Abstract
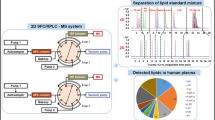
A new continuous comprehensive two-dimensional liquid chromatography–electrospray ionization mass spectrometry method has been developed for the lipidomic characterization of complex biological samples. The reversed-phase ultra-high-performance liquid chromatography with a C18 column (150 mm × 1 mm, 1.7 μm) used in the first dimension makes the separation of numerous lipid species differing in their hydrophobic part of the molecule, mainly fatty acyl chain lengths and the number and positions of double bonds, possible. Coeluted lipid species in the first dimension are resolved by the fast hydrophilic interaction liquid chromatography separation (50 mm × 3 mm, 2.7 μm, core–shell particles) of lipid classes according to their different polarities in the second dimension. Retention times in both dimensions, accurate m/z values, and tandem mass spectra provide high confidence in the identification of lipid species. The retention behavior of individual lipids in reversed-phase mode follows the equivalent carbon number pattern, which provides an additional tool for unambiguous identification. This analytical method is applied for the lipidomic characterization of total lipid extracts of human plasma and porcine brain samples, which resulted in the identification of 143 lipid species from four lipid categories and ten lipid classes.

2D-LC/ESI-MS of porcine brain lipid extract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipids have numerous important functions in cells, tissues, and body fluids, such as the formation of lipid bilayers, cell signaling, and energy storage [1]. Lipids are defined as small molecules formed by carbanion-based condensations of thioesters and/or by carbocation-based condensations of isoprene units. The most widely used LIPID MAPS classification system divides lipids into eight categories, namely, fatty acyls, glycerolipids, glycerophospholipids (GP), sphingolipids (SP), sterol lipids (ST), prenol lipids, saccharolipids, and polyketides, with many further classes and subclasses [1–3]. Lipidomics is a subset of metabolomics, which is aimed at the comprehensive analysis of lipids isolated from cells, tissues, or biological fluids and the study of their biological roles with respect to health and disease states [4–6]. Three major analytical platforms are known in lipidomic analysis [7]: shotgun electrospray ionization (ESI) mass spectrometry (MS) using direct infusion without any chromatographic separation [8–10], liquid chromatography (LC)/MS using various chromatographic modes [11–19], and MS imaging, providing information on the spatial lipid distribution in tissues [20, 21].
Shotgun lipidomics is a simple approach for lipidomic characterization, where the crude lipid extract typically in chloroform–methanol–2-propanol containing additives is infused directly into an ESI tandem MS (MS/MS) instrument. The aim is to identify and quantify as many lipid molecular species as possible from biological samples [8–10]. The advantages of the shotgun approach are the short analysis time and easy automation, but on the other hand it may be difficult to obtain information on isomeric lipids, and attention must be paid to ion suppression effects. In practice, shotgun lipidomics is often used for the high-throughput automated quantitation of lipids, whereas LC/MS is preferred for more demanding tasks, including isomeric resolution.
Several chromatographic modes can be used in a liquid-phase separation of various types of lipid isomers, such as reversed-phase (RP) LC [11], normal-phase (NP) LC [12, 13], hydrophilic interaction LC (HILIC) [14, 15], silver-ion LC [16–18], and chiral LC [19]. RP-LC is used for lipid separation according to the length of the fatty acyl chains and the number and positions of double bonds (DB) [11]. NP-LC is typically used for the separation of only nonpolar lipid classes on the basis of their polarity using conventional NP mobile phase systems using hexane with polar additives [12]. However, the separation of polar lipid classes can be achieved on a poly(vinyl alcohol)–silica column with a more polar mobile phase (2-propanol–methyl tert-butyl ether–methanol with 4 mM ammonium formate), but the resolution of nonpolar lipid classes is sacrificed [13]. HILIC is frequently used for the analysis of polar compounds using various types of chromatographic columns, such as bare silica gel, polar stationary phases (amino, cyano, diol, etc.) chemically bonded to the silica gel support, and zwitterionic or ion-exchanger materials [22–26]. HILIC on silica columns provides very good lipid class separation [14, 15]. The previously discussed work [13] using more polar mobile phases results in a chromatogram very similar to that obtained using HILIC, so the retention mechanisms are probably similar as well. Silver-ion LC [16–18] is a special chromatographic mode typically used for the analysis of triacylglycerols (TG) and other nonpolar lipids and is based on the formation of weak reversible complexes of silver ions in the stationary phase with the π electrons of DB. Chiral LC has been successfully applied for the enantiomeric separation of TG [19].
However, one-dimensional separation may not be sufficient for complex lipidomic mixtures. Two dimensional (2D) LC is based on the combination of two different chromatographic modes, where the orthogonality of the separation mechanisms in both dimensions is a major requirement for such systems [22–26]. Various off-line and online 2D configurations are known, and each have their advantages and limitations. The commonest combination of separation mechanisms is based on lipid class separation in one dimension using either the NP mode or the HILIC mode coupled with lipid species separation in another dimension using RP separation [24–27]. The alternative for nonpolar lipid classes is the combination of silver-ion LC and nonaqueous RP separation in either an off-line setup [28] or an online setup [29, 30]. The advantage of the offline configuration [27, 28] is the possibility of full optimization in both dimensions regardless of the analysis time, which could yield the highest number of identified species, but a serious limitation is the very long analysis times, which are not suitable for high throughput or automation. The online combination [22–26] offers three alternative methods: (1) heart-cut 2D-LC is used for the analysis of a selected fraction [31, 32], (2) stopped-flow 2D-LC is a hybrid approach combining the advantages of the offline and online approaches, where the analysis in the first dimension is stopped for the time of the second-dimension analysis and then the flow is reestablished [33, 34], (3) the continuous comprehensive 2D-LC system allows all fractions eluted from the first dimension to be analyzed in the second dimension as well, and this is the fastest approach and the one with the greatest potential for automation. The major drawback of the continuous comprehensive 2D-LC setup is the limited analysis time in the second dimension caused by the modulation time, which compromises the resolution in the second dimension. To the best of our knowledge, no article has been published on continuous comprehensive 2D-LC/MS of multiple lipid classes. Another possibility on the edge between stopped-flow and continuous comprehensive 2D-LC is the use of a trapping column with a non-stopped-flow system [35, 36].
The main goal of our work was the development of a new continuous comprehensive 2D-LC/MS method for the characterization of the lipidomic composition of various biological samples. For this purpose, RP-LC mode with a C18 column is used in the first dimension, and HILIC with a silica column was used in the second dimension of a comprehensive 2D-LC/MS setup applied for lipid extracts of human plasma and porcine brain, where lipids were identified on the basis of retention times in two dimensions, and accurate m/z values of molecular adducts and characteristic fragment ions in their MS/MS spectra.
Experimental
Chemicals and standards
Acetonitrile, 2-propanol, methanol (all LC/MS gradient grade), chloroform (LC grade, stabilized by 0.5–1 % ethanol), ammonium acetate, sodium formate, sodium chloride, and cholest-5-en-3β-yl nonadecanoate [cholesteryl ester (CE) 19:0] were purchased from Sigma-Aldrich (St Louis, MO, USA). Deionized water was prepared with a Demiwa 5-roi purification system (Watek, Ledeč nad Sázavou, Czech Republic) and with an Ultra CLEAR UV apparatus (SG, Hamburg, Germany). Standards of polar lipid classes—1,2-di-[(9Z)-octadecenoyl)]-sn-glycero-3-phosphoethanolamine [phosphatidylethanolamine (PE) 18:1/18:1], 1,2-di-[(9Z)-octadecenoyl)]-sn-glycero-3-phosphocholine [phosphatidylcholine (PC) 18:1/18:1], 1-[(9Z)-octadecenoyl)]-sn-glycero-3-phosphocholine [lysophosphatidylcholine (LPC) 18:1], N-(octadecanoyl)-sphing-4-enine-1-phosphocholine [sphingomyelin (SM) 18:1]—were purchased from Avanti Polar Lipids (Alabaster, AL, USA). The standard of 1,2,3-tri-[(9Z)-octadecenoyl)]-sn-glycerol (TG 18:1/18:1/18:1) was purchased from Nu-Chek Prep (Elysian, MN, USA). The lipid nomenclature follows the shorthand notation for lipid structures published by Liebisch et al. [37] and the LIPID MAPS [1] classification system.
Sample preparation
Total lipid extracts of human plasma and porcine brain were prepared according to the well-established Folch procedure [38] using a chloroform–methanol–water system with minor modifications [14, 15]. Human plasma (500 μL) or porcine brain (500 mg) was homogenized with 10 mL of a chloroform–methanol mixture (2:1, v/v). The homogenate was filtered using a coarse filter paper. Then, 2 mL of 1 M NaCl was added, and the mixture was centrifuged at 2,500 rpm for 3 min under ambient conditions. The chloroform (bottom) layer containing lipids was collected, evaporated by a gentle stream of nitrogen, and redissolved in a chloroform–2-propanol (1:2, v/v) mixture for analysis.
Continuous comprehensive 2D-LC conditions
Continuous comprehensive 2D-LC analysis of lipids was performed with an Agilent 1290 Infinity 2D-LC Solution system (Agilent Technologies, Santa Clara, CA, USA) containing two Agilent 1290 Infinity pumps, a thermostatted column compartment with a two-position eight-port switching valve (pressure limit 1,200 bar) for 2D-LC, and two identical 20-μL sampling loops. An Acquity UPLC BEH C18 column (150 mm × 1 mm, 1.7 μm, Waters, Milford, MA, USA) was used in the first dimension under the following conditions: flow rate of 20 μL/min; injection volume of 1 μL; column temperature of 25 °C; mobile phase gradient of 78.5 % solvent B at 0 min and 100 % solvent B at 150 min, where solvent A was 5 mM aqueous ammonium acetate and solvent B was a mixture of 99.5 % acetonitrile and 2-propanol (1:2, v/v) and 0.5 % 5 mM aqueous ammonium acetate. A core–shell silica CORTECS HILIC column (50 mm × 3 mm, 2.7 μm, Waters) was used in the second dimension under the following conditions: flow rate of 5 mL/min; column temperature of 40 °C; mobile phase gradient 92 % solvent B at 0 min, 80 % solvent B at 0.7 min, and 92 % solvent B at 1 min, where solvent A was 5 mM aqueous ammonium acetate and solvent B was acetonitrile. The modulation time between dimensions was 60 s. A T-piece mobile phase splitter was used before the effluent introduction into the MS system at a splitting ratio of 8 (MS system):100 (waste).
MS conditions
A hybrid quadrupole time-of-flight mass spectrometer (micrOTOF-Q, Bruker Daltonics, Bremen, Germany) with an ESI source was used as the detector with the following parameter settings: capillary voltage of 4.5 kV; nebulizing gas pressure of 1.6 bar; drying gas flow rate of 12 L/min; drying gas temperature of 220 °C. ESI mass spectra were measured in the range of m/z 50–1,500 in positive-ion mode. Negative-ion MS/MS mode was used only for the identification of fatty acyls in case of PE. A collision energy of 20–25 eV and argon as the collision gas were used for MS/MS experiments. The external calibration of the mass scale was performed with sodium formate clusters before individual measurements. The data were acquired and evaluated using DataAnalysis (Bruker Daltonics) and were visualized with GCImage (University of Nebraska, Lincoln, NE, USA).
Results and discussion
General considerations for the method development
The Agilent 1290 Infinity 2D-LC Solution system with a two-position eight-port switching valve was used for the development of the continuous comprehensive 2D-LC/MS method for lipidomic characterization, where a fully porous 1.7-μm-particle column was used in the first dimension and a core–shell sub-3-μm-particle column was used in the second dimension. The principal issue of the method development is the selection of the orthogonal separation mechanisms and also the appropriate order of these modes in the 2D arrangement. One separation mechanism should be applied for the lipid class separation, where NP and HILIC modes can be considered. NP-LC is typically used only for nonpolar lipid classes owing to the too strong retention of polar GP and SP classes [12], but the meaningful separation of both nonpolar and polar lipids under NP conditions has also been reported [13]. In our case of continuous comprehensive 2D-LC, HILIC separation is preferred owing to easy reequilibration and good compatibility with the aqueous mobile phases used in RP-LC. The RP mode is an obvious choice for the separation of lipids from all classes according to differences in the hydrophobic part of the molecule, mainly the length of the fatty acyl chains, and the number and positions of DB. The order of the RP and HILIC modes in the comprehensive 2D-LC concept is determined by a simple fact that RP-LC cannot provide a highly efficient separation of so many species within only 1 min as a typical modulation time used for comprehensive 2D-LC, but the HILIC class separation can be achieved in such a time frame with a certain level of compromise. Moreover, HILIC allows a partial separation of lipid species within individual classes (Table 1), which is maintained only in the case of the RP–HILIC configuration, but these minor retention differences would be lost in the opposite HILIC–RP configuration. This explains why our choice of RP–HILIC is superior to the HILIC–RP arrangement in continuous comprehensive 2D-LC/MS lipidomic applications. The opposite configuration is typically selected for off-line, stopped-flow, and heart-cut 2D-LC setups [29–32], where the analysis time in the second dimension is not limited as strictly as in the case of continuous comprehensive 2D-LC.
HILIC separation when dealing with phospholipid analysis has the important advantage of achieving a partial separation of molecular species within a single phospholipid class. The order of RP separation and HILIC separation in a consecutive manner is fully understandable. In fact, in “continuous” 2D-LC especially when analyzing lipids with a broad range of equivalent carbon numbers (ECN), the separation time frame becomes critical.
Optimization of RP separation in the first dimension
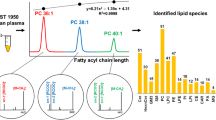
The basic prerequisites for the RP ultra-high performance LC (UHPLC) method optimization in the first dimension are as follows: a long column (i.e., 150 mm) packed with sub-2-μm particles to provide the best efficiency, a narrow column diameter (i.e., 1 mm) to maintain flow rates lower than 50 μL/min, which are essential for comprehensive 2D-LC/MS coupling, and sufficient peak widths to ensure that each chromatographic peak from the first dimension appears in at least two fractions in the second dimension [22–25]. These requirements result in a rather low flow rate in the first dimension (20 μL/min) and a long analysis time (165 min). The best separation is achieved on an Acquity UPLC BEH C18 column (150 mm × 1 mm, 1.7 μm, Waters) using an aqueous ammonium acetate–acetonitrile–2-propanol gradient (details are given in “Experimental”) optimized on the basis of our previous experience [15, 27]. The overlay of reconstructed ion current (RIC) chromatograms of RP-UHPLC/ESI-MS analysis is shown for the mixture of lipid standards in Fig. 1a and the total ion current chromatogram for the lipid extract of human plasma is shown in Fig. 1b. Figure S1a shows the total ion current chromatogram of RP-UHPLC/ESI-MS analysis of the porcine brain extract, which is selected owing to it having a high lipidomic complexity in comparison with plasma samples, mainly in the area of ceramides and hexosylated ceramides occurring in brain tissues (see Table S1 for a detailed comparison).
Reversed-phase ultra-high-performance liquid chromatography (RP-UHPLC)/electrospray ionization mass spectrometry (ESI-MS) chromatograms: a overlay of reconstructed ion current (RIC) chromatograms of the mixture of lipid standards; b total ion current chromatogram of the lipid extract of human plasma. The RP-UHPLC conditions are reported in “Experimental.” CE cholesteryl esters, Chol cholesterol, GP glycerophospholipids, LPC lysophosphatidylcholines, PC phosphatidylcholines, PE phosphatidylethanolamines, SM sphingomyelins, SP sphingolipids, TG triacylglycerols
The retention of individual lipid species is governed by the ECN, which is calculated as the total carbon number of fatty acyls minus two times the DB number. The ECN concept of the retention behavior was extensively discussed in our previous works on TG analysis [39–41], but it is also applicable for the other lipid classes analyzed in this work (Table 1). The LPC class exhibits the lowest retention (7–14 min) in RP mode, because members of this class have the smallest hydrophobic part of the molecule, i.e., one fatty acyl only in comparison with two fatty acyls for GP and SP and three fatty acyls for TG (Table 1). Moreover, the free hydroxyl group also reduces the retention in RP mode. Seven classes of polar lipids (LPC, SM, ceramides, hexosyl ceramides, dihexosyl ceramides, PC, and PE) and cholesterol are eluted over a broad range of retention times in accordance with the literature [11] . Nonpolar lipid classes (TG and CE) exhibit the highest retention in RP mode (120–160 min). Lipid species are partially resolved within ECN groups. Palmitoyl-containing TG typically exhibit higher retention than oleoyl-containing TG with the same ECN, e.g., for ECN = 44 (TG 18:1_18:2_18:2 < TG 16:0_18:2_18:2), ECN = 48 (TG 18:1_18:1_18:1 < TG 16:0_18:1_18:1 < TG 16:0_16:0_18:1), etc., in accordance with our previous works [39–41]. Lipid species are well separated within lipid classes according to their ECN, but overlap of retention may occur among different classes, which is solved by HILIC separation in the second dimension.
Optimization of HILIC separation in the second dimension
Our initial intention was to use a short column (50 mm) with a wider diameter (3 mm) and sub-2 μm particles in the second dimension. An Acquity UPLC BEH HILIC column (50 mm × 3 mm, 1.7 μm, Waters) with an aqueous ammonium acetate–acetonitrile gradient was used for this purpose, but the column back pressure limited the maximum flow rate to only 4 mL/min. Anyway, relatively good separation with a total analysis time of 1 min was achieved (Fig. S2) in the comprehensive 2D-LC setup, but a serious problem arose from the limited column lifetime under such flow rates. The column back pressure gradually increased depending on the number of analyses until the system pressure limit (1,200 bar) was reached or complete deterioration of the column was observed, as experienced with three columns tested. For this reason, we changed the column to a core–shell sub-3-μm column with 50-mm length to develop a robust method capable to reaching the maximum flow rate of 5 mL/min in the second dimension, good separation of lipid classes, and improved column lifetime. This goal was achieved with a CORTECS HILIC column (50 mm × 3 mm, 2.7 μm, Waters) and an aqueous ammonium acetate–acetonitrile gradient (details in “Experimental”), as illustrated by the overlay of the RIC chromatograms in Fig. 2a for the mixture of lipid standards, Fig. 2b for the lipid extract of human plasma, and Fig. S1b for the lipid extract of porcine brain. Nonpolar lipid classes (TG and CE) together with cholesterol are eluted in the void volume of the system, similarly as in our previous works [12, 27], whereas polar lipid classes (PE, PC, SM, and LPC) exhibit acceptable separation according to their polarity within a 1-min separation window. A T-piece flow splitter was used between the second-dimension column and the mass spectrometer with a splitting ratio of 8 (MS system):100 (waste) so that the flow was within the limit of the quadrupole time-of-flight mass spectrometer.
Hydrophilic interaction liquid chromatography (HILIC)/ESI-MS chromatograms: a overlay of RIC chromatograms of the mixture of lipid standards; b total ion current chromatogram of the lipid extract of human plasma. The HILIC conditions are reported in “Experimental”
Identification of individual lipids based on ESI mass spectra
The ionization and fragmentation behavior of individual lipid classes and species within classes are well known from previous works [7–11, 15], so this knowledge was applied for the identification of individual lipids in this work. Table S1 reports only unambiguous identifications for human plasma (Fig. 3a) and porcine brain (Fig. 3b) samples; no tentative suggestions without confident conclusions are reported here. The slash separator shows the known preference of the sn position according to the shorthand lipid notation suggested by Liebisch et al. [37], but it does not refer to a 100 %/0 % ratio of possible lipid regioisomers, because in most cases both regioisomers are present in a mixture with a certain stereospecific preference. The underscore separator is used when sn position is not known. The following essential requirements are taken into account for the identification of the lipids reported in Table 1: (1) retention times in both dimensions determined using RIC chromatograms, (2) accurate m/z values (mostly better than 5 ppm), and (3) characteristic fragmentation behavior in positive-ion and/or negative-ion MS/MS spectra depending on the signal. The characteristic fragment ion for all lipid classes containing a choline moiety (PC, SM, and LPC) is m/z 184, which can be used for better visualization of these classes using the RIC of m/z 184 in a 2D projection, as shown in Fig. 4, chromatogram A for human plasma and Fig. S3, chromatogram A for porcine brain. The fragment ion m/z 369 is characteristic of all lipid species containing cholesterol (Fig. 4, chromatogram B and Fig. S3, chromatogram B). ESI mass spectra of ceramides, hexosyl ceramides, and dihexosyl ceramides exhibit characteristic ions related to the type of base, e.g., m/z 264 for 18:1 and m/z 266 for 18:0, which makes possible the accurate identification of fatty acyl positions (Table 1). The neutral loss of Δm/z 141 is observed in positive-ion ESI mass spectra of PE, whereas negative-ion ESI-MS/MS provides information on the positions of attached fatty acyls based the ratio of [R1COO]-and [R2COO]- ions, where the [R2COO]- ion is more abundant. This ratio changes for the combination of saturated and highly polyunsatured (C20:5 or C22:6) fatty acyls in PE owing to the formation of [Ri]- ions from [RiCOO]- ions caused by the neutral loss of carbon dioxide [42]. The interpretation of positive-ion ESI mass spectra of TG is based on [M + NH4]+ and [M + H − RiCOOH]+ ions.
Two-dimensional liquid chromatography (2D-LC)/ESI-MS chromatograms of total lipid extracts: a human plasma; b porcine brain. The conditions for the first dimension (D1) and the second dimension (D2) are identical to those for Figs. 1 and 2, respectively. The annotation of the peak numbers corresponds to the numbers in Table 1. Cer ceramides, HexCer hexosyl ceramides, Hex2Cer dihexosyl ceramides
Continuous comprehensive 2D-LC/MS of lipids
The optimized continuous comprehensive 2D-LC method was applied for the analysis of human plasma (Fig. 3a) and porcine brain (Fig. 3b) lipidomic extracts. Human plasma is one of the most frequently analyzed biofluids, with the potential for biomarker screening of some diseases. The lipidome of brain tissue is a rather complex biological tissue containing numerous lipid classes, including ceramides and hexosylated ceramides. Use of porcine brain is preferred over use of human brain for ethical reasons. We visualized 2D chromatograms with GCImage using the conventional color scheme, where red corresponds to the most abundant species, and orange, yellow, and green correspond to species of decreasing abundance (Figs. 3 and 4, S3). The absence of a peak is represented by white. Figures 4 and S3 illustrate the potential of RIC chromatograms for better visualization of selected compound classes with common features in their mass spectra; for example, Fig. 4, chromatogram A and Fig. S3, chromatogram A highlight lipid classes containing a choline moiety (PC, SM, and LPC) using the RIC of m/z 184, whereas Fig. 4, chromatogram B and Fig. S3, chromatogram B show the RIC of m/z 369, which is characteristic of cholesterol and CE. Table 1 shows the individual lipid species identified in the samples studied. In total, we identified 97 lipid species from seven different lipid classes (LPC, cholesterol, SM, PC, PE, TG, and CE) in the human plasma sample and 115 lipid species from ten different lipid classes (LPC, ST, ceramides, hexosyl ceramides, dihexosyl ceramides, SM, PC, PE, TG, and CE) in the porcine brain sample. The typical fatty acyls occurring in glycerolipids, GP, SP, and ST are 16:0, 16:1, 18:0, 18:1, 18:2, 20:0, 20:3, 20:4, 20:5, and 22:6. Highly polyunsaturated fatty acyls with five or six DB are not common for TG, but 18:3 is quite common and shorter fatty acyls 12:0 and 14:0 may occur as well. The occurrence of very long fatty acyls is observed for ceramides and hexosylated ceramides, such as 23:0, 24:0, 24:1, 26:0, and 26:1 (see Table 1 for details).
Conclusions
This work shows for the first time the application of continuous comprehensive 2D-LC/ESI-MS for the complex lipidomic characterization of polar and nonpolar lipid classes in human plasma and porcine brain samples. The logical combination for 2D-LC separation of lipids is species separation in one dimension (RP mode) and lipid class separation in another dimension (HILIC mode). An RP-UHPLC column with sub-2-μm particles was used in the first dimension to obtain the best separation of the maximum number of lipid species according to acyl chain lengths and the number of DB, and the fast HILIC analysis in the second dimension allows the separation of overlapped lipids in the first dimension on the basis of their different polarities. The advantages of our continuous comprehensive 2D-LC/MS method are the shorter analysis time and easier automation, but a drawback is the reduced number of identified lipids in comparison with off-line and stopped-flow 2D-LC/MS configurations. The present work is a proof of concept of possible application of continuous comprehensive 2D-LC/MS in lipidomics, but its implementation into quantitative lipidomic workflows requires further research and system improvements. Another aspect for future improvement is the sensitivity, because well-optimized one-dimensional LC/MS yields a higher number of identified lipid species compared with 2D-LC/MS systems using the identical sample [14, 15, 27].
References
LIPID MAPS (2014) LIPID MAPS Lipidomics Gateway. http://www.lipidmaps.org/. Accessed 2 Dec 2014
Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Murphy RC, Raetz CRH, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA (2005) A comprehensive classification system for lipids. Eur J Lipid Sci Technol 107:337–364
Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CRH, Shimizu T, Spener F, van Meer G, Wakelam MJO, Dennis EA (2009) Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res 50:S9–S14
Santos CR, Schulze A (2012) Lipid metabolism in cancer. FEBS J 279:2610–2623
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124
Oresič M, Hanninen VA, Vidal-Puig A (2008) Lipidomics: a new window to biomedical frontiers. Trends Biotechnol 26:647–652
Li M, Yang L, Bai Y, Liu HW (2014) Analytical methods in lipidomics and their applications. Anal Chem 86:161–175
Han XL, Gross RW (2005) Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev 24:367–412
Han X, Yang K, Gross RW (2012) Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev 31:134–178
Papan C, Penkov S, Herzog R, Thiele C, Kurzchalia T, Shevchenko A (2014) Systematic screening for novel lipids by shotgun lipidomics. Anal Chem 86:2703–2710
Sandra K, Pereira AD, Vanhoenacker G, David F, Sandra P (2010) Comprehensive blood plasma lipidomics by liquid chromatography/quadrupole time-of-flight mass spectrometry. J Chromatogr A 1217:4087–4099
Holčapek M, Cífková E, Červená B, Lísa M, Vostálová J, Galuszka J (2015) Determination of nonpolar and polar lipid classes in human plasma, erythrocytes and plasma lipoprotein fractions using ultrahigh-performance liquid chromatography-mass spectrometry. J Chromatogr A 1377:85–91
Sokol E, Almeida R, Hannibal-Bach HK, Kotowska D, Vogt J, Baumgart J, Kristiansen K, Nitsch R, Knudsen J, Ejsing CS (2013) Profiling of lipid species by normal-phase liquid chromatography, nanoelectrospray ionization, and ion trap–orbitrap mass spectrometry. Anal Biochem 443:88–96
Cífková E, Holčapek M, Lísa M, Ovčačíková M, Lyčka A, Lynen F, Sandra P (2012) Nontargeted quantitation of lipid classes using hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry with single internal standard and response factor approach. Anal Chem 84:10064–10070
Cífková E, Holčapek M, Lísa M (2013) Nontargeted lipidomic characterization of porcine organs using hydrophilic interaction liquid chromatography and off-line two-dimensional liquid chromatography-electrospray ionization mass spectrometry. Lipids 48:915–928
Lísa M, Netušilová K, Franěk L, Dvořáková H, Vrkoslav V, Holčapek M (2011) Characterization of fatty acid and triacylglycerol composition in animal fats using silver-ion and non-aqueous reversed-phase high-performance liquid chromatography/mass spectrometry and gas chromatography/flame ionization detection. J Chromatogr A 1218:7499–7510
Adlof RO (1997) Normal-phase separation effects with lipids on a silver ion high-performance liquid chromatography column. J Chromatogr A 764:337–340
Holčapek M, Dvořáková H, Lísa M, Girón AJ, Sandra P, Cvačka J (2010) Regioisomeric analysis of triacylglycerols using silver-ion liquid chromatography–atmospheric pressure chemical ionization mass spectrometry: comparison of five different mass analyzers. J Chromatogr A 1217:8186–8194
Lísa M, Holčapek M (2013) Characterization of triacylglycerol enantiomers using chiral HPLC/APCI-MS and synthesis of enantiomeric triacylglycerols. Anal Chem 85:1852–1859
Murphy SA, Nicolaou A (2013) Lipidomics applications in health, disease and nutrition research. Mol Nutr Food Res 57:1336–1346
Römpp A, Spengler B (2013) Mass spectrometry imaging with high resolution in mass and space. Histochem Cell Biol 139:759–783
Dugo P, Cacciola F, Kumm T, Dugo G, Mondello L (2008) Comprehensive multidimensional liquid chromatography: theory and applications. J Chromatogr A 1184:353–368
François I, Sandra K, Sandra P (2009) Comprehensive liquid chromatography: Fundamental aspects and practical considerations—a review. Anal Chim Acta 641:14–31
Česla P, Hájek T, Jandera P (2009) Optimization of two-dimensional gradient liquid chromatography separations. J Chromatogr A 1216:3443–3457
D'Attoma A, Grivel C, Heinisch S (2012) On-line comprehensive two-dimensional separations of charged compounds using reversed-phase high performance liquid chromatography and hydrophilic interaction chromatography. Part I: orthogonality and practical peak capacity considerations. J Chromatogr A 1262:148–159
Jandera P, Hájek T, Staňková M, Vyňuchalová K, Česla P (2012) Optimization of comprehensive two-dimensional gradient chromatography coupling in-line hydrophilic interaction and reversed phase liquid chromatography. J Chromatogr A 1268:91–101
Lísa M, Cífková E, Holčapek M (2011) Lipidomic profiling of biological tissues using off-line two-dimensional high-performance liquid chromatography mass spectrometry. J Chromatogr A 1218:5146–5156
Holčapek M, Velínská H, Lísa M, Česla P (2009) Orthogonality of silver-ion and non-aqueous reversed-phase HPLC/MS in the analysis of complex natural mixtures of triacylglycerols. J Sep Sci 32:3672–3680
Dugo P, Kumm T, Crupi ML, Cotroneo A, Mondello L (2006) Comprehensive two-dimensional liquid chromatography combined with mass spectrometric detection in the analyses of triacylglycerols in natural lipidic matrixes. J Chromatogr A 1112:269–275
Yang Q, Shi X, Gu Q, Zhao S, Shan Y, Xu G (2012) On-line two dimensional liquid chromatography/mass spectrometry for the analysis of triacylglycerides in peanut oil and mouse tissue. J Chromatogr B 895–896:48–55
Ling YS, Liang HJ, Lin MH, Tang CH, Wu KY, Kuo ML, Lin CY (2014) Two-dimensional LC-MS/MS to enhance ceramide and phosphatidylcholine species profiling in mouse liver. Biomed Chromatogr 28:1284–1293
Sun C, Zhao Y-Y, Curtis JM (2014) Elucidation of phosphatidylcholine isomers using two dimensional liquid chromatography coupled in-line with ozonolysis mass spectrometry. J Chromatogr A 1351:37–45
Dugo P, Fawzy N, Cichello F, Cacciola F, Donato P, Mondello L (2013) Stop-flow comprehensive two-dimensional liquid chromatography combined with mass spectrometric detection for phospholipid analysis. J Chromatogr A 1278:46–53
Wang SY, Li J, Shi XZ, Qiao LZ, Lu X, Xu GW (2013) A novel stop-flow two-dimensional liquid chromatography-mass spectrometry method for lipid analysis. J Chromatogr A 1321:65–72
Li M, Feng BS, Liang Y, Zhang W, Bai Y, Tang W, Wang T, Liu HW (2013) Lipid profiling of human plasma from peritoneal dialysis patients using an improved 2D (NP/RP) LC-QToF MS method. Anal Bioanal Chem 405:6629–6638
Bang DY, Moon MH (2013) On-line two-dimensional capillary strong anion exchange/reversed phase liquid chromatography-tandem mass spectrometry for comprehensive lipid analysis. J Chromatogr A 1310:82–90
Liebisch G, Vizcaino JA, Kofeler H, Trotzmuller M, Griffiths WJ, Schmitz G, Spener F, Wakelam MJO (2013) Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res 54:1523–1530
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Holčapek M, Jandera P, Fischer J, Prokeš B (1999) Analytical monitoring of the production of biodiesel by high-performance liquid chromatography with various detection methods. J Chromatogr A 858:13–31
Holčapek M, Jandera P, Zderadička P, Hrubá L (2003) Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 1010:195–215
Lísa M, Holčapek M (2008) Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 1198–1199:115–130
Berdeaux O, Juaneda P, Martine L, Cabaret S, Bretillon L, Acar N (2010) Identification and quantification of phosphatidylcholines containing very-long-chain polyunsaturated fatty acid in bovine and human retina using liquid chromatography/tandem mass spectrometry. J Chromatogr A 1217:7738–7748
Acknowledgments
This work was supported by ERC CZ project no. LL1302 sponsored by the Ministry of Education, Youth, and Sports of the Czech Republic. E.C. acknowledges the support of project no. CZ.1.07/2.3.00/30.0021 sponsored by the Ministry of Education, Youth, and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Lipidomics with guest editor Michal Holčapek.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 461 kb)
Rights and permissions
About this article
Cite this article
Holčapek, M., Ovčačíková, M., Lísa, M. et al. Continuous comprehensive two-dimensional liquid chromatography–electrospray ionization mass spectrometry of complex lipidomic samples. Anal Bioanal Chem 407, 5033–5043 (2015). https://doi.org/10.1007/s00216-015-8528-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8528-2