Abstract
The occurrence of 26 commonly used cytostatic compounds in wastewaters was evaluated using an automated solid-phase extraction (SPE) method with liquid chromatography–high-resolution mass spectrometry (LC–HRMS). Detection was optimized using Oasis HLB SPE cartridges at pH 2. Two hospital effluents and their two receiving wastewater treatment plants were sampled over five days. In hospital effluents, eight cytostatics were detected at levels up to 86.2 μg L−1 for ifosfamide, 4.72 μg L−1 for cyclophosphamide, and 0.73 μg L−1 for irinotecan, the three most relevant compounds identified. Cyclophosphamide and megestrol acetate were found in wastewaters at concentrations up to 0.22 μg L−1 for the latter. The predicted environmental concentrations (PEC) in sewage effluents of ifosfamide (2.4–4.3 ng L−1), capecitabine (11.5–14.2 ng L−1), and irinotecan (0.4–0.6 ng L−1), calculated from consumption data in each hospital, published excretion values for the target compounds, and wastewater elimination rates, were in agreement with experimental values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals designed for hospital use are suspected to have more risks than other pharmaceuticals regarding their effect on the aquatic environment. Among these pharmaceuticals, drugs used for cancer treatment have received the most attention because of their potential cytotoxicity, genotoxicity, mutagenicity, and teratogenicity [1]. These compounds are classified in the anatomical therapeutical classification (ATC) scheme by the WHO (www.whocc.no/atcddd) under class L, which covers antineoplastic and immunomodulating agents. Two main subgroups are currently used: antineoplastic drugs (class L01) and endocrine therapy (class L02). The L01 subgroup is subdivided into alkylating agents (L01A), antimetabolites (L01B), plant alkaloids and natural products (L01C), cytotoxic antibiotics and related substances (L01D), and other antineoplastic agents (L01X), and class L02 has the two subdivisions L02A and L02B, for hormones and hormone antagonists, respectively. Hospital effluents, which are rarely subject to any treatment, are regarded as one of the sources of pharmaceuticals in the sewage networks. In this context, the contribution to wastewaters of pharmaceuticals [2–6] and cytostatics [7–11] originating from hospitals has been investigated. Recent work has revealed a limited contribution of hospitals to the load of pharmaceuticals. Ort et al. and Verlicchi et al. studied 59 and 73 pharmaceuticals, respectively, and proved that the contribution of hospitals to the wastewater load was usually below 15 % [2, 12]. Le Corre et al. found the same percentage (15 %) for 63 to 84 % of pharmaceuticals investigated in six Australian hospitals [13], and Langford and Thomas revealed that the contribution of studied pharmaceuticals to the wastewater load was for the most part only 1–2 % [5].
European hospitals typically use 50 different active substances to treat oncology patients [14]. When studying the contribution of cytostatics, it must be taken into account that approximately 75 % of oncology patients are outpatients, receiving treatment at oncology wards and leaving for home after the infusion or injection has been administered [10, 15, 16]. These patients may excrete part of the cytostatics in the hospital, because the treatment takes up to two hours, and the pharmacokinetics of some cytostatics is relatively fast. In an exhaustive study, Besse et al. calculated the theoretical input pathways for anticancer drugs in the aquatic environment from both French national data consumption and a specialized cancer center. The study reports that 86.2 % of delivered drugs enter WWTP from the sewer system, whereas 13.8 % come from hospital effluents, noting that 80 % of cancer drugs delivered to outpatients are consumed and excreted outside hospital premises [17]. A shift in consumption patterns was observed for almost all classes of anticancer drug, and notably for antimetabolites, in France between 2004–2008: during this time, the amount of anticancer drugs delivered in hospitals dropped from 82 % to 35 % [17]. The concentrations of cytostatics measured at hospitals can account for a moderate percentage of total consumption [7] because, most often, oncological treatments are administered at the hospital and patients then leave for home, meaning their household discharges are another way in which cytostatics enter the aquatic environment [14, 18]. Thus, Weissbrodt et al. proved that only 5.5 % of cytostatics were excreted within the hospital [7]. Cytostatics which are excreted as both parent compounds and metabolites have been reported to have low biodegradability and poor removal with both conventional and advanced wastewater treatments [10, 19, 20], leading to their identification in surface waters [14, 15, 17, 21–24].
Since the studies of Steger-Hartmann et al., who determined cyclophosphamide and ifosfamide in hospital effluents by use of solid-phase extraction (SPE) as the preconcentration step followed by gas chromatography coupled to mass spectrometry (GC–MS) [25], other compounds including tamoxifen and 5-fluorouracil have also been analyzed by GC–MS [26, 27]. More recently, analysis of cytostatics has used liquid chromatography coupled to tandem mass spectrometry (LC–MS–MS) [8, 11, 14, 15, 20–22, 28–33] and, lately, LC–high resolution mass spectrometry (HRMS) using Orbitrap instruments [29, 30].
Spain, after the USA, has produced the second-highest number of studies on the presence of pharmaceuticals in the aquatic environment, determined on the basis of data collected from river systems around the world [34]. However, there are very few papers dealing with the presence of cytostatics in Spanish water samples. Table 1 summarizes the levels found in these studies, using the ATC classification scheme as a framework to organize the data, as suggested by Daughton [35]. Sixteen cytostatics have been reported, mainly from hospital effluents and wastewaters and less frequently from Spanish river waters [8, 21, 22, 30, 36–39]. Of these, Martin et al. identified six out of 14 compounds in wastewater [22]; Ferrando-Climent et al. determined nine compounds in hospital effluents and wastewater influents [8]; and Negreira et al. identified five cytostatics out of 17 investigated in wastewaters [21]. These are among the few worldwide studies dealing with multiresidue methods for investigating these compounds [11, 14, 31]. Tamoxifen, cyclophosphamide, and ifosfamide are the most studied cytostatics in Spain, and global reviews of their presence in the aquatic environment have also been recently published [14, 17, 24]. Tamoxifen seemed to be the most ubiquitous of the compounds studied, with values ranging from 12.4– 20.1 ng L−1 in the Ebro river [37], 11.2–223 ng L−1 in groundwater underlying Barcelona [36], and up to 17.2 ng L−1 in raw WWTP [28, 38]. Cyclophosphamide and ifosfamide were not found in river waters [22] but were present in influent and effluent wastewaters at ng L−1–μg L−1 levels [8, 21, 28, 30], with a maximum concentration of 13.1 μg L−1 [30]. Predicted environmental concentrations (PEC) for cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine in sewage effluents and surface waters from a variety of European countries have been recently reported [23], and PEC for 13 cytostatics in drinking waters of the Thames catchment in the UK [40]. An estimated risk assessment and prediction of cytostatics concentrations for surface and drinking waters has also been reported [16, 23, 40–43].
In view of the scarce data on the occurrence of cytostatics in the aquatic environment of our country, the objective of this work was to determine the occurrence of 23 commonly used cytostatic drugs, belonging to six different ATC classes (L01 and L02), and of three miscellaneous compounds (ATC codes G03 and H02) in two hospital effluents and in wastewaters receiving these hospital effluents. Nine of them (chlorambucil, melphalan, fludarabine, vinblastine, vincristine, leuprolide, goserelin, aminoglutethimide, and cyproterone) have not been previously studied in the aquatic environment. The data obtained were used, with publicly available consumption data, published excretion values for the target compounds, and wastewater elimination rates, to predict the range of concentrations in influent and effluent wastewaters.
Experimental
Chemicals and materials
Twenty-six pure analytical standards of 98–99 % purity were acquired from Sigma-Aldrich (St. Louis, USA) and from Toronto Research Chemicals, TRC (Ontario, Canada). Cyclophosphamide-d4 (Santa Cruz Biotechnology, USA) was used as internal standard (IS). All the target compounds, molecular formulae, and relevant physicochemical properties are shown in Table 2. Their chemical structures are displayed in Fig. S1 (Electronic Supplementary Material). Stock standard solutions were prepared at a concentration of 1000 ng μL−1 in methanol (MeOH), except for: cyclophosphamide, aminoglutethimide, irinotecan, megestrol acetate and prednisone, which were prepared in chloroform; cyproterone, which was prepared in dichloromethane (DCM); and chlorambucil, which was prepared in acetone. Working solutions were prepared at 10 and 100 ng μL−1. MeOH, DCM, acetonitrile (ACN), ethyl acetate (EtOAC), acetone (SupraSolv grade), and HPLC water (LiChrosolv grade) were supplied by Merck (Darmstadt, Germany). Formic acid (HCOOH), ammonium hydroxide (NH4OH), and ammonium acetate (NH4OAc) were supplied by Sigma-Aldrich (St. Louis, MO USA). When preparing standards, an exhaustive control on handling procedures, storage conditions, and safety rules was followed, as specified by manufacturers. SPE cartridges, Oasis HLB, and Oasis MCX (6 cc, 200 mg) were purchased from Waters (Mildford, MA, USA). Isolute C18 and ENV + (3 cc, 100 mg) were supplied by Biotage (Uppsala, Sweden).
Sampling procedure and sample preparation
Sampling was performed in July 2013 and comprised wastewaters from two hospitals and from the two WWTP that receive the untreated wastewaters from each hospital. Hospital A is the largest hospital complex in Catalonia and one of the largest in Spain. It is located in the north of Barcelona, has over 1100 beds (60 beds for oncology patients) and performs chemotherapy and radiotherapy treatments every day. The effluent from this hospital is discharged untreated to the Barcelona sewage grid and directed to WWTP A, situated 10 km away. This WWTP treats a flow of 525,000 m3 day−1, corresponding to 2,843,750 inhabitants-equivalent (inhab-eq), and treated waters are finally discharged to the Mediterranean Sea. It treats 65 % of wastewaters from Barcelona city and different municipalities around the capital, and performs biological treatment without nitrogen and phosphorous removal. Hospital B, located in the south of Barcelona, specializes in oncology and has over 450 beds (~80 beds for oncology patients). Wastewaters generated in this hospital are also discharged untreated to the municipal sewage grid and are directed to WWTP B, which is located in the south of Barcelona and, similarly to WWTP A, performs biological treatment without nitrogen and phosphorous removal. It treats a flow of 420,000 m3 day−1, corresponding to 2,275,000 inhab-eq, and effluents are discharged to the Mediterranean Sea. Hospital wastewater samples were collected at different times of day (9 a.m., 11 a.m., 1 p.m., and 3 p.m. for hospital A, and 11 a.m. and 3 p.m. for hospital B), according to the chemotherapy and radiology treatment schedules of each hospital (Table S1, Electronic Supplementary Material). Sampling was repeated on five different days to evaluate the intra-day variability. During the same days, the 24 h composite influent and effluent of both WWTP receiving hospital waters were sampled. Once in the laboratory, samples were kept at 4 ºC and processed within 24–48 h, following previous studies that reported low stability of this class of compounds in water [28, 44]. Samples were centrifuged at 4,000 rpm for 10 min and filtered with 1 μm nylon membrane filters (Whatmann, Sigma-Aldrich, St. Louis, MO USA), and then further filtered with 0.45 μm nylon membrane filters (Whatmann, Sigma-Aldrich, St. Louis, MO USA). Next, samples were acidified at pH 2 with HCl 0.1 N and then extracted using an automated solid-phase extraction apparatus (Dionex Autotrace 280, Thermo Scientific). Because of the high toxicity risk of the samples and of this class of pharmaceuticals, strict safety precautions were undertaken. Standard solution preparation and sampling were performed under a hood, and lab coat, gloves, goggles, and masks of 3rd-level safety were used by all people in contact with the samples.
Extraction method
Method performance was tested first, using Milli-Q water spiked at 0.1 μg L−1 with the target compounds to evaluate the efficiency of the different SPE cartridges: Oasis HLB (6 cc, 200 mg) polymeric reversed-phase sorbent; Oasis MCX (6 cc, 200 mg) mixed-mode cation-exchange sorbent; Isolute ENV + (3 cc, 100 mg) hydroxylated polystyrene–divinylbenzene copolymer; and Isolute C18 (3 cc, 100 mg). 100 mL water was spiked with 10 ng internal standard (IS), which was used as sample control. All cartridges were conditioned following the same procedure: 6 mL MeOH and 6 mL H2O with 100 mmol L−1 NH4OAc was loaded at 2 mL min−1, and the sample was then loaded at a flow of 1 mL min−1. Once preconcentrated, Oasis HLB and ENV + cartridges were washed with 6 mL 100 mmol L−1 NH4OAc in H2O, dried over 30–45 min and eluted with 6 mL MeOH and 6 mL HCOOH:MeOH (5:95). Oasis MCX and C18 cartridges were washed with 6 mL HCOOH:MeOH (5:95), dried over 30–45 min, and eluted using 6 mL MeOH and 6 mL HCOOH:MeOH (5:95). The different SPE procedures are summarized in Table S2, Electronic Supplementary Material. Samples were then evaporated to almost-dryness in a TurboVap under a current of N2 at 25 ºC, and transferred to a 2 mL chromatographic vial with 1 mL ACN as washing solvent. Finally, samples were evaporated to dryness and reconstituted to 500 μL using a 50:50 mixture (0.1 % HCOOH in ACN and 0.1 % HCOOH in HPLC water). Oasis HLB provided better performance and, consequently, was chosen for the analysis of hospital effluents and wastewaters. The suitability of the method was further evaluated with filtered wastewater spiked with all the cytostatic compounds at 0.1 μg L−1, using Oasis HLB. Three different pHs (2, 3.5, and 7) were then tested to refine the extraction conditions of these compounds in wastewater.
LC-Orbitrap-MS analysis
Cytostatic compounds were measured using liquid chromatography coupled to high-resolution mass spectrometry (LC–Orbitrap-MS). An Orbitrap/Exactive mass spectrometer equipped with a heated electrospray ionization (H-ESI) source was used, from Thermo Fischer Scientific (Bremen, Germany). The system was equipped with an HTC PAL autosampler and a Surveyor MS Plus pump. A Luna C18 column (150 mm × 2 mm ID, particle size 5 μm, Phenomenex, Torrance, USA) was chosen on the basis of a previous optimization study [29]. The mobile phase composition consisted of binary mixtures of 0.1 % HCOOH in water (A) and 0.1 % HCOOH in acetonitrile (B). Gradient elution started at 95 % A and 5 % B, increased to 70 % B in 30 min, then increased to 100 % B in 1 min, and then held for 10 min. Initial conditions were attained in 4 min and the system was stabilized for 5 min. The flow was set at 200 μL min−1 and 10 μL was injected. Cytostatics were measured under positive electrospray ionization (ESI+). Full scan acquisition was performed over a mass range of 50–1000 Da at 50,000 full width at half maximum (FWHM), with the spray voltage at 3.5 kV, capillary voltage at 30 V, skimmer voltage at 28 V, and tube lens voltage at 130 V. Mass spectral characterization of cytostatic compounds is indicated in Table S3, Electronic Supplementary Material. Different confirmation criteria were established to ensure unequivocal identification of target compounds, preventing false positives. On the basis of Directive 2002/657/EC, the criteria used were:
-
1.
the retention time shift between the standards and the samples should be lower than 2 %;
-
2.
the accurate mass measurements of the molecular and the product ions should have an error <5 ppm, with a high resolving power of 50,000 FWHM, m/z 200;
-
3.
the four decimal numbers should be used to identify precursor and fragment ions; and
-
4.
when possible, the isotopic pattern should have a better than 90 % fit to be accepted as a positive sample [45].
Quality assurance
Calibration was performed over a concentration range from 0.001 to 2 ng μL−1, using 13 calibration points. Cyclophosphamide-d4 was used as IS at 0.1 ng μL−1 as extraction and analytical control. External standard quantification was performed. The instrumental detection limit (IDL) was calculated as the concentration giving a signal intensity of 1 × 103, and afterwards calculated experimentally by injecting a standard concentration that gave this signal intensity. The method detection limit (MDL) was calculated following the same procedure, using spiked wastewater samples at a concentration of 0.1 μg L−1. Intra-assay variation was assessed using five consecutive injections of 1 ng μL−1 standard solution, and inter-assay variation was determined by measuring the same standard solution on four different days. Solvent blanks did not contain any of the investigated analytes, indicating no carry-over effect during LC-Orbitrap runs. Recovery studies were performed in triplicate, using a water sample which consisted of a mixture of influent and effluent wastewater (1:1) spiked at 0.1 μg L−1 with the cytostatic mixture and the IS. Wastewater was previously analyzed and no traces of target compounds were detected. Table S4 (Electronic Supplementary Material) displays the quality characteristics obtained by LC–Orbitrap-MS.
Model used for calculated predicted environmental concentration
A preliminary exposure assessment was implemented by calculating predicted environmental concentrations (PEC), adapting the equation described by Besse et al. [46] to our study. Eq. (1):
PEC are calculated in ng L−1, using the following variables:
consumption is the amount (g day−1) of an active drug consumed by the population over one day in a defined area, in our case in two different hospitals. The Catalan Health Service (CatSalut) provided data as defined daily dose (DDD) of three cytostatic drugs administered during the week of sampling.
% excretion is the excreted fraction of the original drug.
% WWTP removal is the fraction of emission of the drug from WWTP directed to surface water, which can be defined as (1 − WWTP removal fraction). In most cases, WWTP removal fractions were not available and therefore we assume a % WWTP removal value of 0, which corresponds to a worst-case scenario (i.e. no removal by WWTP).
WWTP effluent flow (m3 day−1) is the mean volume of wastewater that each WWTP generates per day.
Results and discussion
Quality characteristics and identification criteria
Quality characteristics of the method are shown in Table 3. Internal standard calibration was used to correct for MS responses and to ensure quantification performance. Good correlation coefficients (R 2 > 0.99) were obtained for 25 compounds. Ten cytostatic compounds were linear from 0.001 to 2 ng μL−1; for the other 10 target compounds, linearity ranged from 0.005 to 2 ng μL−1; cytarabine and etoposide were linear in the range 0.01 to 2 ng μL−1; goserelin and gemtabicyne from 0.02 to 2 ng μL−1; and leuprolide and imatinib in the range 0.05 to 2 ng μL−1. The IDL ranged from 0.005 to 0.25 ng, and intra and inter-day precisions ranged from 0.9 to 20 % and from 0.6 to 21 %, respectively (Table 3).
Using Milli-Q water (pH 2) spiked at 0.1 μg L−1 and C18 SPE-cartridges, 13 cytostatic compounds were recovered within the range 60 ± 4 % to 119 ± 15 %; whereas when Isolute ENV + was used, only nine target compounds were recovered in the range 60 ± 5 % to 121 ± 9 %. Similar results were obtained using Oasis MCX, with only 10 cytostatic compounds recovered, in the range 62 ± 2 % to 108 ± 2 % (Table 3). The best performance was obtained when Oasis HLB was used, with 24 cytostatic compounds recovered in the range 40 ± 1 % to 133 ± 6 %. Aminoglutethimide had poor recovery (29 ± 8 %), and imatinib was not recovered.
The suitability of Oasis HLB was further tested for analysis of wastewater spiked at 1 μg L−1 at three different pHs. At pH 7, 17 cytostatic compounds were detected with recovery values >40 %, whereas at pH 2 and pH 3.5, 19 compounds were effectively recovered (Fig. S2, Electronic Supplementary Material). Because of the higher number of target compounds recovered, pH 2 was chosen for the analysis of hospital effluents and wastewaters. However, cytarabine, gemcitabine, etoposide, paclitaxel, docetaxel, imatinib, and aminoglutethimide either were not recovered or had a recovery below 25 %, and therefore were not included in the analytical method (Table 3). The MDL ranged from 0.7 (tamoxifen) to 61 ng L−1 (daunorubicin) with the exception of that of fludarabine, for which sensitivity was very low (MDL: 164 ng L−1).
Occurrence of cytostatic compounds in hospital effluents
Out of 19 cytostatic compounds, seven were detected in hospital effluents at the low or sub-μg L−1 range: cyclophosphamide, ifosfamide, epirubicin, capecitabine, irinotecan and megestrol acetate, and prednisone. For the last four compounds, this is the first time they have been detected in Spanish hospital effluents. Table 4 summarizes the levels of the cytostatics found in the two hospital effluents, with ifosfamide and irinotecan being present in the highest number of samples. Figure 1 displays, as an example, the LC–HRMS chromatograms of two hospital A samples with the detected cytostatic compounds.
LC–HRMS ion chromatogram for (a–e) hospital A effluent (VH1 sample) and (f) hospital A effluent (VH8 sample). Codes are shown in Table SI1
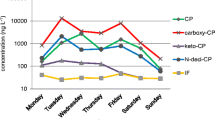
For hospital A ifosfamide, with an established elimination half-life of 6–8 h [24], was present in 17 out of 20 samples, at levels ranging from 0.006 to 86.2 μg L−1 (mean 12.3 μg L−1; median 0.85 μg L−1). The concentration of 86.2 μg L−1 is the highest reported in the literature [24]. On the basis of a daily effluent flow of 3.61 m3 and this maximum effluent concentration, 0.31 g day−1 of ifosfamide were released from hospital A. On the basis of consumption values and percentage excreted, 1.26 g day−1 was calculated, 25 % of which was from hospital cancer patients and the rest from outpatients. The maximum reported level has been found in China (10.64 μg L−1) [11]. High daily variations of ifosfamide were measured during the 9 a.m.–3 p.m. period; this is when the oncological treatments are performed and thus when the greatest amount of the parent compounds is expected to be excreted. In contrast, ifosfamide was detected only in one sample in hospital B, at 2.69 μg L−1. This can be explained by the fact that this hospital has mainly outpatients, whereas hospital A has a substantial number of hospitalized patients. Recently, Ferrando-Climent et al. analyzed 10 cytostatic drugs in three hospitals from Spain and Portugal. In this study, ifosfamide levels ranged from 0.031 ± 0.075 μg L−1 to 0.23 ± 0.013 μg L−1 and cyclophosphamide from 0.035 ± 0.055 μg L−1 to 0.043 ± 0.045 μg L−1 [8]. In our study, cyclophosphamide was present in eight out of 20 samples, with concentrations up to 4.72 μg L−1 (mean and median 0.33 and 0.17 μg L−1, respectively), from hospital A, but only detected in one sample (0.01 μg L−1) from hospital B. The administered dose of both alkylating agents (www.rxlist.com) is usually much higher than that for other cytostatics, and their wide range of use in chemotherapy for a variety of cancer led to their relatively high presence in hospital wastewaters.
Irinotecan was detected in 16 out of 20 samples in hospital A, at levels up to 0.73 μg L−1 (mean 0.08 μg L−1; median 0.02 μg L−1), 10 to 100 times lower than levels of ifosfamide (Table 4). Irinotecan not was detected in hospital B. A study performed in Norway [47] reported the occurrence and fate of irinotecan in a hospital effluent (0.015–0.035 μg L−1) and sewage treatment plant (STP) effluents (0.015–0.03 μg L−1); this is the only precedent study indicating the presence of irinotecan in environmental samples.
Megestrol (12 out of 20 samples) and prednisone (10 out of 20 samples) were frequently detected in samples from hospital A, in the range 0.04 and 1.26 μg L−1 and 0.01 and 0.21 μg L−1, respectively. Mean and median values of, respectively, 0.21 μg L−1 and 0.22 μg L−1 for megestrol and 0.03 μg L−1 and 0.02 μg L−1 for prednisone were obtained. In contrast, megestrol was detected in only one sample from hospital B (0.03 μg L−1) and prednisone was not detected (Table 4). These concentration levels are lower than those detected by other authors who analyzed several glucocorticoids in different hospitals of the Netherlands and detected prednisone in the range 0.117 ± 0.012 μg L−1 to 0.545 ± 0.058 μg L−1 (Schriks et al., 2010).
Capecitabine, the oral form of 5-fluorouracil [48], is usually administered to outpatients, and this may partially explain its low presence in the hospital effluents despite its relatively high consumption per day in both hospitals (Table 5). However, because capecitabine has an elimination half-life of 45 min [24] it is also to be expected that partial excretion takes place in hospitals. Capecitabine was only detected in two samples from hospital A, at 0.28 μg L−1 and 0.49 μg L−1.
Epirubicin was detected only in one sample from hospital B, at 0.06 μg L−1. Little information is available about the presence of this compound in hospital effluent and only Mahnik et al., in 2006, reported levels of this compound in the range 0.1 to 1.4 μg L−1 in a Vienna hospital [9], whereas it was not detected in a hospital effluent in Girona (Spain) [30].
The last compound, goserelin, was identified for the first time in one sample, at 0.35 μg L−1, in hospital A effluent.
Expected emission of cytostatic compounds
The predicted environmental concentrations (PEC) of ifosfamide, capecitabine, and irinotecan in wastewater effluents were calculated from daily consumption data provided by the two hospitals studied (CatSalut). Table 5 summarizes the results obtained. For megestrol and prednisone, no data was available. Calculations were also made taking into account the amount of drugs administered to patients receiving treatment, the removal rate of the drugs, and the effluent volume of each WWTP.
Data from excretion has been obtained from different sources, as described below. It has been reported that ifosfamide is excreted unchanged in the range 13–15 % [15]. For capecitabine the figure ranges from 2.6 % to 3.4 %, with a mean of 3.1 % [23, 49], and for irinotecan the excretion of unchanged product was in the range 11–20 % [24]. There are few reports on the removal of cytostatics in WWTP. Buerge et al. found no elimination of ifosfamide by STP [15]; for capecitabine, removal of 15 % was reported [50]; and for irinotecan no data was available and a worst-case scenario (0 % removal) was assumed.
PEC of ifosfamide in the wastewater effluents from hospital A and hospital B were 2.4 and 4.3 ng L−1, respectively. These values agree relatively well with different PEC reported in the literature for ifosfamide. An average value of ~1.4 ng L−1 (considering 13–15 % excretion and 0 % removal) was predicted in treated water [15], in good agreement with the PEC wastewater effluent range from 2 to 14 ng L−1 (12–90 % excretion and 0 % removal) reported by Tauxe-Wersch et al. [27] (both studies performed in Switzerland). Besse et al. reported a refined PEC (considering 50 % excretion and no removal) of 1.18 ng L−1 in France [17], whereas Kümmerer et al. reported German nationwide PEC in surface waters to range from 0.6 to 1.0 ng L−1, and PEC in surface waters receiving local STP effluent to range from 10.9 to 206 ng L−1 [42].
The calculated PEC of capecitabine for sewage effluents of the two studied hospitals ranged from 11.5–14.2 ng L−1. Recently, Johnson et al. reported PEC of capecitabine from different European nations with values between 8.5 ng L−1 (Norway) and 87 ng L−1 (Czech Republic), which were calculated on the basis of 3 % excretion of the parent compound and a mean of 50 % WWTP removal (range 25–75 %) [23]. A similar value, 23.1 ng L−1, was recently reported by Booker et al. in sewage effluents of NW England [51]. PEC in another English sewage effluents were studied for 11 cytostatics including capecitabine, for which a value of 13.7 ng L−1 was calculated on the basis of excretion of 7–11 % of the unchanged original drug in urine and a range of 92–99 % sewage treatment plant removal [40]. In France, the refined nationwide PEC of capecitabine in surface water was 3.52 ng L−1, calculated on the basis of 3 % excretion and no removal by WWTP. According to Besse et al., capecitabine is one of three cytostatics, together with hydroxycarbamide and 5-fluorouracil, having a conservative PEC (worst-case scenario, i.e. 100 % excretion and 0 % removal in WWTP) greater than 10 ng L−1 [17]. Our calculated PEC fairly agrees with those PEC published despite the very few and different WWTP removal rates used in the calculations.
For irinotecan, PEC in the two studied sewage effluents ranged from 0.4 to 0.6 ng L−1. Besse et al. reported a PEC value of >0.53 ng L−1, in a French national study assuming an excretion of the parent compound >50 % and 0 % removal in WWTP. To the best of our knowledge this is the only report available, and therefore the lack of data prevents any conclusion. On the basis of calculated PEC and of available pharmacological, metabolism, and biodegradation data, capecitabine and ifosfamide are included in the preferential list of anticancer drugs for the aquatic environment, whereas data is too scarce to determine whether irinotecan should be considered [17].
Occurrence of cytostatic compounds in WWTP
There were low levels of the cytostatic compounds in the two WWTP receiving the hospital effluents. Ifosfamide, the compound most often detected in the hospital effluents, was not detected in the influents of the two WWTP (<6 ng L−1). These results agree with its predicted PEC of 2.4–4.3 ng L−1. Ifosfamide was previously reported in Spanish WWTP at levels ranging from 7.3 to 43 ng L−1, in raw wastewater from a WWTP located in Catalonia (NE Spain) [21].
A similar observation can be made for capecitabine, with levels of <15 ng L−1 in both influents agreeing with the 11.2–14.2 ng L−1 predicted PEC for this compound. Capecitabine has already been detected in Spain, in a wastewater influent at a maximum concentration of 27 ng L−1 [21].
Irinotecan was not detected in either WWTP effluents (<4.5 ng L−1). However, improvement of the analytical method for this compound is required to detect values closer to its PEC, estimated to be in the 0.4–0.6 ng L−1 range for the two WWTP studied.
Cyclophosphamide was only detected in one sample out of 10 analyzed from the WWTP A influents. Although non-removal of cyclophosphamide by WWTP [15, 23, 40] has been reported, we measured 10 ng L−1 and 5 ng L−1 for the influent and effluent, respectively (Table 4). Ferrando-Climent et al. detected cyclophosphamide in Spanish wastewaters, at 25 ng L−1 in influent wastewater [8]. Johnson et al. reported a mean European concentration of cyclophosphamide in sewage effluents of 11 ng L−1 (maximum 40 ng L−1, in Sweden) [23], and reported 4–5.6 ng L−1 for a local German WWTP [42] and 70 ng L−1 in the Thames catchment [40], values which are not far from the maximum concentration level measured in the WWTP A effluent.
Megestrol acetate, the most common progestogen used in medicine, was detected in two influents and one effluent from WWTP A on two different days. Levels detected were 150 ng L−1 in the influent and 20 ng L−1 in the effluent (Table 4). Megestrol acetate was the only compound detected in WWTP B, at a maximum concentration of 220 ng L−1 in the influent. Guedes-Royco et al. studied the presence of megestrol acetate and other progestogens in wastewaters from Gran Canaria (Canary Islands, Spain) but none were detected [52]. In contrast, Chang et al. analyzed several synthetic progestogens in WWTP of Beijing, China, and detected megestrol acetate in all the influent wastewaters analyzed at levels of 41 ± 25 ng L−1, with a removal rate of 96 ± 9.4 % in the wastewater effluents [53].
Neither goserelin, epirubicin, nor prednisone, which were identified in several samples from hospital effluents, could be detected in WWTP influents. This indicates that cytostatic compounds are either degraded or diluted during passage through the sewage grid.
Concluding remarks
A comprehensive optimization of an automated SPE followed by LC–Orbitrap-HRMS was performed for the unequivocal identification of 19 cytostatic compounds in hospital effluents and wastewaters. Seven compounds (cyclophosphamide, ifosfamide, epirubicin, capecitabine, irinotecan, megestrol acetate, and prednisone) were detected in hospital effluents at levels ranging from 0.02 to 86.2 μg L−1, with ifosfamide, irinotecan, and megestrol acetate being the most ubiquitous compounds. Cyclophosphamide and megestrol acetate were the only compounds detected in the WWTP. From these results we can conclude that cytostatic compounds are metabolized, degraded by hydrolysis, or diluted during the sewage passage. To assess the estimated levels of cytostatic compounds in WWTP effluents, predicted environmental concentrations (PEC) of ifosfamide, capecitabine, and irinotecan were calculated. The predicted concentrations were in good agreement with measured concentrations of these three cytostatic compounds. Variations in the unchanged excreted amount of each cytostatic compound and the percentage of WWTP removal may change the PEC values. So, although PEC values can be used as a first approximation, measured environmental concentrations should be preferred for environmental risk assessment.
References
Kümmerer K (2001) Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources - A review. Chemosphere 45(6–7):957–969
Verlicchi P, Al Aukidy M, Galletti A, Petrovic M, Barceló D (2012) Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ 430:109–118
Santos LHMLM, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barceló D, Montenegro MCBSM (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci Total Environ 461–462:302–316
Jean J, Perrodin Y, Pivot C, Trepo D, Perraud M, Droguet J, Tissot-Guerraz F, Locher F (2012) Identification and prioritization of bioaccumulable pharmaceutical substances discharged in hospital effluents. J Environ Manag 103:113–121. doi:10.1016/j.jenvman.2012.03.005
Langford KH, Thomas KV (2009) Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ Int 35(5):766–770
Verlicchi P, Galletti A, Petrovic M, Barceló D (2010) Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J Hydrol 389(3–4):416–428
Weissbrodt D, Kovalova L, Ort C, Pazhepurackel V, Moser R, Hollender J, Siegrist H, McArdell CS (2009) Mass flows of x-ray contrast media and cytostatics in hospital wastewater. Environ Sci Technol 43(13):4810–4817
Ferrando-Climent L, Rodriguez-Mozaz S, Barceló D (2013) Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool (IDA) in sewage samples. Anal Bioanal Chem 405(18):5937–5952
Mahnik SN, Rizovski B, Fuerhacker M, Mader RM (2006) Development of an analytical method for the determination of anthracyclines in hospital effluents. Chemosphere 65(8):1419–1425
Mahnik SN, Lenz K, Weissenbacher N, Mader RM, Fuerhacker M (2007) Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 66(1):30–37
Yin J, Shao B, Zhang J, Li K (2010) A preliminary study on the occurrence of cytostatic drugs in hospital effluents in Beijing, China. Bull Environ Contam Toxicol 84(1):39–45
Ort C, Lawrence MG, Reungoat J, Eaglesham G, Carter S, Keller J (2010) Determining the fraction of pharmaceutical residues in wastewater originating from a hospital. Water Res 44(2):605–615. doi:10.1016/j.watres.2009.08.002
Le Corre KS, Ort C, Kateley D, Allen B, Escher BI, Keller J (2012) Consumption-based approach for assessing the contribution of hospitals towards the load of pharmaceutical residues in municipal wastewater. Environ Int 45:99–111. doi:10.1016/j.envint.2012.03.008
Kosjek T, Heath E (2011) Occurrence, fate and determination of cytostatic pharmaceuticals in the environment. Trends Anal Chem 30(7):1065–1087
Buerge IJ, Buser HR, Poiger T, Müller MD (2006) Occurrence and fate of the cytostatic drugs cyclophosphamide and ifosfamide in wastewater and surface waters. Environ Sci Technol 40(23):7242–7250
Johnson AC, Jürgens MD, Williams RJ, Kümmerer K, Kortenkamp A, Sumpter JP (2008) Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study. J Hydrol 348(1–2):167–175
Besse JP, Latour JF, Garric J (2012) Anticancer drugs in surface waters. What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ Int 39(1):73–86
Bound JP, Voulvoulis N (2005) Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ Health Perspect 113(12):1705–1711
Kazner C, Lehnberg K, Kovalova L, Wintgens T, Melin T, Hollender J, Dott W (2008) Removal of endocrine disruptors and cytostatics from effluent by nanofiltration in combination with adsorption on powdered activated carbon. Water Sci Technol 58(8):1699–1706. doi:10.2166/wst.2008.542
Kovalova L, Siegrist H, Singer H, Wittmer A, McArdell CS (2012) Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination. Environ Sci Technol 46(3):1536–1545
Negreira N, López de Alda M, Barceló D (2013) On-line solid phase extraction-liquid chromatography-tandem mass spectrometry for the determination of 17 cytostatics and metabolites in waste, surface and ground water samples. J Chromatogr A 1280:64–74
Martín J, Camacho-Muñoz D, Santos JL, Aparicio I, Alonso E (2011) Simultaneous determination of a selected group of cytostatic drugs in water using high-performance liquid chromatography-triple-quadrupole mass spectrometry. J Sep Sci 34(22):3166–3177
Johnson AC, Oldenkamp R, Dumont E, Sumpter JP (2013) Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of Europe. Environ Toxicol Chem 32(9):1954–1961. doi:10.1002/etc.2311
Zhang J, Chang VWC, Giannis A, Wang JY (2013) Removal of cytostatic drugs from aquatic environment: A review. Sci Total Environ 445–446:281–298
Steger-Hartmann T, Kümmerer K, Schecker J (1996) Trace analysis of the antineoplastics ifosfamide and cyclophosphamide in sewage water by two step solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr A 726(1–2):179–184. doi:10.1016/0021-9673(95)01063-7
Kosjek T, Perko S, Žigon D, Heath E (2013) Fluorouracil in the environment: Analysis, occurrence, degradation and transformation. J Chromatogr A 1290:62–72. doi:10.1016/j.chroma.2013.03.046
Tauxe-Wuersch A, De Alencastro LF, Grandjean D, Tarradellas J (2006) Trace determination of tamoxifen and 5-fluorouracil in hospital and urban wastewaters. Int J Environ Anal Chem 86(7):473–485
Negreira N, Mastroianni N, López de Alda M, Barceló D (2013) Multianalyte determination of 24 cytostatics and metabolites by liquid chromatography-electrospray-tandem mass spectrometry and study of their stability and optimum storage conditions in aqueous solution. Talanta 116:290–299. doi:10.1016/j.talanta.2013.04.070
Gómez-Canela C, Cortés-Francisco N, Ventura F, Caixach J, Lacorte S (2013) Liquid chromatography coupled to tandem mass spectrometry and high resolution mass spectrometry as analytical tool to characterize multi-class cytostatic compounds. J Chromatogr A 1276:78–94
Gómez-Canela C, Cortés-Francisco N, Oliva X, Pujol C, Ventura F, Lacorte S, Caixach J (2012) Occurrence of cyclophosphamide and epirubicin in wastewaters by direct injection analysis-liquid chromatography-high-resolution mass spectrometry. Environ Sci Pollut Res 19(8):3210–3218
Nussbaumer S, Fleury-Souverain S, Antinori P, Sadeghipour F, Hochstrasser DF, Bonnabry P, Veuthey JL, Geiser L (2010) Simultaneous quantification of ten cytotoxic drugs by a validated LC-ESI-MS/MS method. Anal Bioanal Chem 398(7–8):3033–3042
Llewellyn N, Lloyd P, Jürgens MD, Johnson AC (2011) Determination of cyclophosphamide and ifosfamide in sewage effluent by stable isotope-dilution liquid chromatography-tandem mass spectrometry. J Chromatogr A 1218(47):8519–8528
Nussbaumer S, Bonnabry P, Veuthey JL, Fleury-Souverain S (2011) Analysis of anticancer drugs: A review. Talanta 85(5):2265–2289
Hughes SR, Kay P, Brown LE (2013) Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ Sci Technol 47(2):661–677
Daughton C Pharmaceutical ingredients in drinking water: overview of occurrence and significance of human exposure. In: Emerging contaminants: Pharmaceuticals, personal care products. ACS Symposium Series, 2010.
López-Serna R, Jurado A, Vázquez-Suñé E, Carrera J, Petrović M, Barceló D (2013) Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ Pollut 174:305–315
López-Serna R, Petrović M, Barceló D (2012) Occurrence and distribution of multi-class pharmaceuticals and their active metabolites and transformation products in the Ebro River basin (NE Spain). Sci Total Environ 440:280–289
Martinez Bueno MJ, Hernando MD, Herrera S, Gomez MJ, Fernández-Alba AR, Bustamante I, Garcia-Calvo E (2010) Pilot survey of chemical contaminants from industrial and human activities in river waters of Spain. Int J Environ Anal Chem 90(3–6):321–343
Valcárcel Y, González Alonso S, Rodríguez-Gil JL, Gil A, Catalá M (2011) Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 84(10):1336–1348
Rowney NC, Johnson AC, Williams RJ (2009) Cytotoxic drugs in drinking water: A prediction and risk assessment exercise for the Thames catchment in the United Kingdom. Environ Toxicol Chem 28(12):2733–2743. doi:10.1897/09-067.1
Oldenkamp R, Huijbregts MAJ, Hollander A, Versporten A, Goosens H, RAM J (2013) Spatially explicit prioritization of human antibiotics and antineoplastics in Europe. Environ Int 51:13–26
Kümmerer K, Al-Ahmad A (2010) Estimation of the cancer risk to humans resulting from the presence of cyclophosphamide and ifosfamide in surface water. Environ Sci Pollut Res 17(2):486–496
Martins AF, Vasconcelos TG, Henriques DM, Frank CS, König A, Kümmerer K (2008) Concentration of Ciprofloxacin in Brazilian Hospital Effluent and Preliminary Risk Assessment: A Case Study. Clean Soil Air Water 36(3):264–269. doi:10.1002/clen.200700171
Gómez-Canela C, Campos B, Barata C, Lacorte S (2013) Degradation and toxicity of mitoxantrone and chlorambucil in water. Int J Environ Sci Technol. doi:10.1007/s13762-013-0454-2
European Communities (EC) (2002) Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, vol 2002/657/EC.
Besse JP, Kausch-Barreto C, Garric J (2008) Exposure assessment of pharmaceuticals and their metabolites in the aquatic environment: Application to the French situation and preliminary prioritization. Hum Ecol Risk Assess 14(4):665–695
Schlabach M, Dye C, Kaj L, Klausen S, Langford K, Leknes H, Moe MK, Remberger M, Schøyen M, Thomas K, Vogelsang. C (2008) Human and hospital-use pharmaceuticals, aquaculture medicines and personal care products. Norwegian Pollution Control Authority, SPFO-rapport: 1046/2009:1–114
Kosjek T, Perko S, Žigon D, Heath E (2013) Fluorouracil in the environment: Analysis, occurrence, degradation and transformation. J Chromatogr A 1290:62–72
Roche Australia (2013) Pharmaceuticals. Xeloda. http://www.roche-australia.com/fmfiles/re7229005/downloads/oncology/xeloda-pi.pdf. Accessed 05/11/2013
Straub JO (2010) Combined environmental risk assessment for 5-fluorouracil and capecitabine in Europe. Integr Environ Assess Manag 6 (SUPPL. 1):540–566
Booker V, Halsall C, Llewellyn N, Johnson A, Williams R (2014) Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci Total Environ 473–474:159–170
Guedes-Alonso R, Sosa-Ferrera Z, Santana-Rodríguez JJ (2013) Simultaneous determination of hormonal residues in treated waters using ultrahigh performance liquid chromatography-tandem mass spectrometry. J Anal Methods Chem 2013
Chang H, Wan Y, Wu S, Fan Z, Hu J (2011) Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res 45(2):732–740
Acknowledgments
WWTP and hospital workers are acknowledged for their administrative and logistic support in the sampling campaigns. Alejandro Delgado and Sen Lin are acknowledged for their hospital and sewage wastewater sampling assistance, and Cintia Flores and Oscar Palacios are acknowledged for mass spectrometric assistance with LC–Orbitrap-MS. The Spanish Ministry of Science and Innovation project (CTQ2011-25875) is acknowledged for financial support. Mª Rosa Boleda (Aigües de Barcelona) is acknowledged for granting us use of all the equipment in Aigües de Barcelona, including Dionex Autotrace. The Servei Català de la Salut (CatSalut) is also acknowledged for supplying information on the cytostatic compounds administered.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.42 MB)
Rights and permissions
About this article
Cite this article
Gómez-Canela, C., Ventura, F., Caixach, J. et al. Occurrence of cytostatic compounds in hospital effluents and wastewaters, determined by liquid chromatography coupled to high-resolution mass spectrometry. Anal Bioanal Chem 406, 3801–3814 (2014). https://doi.org/10.1007/s00216-014-7805-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7805-9