem. 2003;49(7):1105–13.
Anisimov AP, Lindler LE, Pier GB. Intraspecific diversity of Yersinia pestis. Clin Microbiol Rev. 2004;17(2):434–64.
Skrzypek E, Straley SC. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177(9):2530–42.
Kobiler D, Weiss S, Levy H, Fisher M, Mechaly A, Pass A, et al. Protective antigen as a correlative marker for anthrax in animal models. Infect Immun. 2006;74(10):5871–6. doi:10.1128/IAI.00792-06.
Jernigan D, Raghunathan P, Bell B, Brechner R, Bresnitz E, Butler JC, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8(10):1019–28. doi:10.3201/eid0810.020353.
Wein LM, Liu Y. Analyzing a bioterror attack on the food supply: the case of botulinum toxin in milk. Proc Natl Acad Sci U S A. 2005;102(28):9984–9. doi:10.1073/pnas.0408526102.
Cohen S, Mendelson I, Altboum Z, Kobiler D, Elhanany E, Bino T, et al. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect Immun. 2000;68(8):4549–58.
Ben-Gurion R, Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981;5(2):183–7.
Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, et al. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69(5):2888–93. doi:10.1128/IAI.69.5.2888-2893.2001.
Diamant E, Lachmi BE, Keren A, Barnea A, Marcus H, Cohen S, et al. Evaluating the synergistic neutralizing effect of anti-botulinum oligoclonal antibody preparations. PLoS One. 2014;9(1):e87089. doi:10.1371/journal.pone.0087089.
Levy Y, Flashner Y, Tidhar A, Zauberman A, Aftalion M, Lazar S, et al. T cells play an essential role in anti-F1 mediated rapid protection against bubonic plague. Vaccine. 2011;29(40):6866–73. doi:10.1016/j.vaccine.2011.07.059.
Zahavy E, Fisher M, Bromberg A, Olshevsky U. Detection of frequency resonance energy transfer pair on double-labeled microsphere and Bacillus anthracis spores by flow cytometry. Appl Environ Microbiol. 2003;69(4):2330–9.
Flashner Y, Fisher M, Tidhar A, Mechaly A, Gur D, Halperin G, et al. The search for early markers of plague: evidence for accumulation of soluble Yersinia pestis LcrV in bubonic and pneumonic mouse models of disease. FEMS Immunol Med Microbiol. 2010;59(2):197–206. doi:10.1111/j.1574-695X.2010.00687.x.
Knepp AM, Grunbeck A, Banerjee S, Sakmar TP, Huber T. Direct measurement of thermal stability of expressed CCR5 and stabilization by small molecule ligands. Biochemistry. 2011;50(4):502–11. doi:10.1021/bi101059w.
Vagima Y, Levy Y, Gur D, Tidhar A, Aftalion M, Abramovich H, et al. Early sensing of Yersinia pestis airway infection by bone marrow cells. Front Cell Infect Microbiol. 2012;2:143. doi:10.3389/fcimb.2012.00143.
Turro N, Ramamurthy V, Scaiano J. Modern molecular photochemistry of organic molecules. New York: Wiley Online Library; 2012.
Janzen TW, Thomas MC, Goji N, Shields MJ, Hahn KR, Amoako KK. Rapid detection method for Bacillus anthracis using a combination of multiplexed real-time PCR and pyrosequencing and its application for food biodefense. J Food Prot. 2015;78(2):355–61. doi:10.4315/0362-028X.JFP-14-216.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were performed in accordance with Israeli law and were approved by the Ethics Committee for Animal Experiments at the Israel Institute for Biological Research.
Conflict of interest
The authors state that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cohen, N., Zahavy, E., Zichel, R. et al. An internal standard approach for homogeneous TR–FRET immunoassays facilitates the detection of bacteria, biomarkers, and toxins in complex matrices. Anal Bioanal Chem 408, 5179–5188 (2016). https://doi.org/10.1007/s00216-016-9602-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9602-0