Abstract
The use of small scale renewable sorbent material for automated solid phase extraction of multi-residue pharmaceuticals in environmental samples exploiting the sequential injection analysis-bead injection with direct coupling to liquid chromatography-electrospray ionization tandem mass spectrometry (SIA-BI-μSPE-LC-ESI-MS/MS) is presented to determine beta-blockers, namely atenolol, sotalol, pindolol, acebutolol, timolol, metoprolol, labetalol, carazolol, propranolol and betaxolol. These compounds yielded the same product ions, therefore were affected in terms of quantification when flow injection analysis-mass spectrometry (FIA-MS) was used. Thus, analytes and matrix present in the sample travel together into the ionization source which can seriously affect the ionization efficiency and analyte signals due to monitoring over a short time period.

A two-dimensional analysis involving a time dimension (retention time) and an m/z dimension (fragmentation ion) is promising for the various sample types. Using the developed method, absolute recoveries percentages of 10 mL of sample loading volume were >91 % for all β-blockers with enrichment factor of 62–74, limits of detection of 0.005–0.07 μg L−1, limits of quantification of 0.01–0.23 μg L−1, enrichment factor of 62–72 and repeatability within range 7–12 %. This developed method is suggested to be used as quantitative screening technique for drugs of abuse or persistent contamination using different kinds of sorbent materials and complex matrix such as biological fluid sample as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the low concentrations of pharmaceutical residues in the aquatic environment, mass spectrometry has emerged as the dominant detection technique which provides a sensitive, selective and reliable tool for multi-residue analysis [1, 2]. However, even the high selectivity offered by mass spectrometry or tandem mass spectrometry does not always guarantee the effective recognition and determination of target compounds from endogenous impurities when used on its own without sample pretreatment processes since the instruments struggle with matrix complexity inasmuch as most samples are not ready for direct introduction into the analytical systems for quantification [3]. In addition, analyte ionization approaches, such as electrospray ionization (ESI) as the preferred mode for applications involving pharmaceuticals in the environment, can be substantially affected by ion suppression or ion enhancement caused by the presence of matrix components or method-related interferences. Therefore, to improve the reliability of analysis, separation of the sample is generally required before mass spectrometry. This generally includes both preparative and analytical separation components to achieve the desired sensitivity and selectivity for pharmaceutical residues present typically at the nanograms per litre range in the aqueous environment in particular [4].
Commercial robotic on-line SPE or parallel-flow systems, namely Spark-Holland SymbiosisTM Pro, Spark-Holland SymbiosisTM Pharma, MultiPurpose Sampler MPS XL with GERSTEL SPE and Prospekt-2 systems, which can be hyphenated to chromatographic systems (either LC or GC) implementing a mini-cartridge, a 96-well plate, a short packed column as a trapping column, can simultaneously measure and extract samples for the next separation, thereby increasing throughput. These systems have been successfully applied for the rapid and sensitive detection of a variety of drugs in biological matrices [5, 6]. However, limitations of this technique may include increased pressure in the system, column clogging and the potential for carry over [7]. Notwithstanding, the sample enrichment factor is not easily calculated. In addition, technical limitations could be reduced if the manufacturers of robotic instrumentation collaborated with cartridge manufactures to develop, design and facilitate the simultaneous use of expensive robotic systems for multiple analyses capable of reducing many different manual generic SPE steps to a few automated SPE steps.
The combination of on-line SPE with direct coupling to different analytical detection systems has provoked an increase in research and development; therefore, any new analytical strategy has to take this trend into account. In fact, a fast analytical development, namely flow injection analysis-mass spectrometry (FIA-MS) has been developed for the simultaneous quantitative screening of pesticides and has claimed an affordable increase of sample throughput [8, 9]. However, efforts to increase sample throughput have placed a significant challenge on the reliability of this analytical approach, not least where matrix suppression is expected to occur and since this approach lacks the added selectivity offered by a high pressure liquid chromatography pre-separation stage (despite a subsequent loss of throughput). To overcome this problem, different approaches can be applied including powerful sample preparation tools for cleanup, enrichment and, most importantly, a more efficient chromatographic separation prior to mass spectrometric detection. Consequently, the integration of solid phase extraction in a SIA configuration manifold with liquid chromatographic separation and electrospray ionization tandem mass spectrometry detection was investigated in this study.
To the best of our knowledge, the use of small scale renewable sorbent material for automated SPE of multi-residue pharmaceuticals in environmental samples exploiting the sequential injection analysis-bead injection (SIA-BI-μSPE) with direct coupling to liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) has not yet been reported. Particularly, the compatibility of the various parameters of SIA-BI-μSPE and LC-ESI-MS/MS for fully automated control in terms of e.g., sample volume, solvents required, eluate transfer and injection, mobile phase composition, analysis time and liquid flow rate make this work a very useful and promising alternative for this application. The on-line SIA-BI-μSPE-LC-ESI-MS/MS configuration, optimization and analytical performance were examined. In addition, the quantification of target compounds as β-blockers in wastewater using this developed technology was performed.
Experimental
Chemicals and reagents
Analytical grade (>98 % purity) β-blocker standards of pindolol, propranolol hydrochloride, (±) metoprolol (+)-tartrate salt, atenolol, sotalol, carazolol, betaxolol hydrochloride, labetalol hydrochloride and acebutolol hydrochloride were purchased from Sigma-Aldrich (Steinheim, Germany). The HPLC grade or higher of acetonitrile and methanol were purchased from Fisher Scientific (Cheshire, UK). Ammonium acetate was obtained from Sigma-Aldrich (Steinheim, Germany). Ultra pure water was obtained from a Milli-Q water purification system with a specific resistance of 18.2 MΩ cm−1 (Millipore, Bedford, MA, USA). Individual stock standard solutions of β-blockers were prepared separately by dissolving an accurately weighted amount of each compound in methanol to achieve a concentration of 1000 μg mL−1. All stock standard solutions were stored in the dark at 4 °C and diluted stepwise to the desired concentration for preparation of working solutions. All working solutions were freshly prepared.
Wastewater sampling, transport and storage
Effluent wastewater was taken in Nalgene bottles (Thermo Fisher Scientific, San Jose, CA, USA) as a 24-h, 30-min time-integrated composite sample from a principal sewage treatment works in the London area. The collected samples were packaged on ice and transported to the laboratory where they were frozen at −20 °C until analysis. Before analysis, wastewater samples were fully thawed in the dark, and vacuum filtered through a 0.45-μm Nylon membrane filter (47-mm diameter, Millipore). No pH adjustment was made. For recovery experiments, wastewater samples were spiked at the 0.5 and 1.0 μg L−1 concentrations with all target analytes.
Sorbent material
HyperSep retain PEP 200 mg × 6 mL barrel-type SPE cartridges with 30–50-μm particle size were obtained from Thermo Fisher Scientific. Cartridges were disassembled, and the sorbent materials were collected in a clean glass container. A frit equipped with SPE cartridge was cut in a small circle to fit in the μSPE column in the SIA manifold.
SIA-BI-μSPE setup
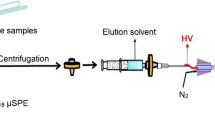
The overall schematic diagram of an on-line SIA-BI-μSPE-LC-ESI-MS/MS system is illustrated in Fig. 1.
Figure 1a shows the SIA-BI-μSPE setup which acts as an automated sample preparation unit. A bi-directional micro-syringe pump (MicroCSP-3000, FIAlab, USA) was used as a liquid handling system for the automated SPE operations. It was equipped with a 2.5-mL glass barrel syringe (Cavro, USA) and connected in block to a 24000-increment high-resolution stepper motor. A three-way Teflon valve was placed on the top of a glass syringe enabling connection with either the carrier reservoir direction (IN) or the flow network direction (OUT). A 10-port multi-position selection valve (MPV) together with its multi-position actuator control module (Cheminert VICI Instrument, USA) was connected to a micro-syringe pump via a holding coil (HC, 2.5 mL volume). The MPV encircled a coincidental central port (CP) and a communication channel (CC) that could be programmed to address each of the peripheral ports for control of solution handling, bead suspension, air and peripheral units (column, detector, etc.). All components were controlled by FIAlab for Windows Software 5.9.321 (FIAlab, USA).
The flow network of SIA-BI-μSPE manifold was built using polytetrafluoroethylene (PTFE) tubing of 1.5 mm i.d. (IDEX Health and Science LLC, WA, USA). The external collector and bead container vessels were made from luer lock plastic syringe barrels (2.5 mL) which were able to be connected to MPV using the Luer Tefzel adaptor (P-624), which were mounted vertically on MPV ports 9, and 10, respectively. Port 8 of the MPV was connected to a cylindrical column (3.0 mm i.d PTFE tubing) and furnished with a transparent Kel-F fluoroplastic piece which works as in-line container for capturing beads (solid sorbent particles) in order to form the μSPE column. The bottom of the μSPE column was equipped with a 10-μm polyethylene frit for efficient trapping of beads without leaking into the LC-ESI-MS/MS system.
A SIA configuration manifold was designed on the basis of the generic SPE procedure of HyperSep retain PEP sorbent. Methanol at MPV port 3 was used to condition and elution for the sorbent material. Five percent (v/v) methanol–water at port 5 was employed to wash non-specific interferences. Water in the carrier reservoir was used as a liquid driver in the SIA system as well as conditioning solvent for sorbent material. Air at port 2 prevented mixing of reagent, sample and carrier solution in the holding coil, and dried the μSPE column before the elution step and homogenizing the extract in the external collector vessel (port 9). The external collector vessel was used as a temporary waste and extract collector. The homogenized extract (port 9) was taken into a small vial through port 7a for off-line analysis using an autosampler (Fig. 1a, dashed line) or the transfer line to injection loop for on-line analysis (Fig. 1a, b, solid blue line 7b). Ammonium acetate (port 4) improved ionization efficiency with the large volume injection into LC-ESI-MS/MS.
Liquid chromatography
A Jasco HPLC system (Jasco Corporation, Tokyo, Japan) comprised of solvent delivery pump units (X-LC 3085Pu), a dynamic mixer (HG-1580-32), a degasser (DG-1580-53), an autosampler (X-LC 3159AS) and a Rheodyne 7725i injection valve (the extra high pressure injection valve for coupling LC to ESI-MS/MS) fitted with a 100 μL injection loop. The Jasco chromatography data system (LC Net II/ADC) was used as an interface between PC and LC components. Chromatographic separation was performed using a reversed-phase Ascentis® Express C-18 column (100 mm × 4.6 mm, 2.7 μm, Supelco, PA, USA). Gradient elution was carried out with 90:10 (v/v) of 10 mM ammonium acetate- acetonitrile (solvent A) and pure acetonitrile (solvent B). The gradient programme was linear from 20 % of B to 40 % of B over 30 min at a flow rate of 0.20 mL min−1. Finally, the mobile phase composition was returned to initial conditions in 2 min and equilibrated for 5 min prior to the subsequent analysis.
Electrospray ionization tandem mass spectrometry
ESI-MS/MS analysis was performed using a LCQ electrospray ion trap tandem mass spectrometer (Thermo Fisher Scientific, San Jose, CA). The ESI source was operated in positive ionization mode. The tune file was created using Tune Plus Software (Thermo Fisher Scientific). Instrument control, data acquisition and data evaluation were carried out by Xcalibur software (Thermo Fisher Scientific). Tuning parameters were optimized using 1 μg mL−1 tuning solution of metoprolol in 1:1 (v/v) methanol–water due to its lowest measured intensity of the chosen β-blocker compounds at the same concentration. Tuning was performed by direct infusion of tuning solution at a flow rate of 0.01 mL min−1 by an integrated syringe pump and merged with the mobile phase composition at the centre point of linear gradient programme at 0.20 mL min−1 through a T-connector towards to the ESI source. The optimized settings for ESI-MS/MS were sheath gas flow rate, 70 arbitrary units (arb); auxiliary gas flow rate, 20 arb; spray current voltage, 4.0 kV; capillary temperature, 250 °C; capillary voltage, 14.0 V; tube lens offset, 5.0 V; peak-to-peak and scan ranges, m/z 100.0–1000.0. Direct infusion of the standard mixture of 10 β-blocker compounds then allowed optimization of the detection parameters for precursor ions, [M + H]+, m/z at 249, 260, 268, 267, 273, 299, 308, 317, 329 and 337 for pindolol, propranolol, metoprolol, atenolol, sotalol, carazolol, betaxolol, timolol, labetalol and acebutolol, respectively. Fragmentation was produced by collision-induced dissociation (CID) at optimum of collision energies, and isolation width of each precursor ion and product ion scanning was acquired (Table 1). Detection and quantification of β-blockers were performed in the selected product ion using the most intense and stable fragment ion, m/z.
Interfacing of the SIA-BI-μSPE to LC-ESI-MS/MS
The SIA-BI-μSPE-LC-ESI-MS/MS consisted of 3 units: (1) an automated sample pretreatment unit (SIA-BI-μSPE; Fig. 1a), (2) a separation unit (LC; Fig. 1b) and (3) a detection unit (ESI-MS/MS; Fig. 1c). The SIA-BI-μSPE unit was connected to the chromatographic system via the transfer line from MPV (port 7b solid blue line in Fig. 1a, b) to the extra high pressure Rheodyne 7725i injection valve at port 6. The dead volume and length of this transfer line was kept as short as possible to minimize broadening of the extract plug. The analytical column was configured between the extra high pressure injection valve at port 3 and ESI source. The compatibility of mobile phase type and composition, flow rate and injection volume of LC and ESI-MS/MS were critical parameters to be optimized.
Analytical procedures of the on-line SIA-BI-μSPE-LC-ESI-MS/MS
An automated sample pretreatment unit was controlled by a user-friendly FIAlab software through a given number of steps, μSPE column packing, conditioning, sample loading, washing, sorbent drying, elution, extract transfer and finally sorbent discarding. Separation, detection, data acquisition and data processing were adopted by Xcalibur software. Both softwares run parallel with their own programmable tasks. The operational details of overall analytical procedures were described below.
μSPE column packing
The micro-syringe pump was set to aspirate (liquid movement direction from external reservoir into the holding coil) consecutively 80 μL of air (port 2), 100 μL of methanol (port 3) and 80 μL of bead suspension in 50 % (v/v) methanol–water (corresponding to 10 mg dry bead, port 10); thereafter, the opposite sequence of the bead suspension, methanol and air plugs were dispensed (liquid movement from HC to reservoirs or other peripheral units) to form μSPE column (port 8) by a flow reversal operation. The flow rate of the bead suspension was fixed to 0.5 mL min−1.
Sorbent conditioning
The μSPE column was conditioned by consecutively delivering 500 μL of methanol and water. Then, 300 μL of air was pumped through the μSPE column to remove remaining liquid before the sample loading step. The liquid waste in the external collector vessel (port 9) was sent to waste 1 (port 1). The flow rate of solution handling was fixed to 2.0 mL min−1.
Sample loading
The micro-syringe pump was programmed to aspirate 80 μL of air and 2 mL of water sample consecutively into HC and then dispense through the μSPE column at 1.0 mL min−1 in an opposite sequence for analytes enrichment. The external collector here acted as the temporary waste container vessel. The loaded sample in the external collector (port 9) was delivered to waste 1. These operational steps were repeated five times in order to handle a total sample volume of 10 mL. Then, an air plug of 300 μL was dispensed through the μSPE column in order to remove the remaining liquid sample in the column.
Washing
Five hundred microlitres of 5 % (v/v) of methanol–water (port 5) was perfused through the μSPE column in order to remove the non-specific interferences. The μSPE column was dried by a flow of air (port 2). The liquid waste in the external collector vessel was sent to waste 1 (MPV port 1), then cleaned with methanol (port 3) and rinsed with water to prevent contamination prior to the elution step.
Elution
Retrieval of the pre-concentrated β-blockers was accomplished by pumping 120 μL of methanol (port 3) at a flow rate of 0.5 mL min−1. The overall extract volume was collected in the cleaned external collector and thoroughly mixed with an air bubble to ensure homogeneity prior to the transfer step.
Transfer and injection
A sandwich of an ammonium acetate extract segmented plug at a ratio of 10:80:10 μL was aspirated into the HC and then dispensed to the transfer line (port 7b) entering into the injection loop, whereupon the high pressure injection valve was switched to the inject position. The LC gradient programme and ESI-MS/MS analysis were then initiated. The switching valve was returned to the load position after 2 min. The automated operational sequence for 10 mL of sample lasted 30 min and re-equilibration of the analytical column took 7 min.
Sorbent disposal
Sorbent material was automatically renewed after processing each individual sample or replicate by back flushing after being moistened with methanol and delivered to waste 1 (port 1).
Cleaning step
The external collector was cleaned by methanol through the waste 1. Then, 200 μL of methanol from MPV port 3 was pumped through the transfer line and injection loop delivered to waste 2 at the load position to eliminate any possible cross-contamination between consecutive runs. The holding coil was cleaned with methanol and carrier solution as well. Hence, the flow system was ready to initiate a new sample extraction cycle with a fresh portion of sorbent material.
Results and discussion
On-line SIA-BI-μSPE-LC-ESI-MS/MS configuration
The configuration of the on-line SIA-BI-μSPE-LC-ESI-MS/MS system was based on a two-pump system which consisted of the micro-syringe pump as a loading pump and analytical pump. The μSPE column was assembled in the SIA-BI manifold. The column configuration arrangement involving the μSPE column and analytical column were connected by an extra high pressure switching valve (Fig. 1b). The flow stream from the μSPE column was direct to the external collector vessel and then permanently transferred to waste 1 in steps of sorbent conditioning, sample loading and washing. The advantages of using an external collector vessel were elimination of air bubbles which possibly occurred within the heart-cutting and shoot approach of the on-line SPE-LC technique and to assure homogeneity of the extract. It also allowed the entire SPE procedure to be fully controlled by software resulting in the high reproducibility of the overall analysis.
Coupling to a chromatographic system often limits the choice of solvents used for back flushing of target compounds from a μSPE column and also limits the choice of receiving mobile phase composition. This is particularly the case in reversed-phase LC where highly aqueous content is often required to achieve separation of moderately polar analytes, yet introduction of a sample plug containing high organic solvent content (required for μSPE elution) may result in self-elution or peak distortion effects [10, 11]. As expected, poor separation was observed using higher percentages of organic solvent for reversed-phase LC separations due to co-elution of polar compounds. This could be improved using a hydrophilic interaction liquid chromatography (HILIC) column for separation. However, HILIC was not pursued here as column re-equilibration times can be significant as well as introducing the potential for reduced reproducibility. These challenges therefore significantly complicate the use of robotic on-line SPE-LC. The initial mobile phase composition for separation in the developed system is always independent from the SPE elution solvent that allows elution with varieties of solvents in comparison to robotic on-line SPE-LC. Generally, the stronger the organic solvent for elution, the higher the recovery achieved. Particularly in the case of molecularly imprinted polymers, the dilute basic or acid content in organic solvents required for SPE extraction which made the on-line SIA-BI-μSPE-ESI-LC-MS/MS superior to the robotic on-line SPE-LC. SIA-BI-μSPE techniques with certain manifold configurations ensure the flexibility of using any kind of solvent, resulting in improved sensitivity and the lower limit of detection of LC-ESI-MS/MS. Furthermore, this approach eliminates additional back pressure, increases sample throughput, decreases solvent consumption and reduces the analysis time interval between sample preparation processes and sample measurement. Lastly and importantly, an enrichment factor can be evaluated much more easily.
Comparison of SIA-BI-μSPE-LC-ESI-MS/MS and FIA-MS
Taking into account the conventional off-line SPE methods are less susceptible to suppression effects because they rely on large differences in chromatographic behaviour for matrix removal. Therefore, such samples were prepared by SPE in a classical way and thereafter analysed via a flow injection into the electrospray source without chromatographic separation to improve throughput. This is generally known as flow injection analysis-mass spectrometry (FIA-MS) [8]. This approach can cause in-source formation of the ionic species of similar isobaric m/z from different analytes or matrix components that are not resolved in time, thus producing artificial signals [3]. Highly specific fragmentation pathways should be evaluated and used for analyte quantitation and confirmation by MS/MS, and this is particularly important when chromatographic separation is not used. Furthermore, whilst increasing sample throughput using infusion or flow injection methods is an advantage for fast analysis by mass spectrometric techniques.
However, mass spectrometric techniques are generally restricted to a limited number of ion transitions that can be monitored simultaneously, and this limitation also becomes a particularly important issue when FIA-MS was used since all analytes are quickly monitored in a short time, approximately 50 s [9]. Thus, analytes and matrix present in the sample travel together into the ionization source at the same time which can seriously affect the ionization efficiency, analyte signals (enhance or suppress the analyte responses), and dirty the system. These problems require an increased frequency of instrument maintenance and might increase maintenance cost as well. Complex samples such as biological fluids are not recommended for FIA-MS determination.
A proof-of-concept for matrix removal by fresh sorbent surfaces extraction along with the addition of a more efficient chromatographic separation for ESI-MS/MS provided a significant improvement of the suppression effect of compounds which might interfere with the ESI process. β-blockers are a good example for FIA-MS analysis complications using this type of mass analyser. Acebutolol, pindolol, acebutolol, metoprolol, carazolol, propranolol and betaxolol yielded product ion at m/z 116, which corresponded to cleavage of the carbon-oxygen bond in the dimethyl-amino propyl side chain. For FIA-MS, these compounds may therefore be affected in terms of quantification. When using the SIA-BI-μSPE-LC-ESI-MS/MS method, the same product ions were detected and analyte discrimination was achieved due to their separation by LC which represents a more efficient approach than FIA-MS. Product ions with m/z values of 191, 183 and 172 corresponding to [M-H2O-C3H7NH]+ were obtained from the pseudo molecular precursor ion ([M + H]+) of metoprolol, propranolol and pindolol, respectively.
Therefore, SIA-BI-μSPE-LC-ESI-MS/MS is defined here as a form of two-dimensional analysis involving a time dimension (retention time) and an m/z dimension (product ion). The crucial advantage of SIA-BI-μSPE-LC-ESI-MS/MS was not only elimination of analysis complications caused by matrix effects but also minimizing the degree of potential instrumentation fouling in comparison to FIA-MS. Analyte selectivity and ion suppression effects were found to be significantly improved (see determination of β-blockers section).
Injection volume
The maximum volume of extract that could be injected for analysis to achieve satisfactory peak shape, sufficient sensitivity and reproducibility was assessed. The extract volume investigated ranged from 10 to 100 μL. Injection volume of 80 μL of a methanolic extract was shown to be suitable for processing residues of β-blockers at trace concentrations in wastewater samples. In our particular application, the sandwich of ammonium acetate extract segment plug (10 μL of ammonium acetate–80 μL extract–10 μL ammonium acetate) tends to decrease band broadening and increased ionization efficiency. However, on-line injection of a pure methanolic extract to LC-ESI-MS/MS did not require the additional step of in-line dilution in comparison to on-line LC-UV [12] or GC-MS [13]. For this purpose, 80 μL of extract was chosen as injection volume for the remaining part of the work.
Determination of β-blockers using SIA-BI-μSPE-LC-ESI-MS/MS
Atenolol, sotalol, pindolol, acebutolol, timolol, metoprolol, labetalol, carazolol, propranolol and betaxolol showed protonated precursor ions, [M + H]+, at m/z 267.08, 273.07, 249.10, 337.24, 317.19, 268.16, 329.28, 299.07, 260.03 and 308.22, respectively, in full scan MS mode (Fig. 2a). These precursor ions were subjected to fragmentation by MS/MS. Figure 2b shows an example of the MS/MS spectra of propranolol which represented the product ion (base peak) at m/z 183.10 and the possible fragmentation patterns. The most intense product ions for atenolol, sotalol, pindolol, acebutolol, timolol, metoprolol, labetalol, carazolol, propranolol and betaxolol are presented in Table 1 and subsequently used for identification and monitoring of drug residues in wastewaters.
In the quantitative part of this study, three-time segment of MS/MS scan with respect to chromatographic separation order was performed (Fig. 3). The first time segment ranging from 0 to12 min was used for atenolol and sotalol; a second interval ranging from 12 to 20 min was used for pindolol, acebutolol, timolol and metoprolol and a third segment which ranged from 20 to 30 min was used for carazolol, propranolol and betaxolol determination. These time segments improved chromatographic peak definition and selectivity of MS/MS measurement due to more residence time scan of target compound per mass at a certain period of time. Quantitative results were summarized in Table 1.
Total ion chromatogram of 10 mL of spiked wastewater at 1.0 μg L−1 of β-blockers standard mixture analysed by the on-line SIA-BI-μSPE-LC-ESI-MS/MS method within the time segment of MS/MS monitoring ranged from (i) 0 to 12.00 min for determination of atenolol and sotalol; (ii) 12.01 to 20.00 min for determination of pindolol, acebutolol, timolol and metoprolol and (iii) 20.01 to 30 min for determination of carazolol, propranolol and betaxolol
Matrix effect
Matrix-dependent signal suppression or enhancement represented a major potential limitation for quantitative analysis with the developed system involving on-line SIA-BI-μSPE-LC-ESI-MS/MS. Matrix effects were evaluated using the post-extraction spiking method which is based on the comparison of the average MS/MS area response obtained for a spiked extract (matrix-matched standard) with those obtained for a standard solution at the 5 μg L−1 concentration. Experiments were conducted as follows: a 5 μg L−1 of β-blockers standard mixture in ultra pure water was analysed as theoretical response, and the average MS/MS area response was obtained (no-extraction with spiked standard, A). Then, a 10-mL wastewater sample was extracted by the SPE protocol, and thereafter, the standard mixture at the same concentration was added (post-extraction with spiked matrix, B). The post-extraction without spiked standard was evaluated (blank extract, C) to subtract the possible signal of existing analytes that appeared in original samples at low concentration levels. Experiments were performed in triplicate.
The percentage of matrix effect (%ME) was defined as following [12]:
A value of 100 % means that there is no matrix effect. A value higher than 100 % means enhancement, whilst signal suppression is found when a value of less than 100 % is obtained.
In our study, %ME between 92 and 109 % with RSDs below 6 % for all compounds (Table 1) were found to be significantly better than those reported for the determination of β-blockers in effluent wastewater samples without a cleanup step before robotic LC-MS/MS determination with ion suppression effects up to 65 % observed [13]. The low %ME values encountered in the developed method considering no matrix effect were due to the cleanup by the renewable SPE and efficient chromatographic separation which simplified the complexity of the matrix.
Analytical performance
In order to assess the performance of the proposed method, the main analytical quality parameters were thoroughly evaluated using standards prepared in ultrapure water. Calibration curves were built in a concentration range of 0.01–10 μg L−1. Correlation coefficients (r) better than 0.9921 were obtained for all compounds. Table 1 summarizes the method validation data. Absolute recoveries of β-blockers were 74 to 86 %. Enrichment factors of target β-blockers ranged from 62 to 74 which were calculated as the ratio of the linear sensitivity of the proposed SIA-BI-μSPE-LC-ESI-MS/MS procedures and that obtained by direct analysis of 80 μL of standard mixture solution by LC-ESI-MS/MS [14]. The results were found to be satisfactory despite lower recoveries since the metered elution volume (120 μL) was applied for elution, however, with relative standard deviation (RSD) less than 10 %.
Method precision was expressed as the relative standard deviations (RSDs) obtained from nine consecutive assays of 10 mL mixed standard solution at 10 μg L−1 level. RSDs ranged from 2 to 6 % and were significantly better than those recently reported for β-blockers in water using robotic LC-MS/MS methods with RSDs higher than 16 % [15]. The inter-day variation in six identical samples on six different days was less than 12 %. The results indicated that the method was considered as acceptable to be used on a regular basis for the screening of the target analytes in environmental samples. LODs and LOQs calculated at a peak-to-peak signal-to-noise ratio (S/N) of extracted MS/MS ion chromatogram of 3 and 10, respectively, for the analysis of 10 mL spiked water ranged from 0.005 to 0.07 and 0.01 to 0.23 μg L−1, respectively. It should be noted that analytical method featured based on renewable sorbent material together with chromatographic separation for on-line LC-ESI-MS/MS under the optimal experimental conditions showed better recovery and reproducibility than those found in robotic on-line LC-MS/MS [16, 17]. Remarkably, no backpressure and no carryover within the renewable μSPE column were detected. No breakthrough volume was observed for any of the target analytes up to 10 mL of water at loading sample and elution at flow rates 1.0 and 0.5 mL min−1, respectively.
Real sample analysis
The on-line SIA-BI-μSPE-LC-ESI-MS/MS method was successfully applied to the analysis of effluent wastewaters. Matrix-matched calibration curves were constructed in the concentration range of 0.01 to 10.0 μg L−1 which covered the spiked concentration levels in the wastewater sample. Figure 3 shows the total ion chromatogram of target species in the spiked wastewater sample. The extracted product ion chromatograms of extract spiked wastewater at 1.0 μg L−1 level are shown in Fig. 4. The peak profiles were symmetric and reproducible for almost all analytes at certain experimental conditions except for the two first compounds, namely atenolol and sotalol; however, they were not significantly affected in terms of quantitation. Broadening of extracted ion chromatogram peaks was not observed for any of the target compounds.
Analytical recoveries and relative standard deviations are illustrated in Table 2. Relative recoveries varied from 91 to 117 % for both samples of spiked wastewater and relative standard deviations ranged from 2 to 12 % for 0.5 and 1.0 μg L−1 concentration levels.
Conclusions
The advantages of renewable sorbent material in an automated SIA-BI-μSPE coupled to chromatographic separation- tandem mass spectrometric detection were demonstrated for the determination of trace levels of β-blocker residues in environmental samples for the first time. On-line SIA-BI-μSPE-LC-ESI-MS/MS offered benefits such as reduction in the amount of solvent used, elimination of human intervention and manual steps, increased sample throughput, diminishing errors and time associated with sample transport and storage and lower limit of detection and quantification, therefore resulting in more reproducible and faster analytical data due to the precise flow programing control. Limits of detection (S/N = 3) and quantification (S/N = 10) for β-blockers in effluent wastewater were found in the range of 0.005 to 0.07 μg L−1 and 0.01 to 0.23 μg L−1, respectively. Repeatability of the overall procedure provided RSDs ranging from 7 to 12 %. The correlation coefficient for all of the target compounds exhibited excellent linearity (r > 0.9921) over the range of 0.01 to 10 μg L−1. The absolute recovery percentages within heart-cutting elution of 10 mL sample loading volume for the overall β-blockers in effluent wastewaters ranged from 91 to 117 %. Based on both automation of renewable sorbent surfaces and liquid chromatographic separation-electrospray ionization tandem mass spectrometry, the proposed approach is promising for the time-efficient and reliable analysis of the large numbers of environmental samples. This developed method is suggested to be used as quantitative screening technique for drugs of abuse or persistent contamination using different kinds of sorbent materials and complex sample matrix such as biological fluid as well.
References
Jakimska A, Śliwka-Kaszyńska M, Reszczyńska J, Namieśnik J, Kot-Wasik A (2014) Anal Bioanal Chem 406:3667–3680
Barron L, Nesterenko E, Hart K, Power E, Quinn B, Kelleher B, Paull B (2010) Anal Bioanal Chem 397:287–296
Nanita SC, Stry JJ, Pentz AM, McClory JP, May JH (2011) J Agric Food Chem 59:7557–7568
U.S. Environmental Protection Agency Office of Water, EPA Method 1694: Pharmaceuticals and personal care products in water, soils, sediments, and biosolids by HPLC/MS-MS (2007). Wahington DC
López-Serna R, Pérez S, Ginebreda A, Petrović M, Barceló D (2010) Talanta 83:410–424
Lin X, Li H-F, He X, Hashi Y, Lin J-M, Wang Z (2012) J Sep Sci 35:2553–2558
Boonjob W, Miro M, Segundo MA, Cerda V (2011) Anal Chem 83:5237–5244
Nanita SC, Pentz AM, Bramble FQ (2009) Anal Chem 81:3134–3142
Nanita SC (2013) Anal Chem 85:11866–11875
Regueiro J, Rossignoli AE, Álvarez G, Blanco J (2011) Food Chem 129:533–540
Kwok WH, Leung DKK, Leung GNW, Wan TSM, Wong CHF, Wong JKY (2010) J Chromatogr A 1217:3289–3296
Yan W, Li Y, Zhao L, Lin JM (2009) J Chromatogr A 1216:7539–7545
Fontanals N, Miralles N, Abdullah N, Davies A, Gilart N, Cormack PAG (2014) J Chromatogr A 1343:55–62
Boonjob W, Yu YL, Miro M, Segundo MA, Wang JH, Cerda V (2010) Anal Chem 82:3052–3060. doi:10.1021/ac100185s
Alnouti Y, Li M, Kavetskaia O, Bi H, Hop CECA, Gusev AI (2006) Anal Chem 78:1331–1336
Gros M, Pizzolato TM, Petrović M, de Alda MJL, Barceló D (2008) J Chromatogr A 1189:374–384
Salem AA, Wasfi IA, Al-Nassibi SS (2012) J Chromatogr B 908:27–38
Acknowledgments
This work is co-financed by the European Social Fund and the State Budget of the Czech Republic (Project no. CZ.1.07/2.3.00/30.0061 (FAF12)). The authors thank to Dr. Petr Chocholouš for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonjob, W., Sklenářová, H., Barron, L. et al. Renewable sorbent material for solid phase extraction with direct coupling of sequential injection analysis-bead injection to liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Bioanal Chem 407, 5719–5728 (2015). https://doi.org/10.1007/s00216-015-8752-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8752-9