Abstract
The mechanism of arsenic toxicity still remains unclear, although enzymatic inhibition, impaired antioxidants metabolism and oxidative stress may play a role. The toxicological effects of trivalent inorganic arsenic on laboratory mouse Mus musculus after oral administration (3 mg/kg body weight/day) were investigated along 12 days, using a metabolomic approach based on direct infusion mass spectrometry to polar and lipophilic extracts from different organs and fluids (liver, kidney, and plasma). Positive and negative acquisition modes (ESI+/ESI−) were used throughout the experiments. The most significant endogenous metabolites affected by exposure were traced by partial least square-discriminant analysis and confirmed by tandem mass spectrometry (MS/MS) and gas chromatography coupled to MS. In this work, the toxic effect of arsenic has been related with important metabolic pathways, such as energy metabolism (e.g., glycolysis, Krebs cycle), amino acids metabolism, choline metabolism, methionine cycle, and degradation of membrane phospholipids (cell apoptosis). In addition, this work illustrates the high reliability of mass spectrometry based on a metabolomic approach to study the biochemical effects induced by metal exposure.

Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is one of the most widely studied elements in relation to mammal toxicity. This element is a widespread pollutant in the environment found in water, soil, and air from natural and anthropogenic sources, occurring in both inorganic and organic chemical forms, which strongly differ in terms of toxicity, accumulation, and involvement of arsenic respect to metabolism of living organisms [1, 2]. Inorganic forms of arsenic (iAs) are highly toxic [arsenite (iAsIII) and arsenate(iAsV)] (LD50 for mice are 4.5 mg kg−1 per body weight and per day (bw/d) and 14–18 mg kg−1 for the arsenite and arsenate by subcutaneous injection, respectively), producing a wide range of adverse health effects, but this toxicity decreases in the methylated forms [methylarsonic acid (MAV) and dimethylarsinic acid (DMAV)] (LD50 for mice are 1,800 and 1,200 mg kg−1 for the MAV and DMAV by subcutaneous injection, respectively), which are considered moderately toxic and can be excreted in urine after iAs ingestion; however, other organic species such as arsenobetaine (AB) (LD50 for mice is 10,000 mg kg−1 by subcutaneous injection), arsenocholine (AsC), and arsenosugars are regarded as innocuous [3–5]. In recent decades, the biomethylation of iAs has been considered as a detoxification mechanism, but methylated trivalent arsenicals, such as methylarsonite (MAIII) and dimethylarsinite (DMAIII), are toxic intermediates that arise during biomethylation process of arsenic in mammals and have been recently identified as cancer promoters [6–8]. Otherwise, pentavalent arsenicals are significantly less toxic than their equivalent trivalent forms [9, 10]. On the other hand, it has been recently demonstrated that arsenic species have high affinity for thiol-containing residues of proteins [11], especially the trivalent form due to its higher reactivity, as a consequence increase the number of proteins involved, such as tubulin and actin [12], hemoglobin [13, 14], metallothionein [15], galectin-I, thioredoxin peroxidase II [16], and others. This fact results in alterations of their biological functions (i.e., enzyme inhibition and metabolic pathways) that markedly contribute to arsenic carcinogenesis [17, 18]. Additionally, it is well known that iAsIII can perturb the metabolism of selenium at environmentally relevant doses [19], which could also be related with its mechanism of arsenic carcinogenesis [20].
In some areas, drinking water containing high levels of inorganic arsenic and industrial pollution are major sources of exposure to iAs for population through oral ingestion. This is an important problem for more than 200 million people in many countries chronically exposed to iAs through contaminated drinking water. Once ingested the soluble forms of As are readily absorbed from the gastrointestinal tract to bloodstream and distributed to organs. iAsV can be metabolized by consecutive reduction to iAsIII and oxidative biomethylation that convert the element to methylarsenic (MAV) and dimethylarsenic (DMAV), which contain arsenic in a pentavalent oxidation state [21]. Human arsenic exposure is related to several health problems such as liver damage and cardiovascular disease [22] and has been demonstrated the capacity of iAsIII and MAV to cross the blood–brain barrier [23, 24]. Moreover, a number of epidemiological studies have shown the association between the presence of arsenic in drinking water and the risk of cancer (skin, lung, bladder, liver, and kidney) [25–28], although the mechanisms involved in these carcinogenic processes and toxic effects caused by inorganic arsenic remain still unclear.
The metabolome is the set of low molecular mass compounds (typically <1,000 Da) present in biological systems [29]. Metabolomics is the analysis of the metabolome under certain endogenous (physiological) or exogenous (exposure) conditions. Metabolomics refers to the measurement of the entirety of metabolites in a cell [30], whereas metabolomics describes “the quantitative measurement of the dynamic multiparametric metabolic response of living systems to pathophysiological stimuli or genetic modification” [31, 32]. Two disciplines have been highlighted by Nicholson as a necessary complement to metabolomics: analytical chemistry and chemometrics [32]. Metabolomic approaches have been developed in many areas of biomedical research including toxicology [33] because they allow for the detection of metabolic response to chemical exposure with the purpose of finding biomarkers about the actual physiological status of living organisms. For this reason, metabolomics plays a key role to understand the results of complex biological processes, such as metal exposure experiments [34]. The main analytical techniques that are employed for metabolomic studies are based on nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). MS is also a major technique for molecular identification of unknown metabolites, especially through the use of tandem MS/MS experiments for fragment ion studies using direct infusion [35–38]. The metabolic fingerprint of biofluid or tissues extracts obtained by MS or NMR-based methods contain few hundreds to thousands signals related to both genetic and environmental contributions. MS approaches used in metabolomics normally requires a previous separation of metabolic components using either gas chromatography (GC), liquid chromatography (LC), or capillary electrophoresis (CE) to avoid traditional isobaric interferences affecting direct infusion. However, a number of studies have proposed the use of direct infusion to the mass spectrometer (DIMS) without any previous separation using high-resolution mass analyzers, such as hybrid systems triple quadrupole-time-of-flight (QqQ-TOF) [34, 38, 39] or Orbitrap [40]. In this sense, DIMS exhibits several advantages in metabolomics such as high-throughput screening capability, fast analysis, and wider nontarget metabolite coverage than chromatographic and electrophoretic couplings, since there is not exclusion of compounds associated to the separation step. For these reasons, DIMS is recommended to be a fast diagnostic method for evaluating the toxicological effects of metals, while LC-MS is necessary when comprehensive screening of biomarkers is required [41]. Considering that a much higher throughput can be obtained without a chromatographic step, DIMS could be developed as a fast metabolomic approach to evaluate the possible alterations in metabolic cycles caused by toxic metals [41]. On the other hand, ability of GC-MS to measure key primary metabolites, such as aminoacids, organic acids, or carbohydrates, involved in essential pathways in the metabolism of organisms (glycolysis, Krebs cycle) makes it an interesting alternative in toxicology and, in particular, in the discovery of potential biomarkers.
A crucial step in metabolomics is to get a suitable sample extraction to recover as many metabolites as possible present in the biological fluid or tissue. Most of strategies consider the use of multiplexed extractions under certain conditions for selective recovery of different families or classes of metabolites, such as lipids [42], or water-soluble metabolites [43], related to certain potential biomarkers in the mass range of typically between 50 and 1,000 m/z. On the other hand, complete extraction of metabolites is one current weak point in metabolomic studies, being necessary the development of extraction procedures for both polar and nonpolar metabolites from matrix to get a nontargeted multi-metabolite analysis to assess about alterations related to the effects of arsenic in mice.
The aim of the present study is to determine the biochemical alterations in the metabolic pathways of mice subjected to acute exposure to arsenic during 12 days by oral administration using mass spectrometry complemented by PLS-DA. For this purpose, DIMS has been applied, in both positive and negative ionization modes, to liver, kidney, and plasma of mice (Mus musculus) to obtain metabolic information. Subsequently, metabolites were identified using MS/MS experiments and a metabolomics database. Additionally, a complementary metabolomic approach based on GC-MS was applied to quantify and validate the metabolic pathways altered by DIMS in plasma from mice affected by acute doses of inorganic arsenic during 12 days of exposure.
Experimental
Standard solutions and reagents
As2O3 (99.995 %, trace metals basis) used to prepare acute doses for oral administration was supplied by Sigma-Aldrich (Steinheim, Germany). All solvents used for sample preparation were of optima grade for mass spectrometry. Methanol and chloroform were purchased from Fisher Chemical (Geel, Belgium), while ethanol, pyridine, and formic acid were supplied by Sigma-Aldrich. Water was purified with a Milli-Q Gradient system (Millipore, Watford, UK). Derivatizing agents, methoxylamine hydrochloride, and N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) containing 1 % (w/v) trimethylchlorosilane (TMCS), were obtained from Sigma-Aldrich. Alanine, valine, isoleucine, proline, glycine, serine, threonine, glutamic acid, phenylalanine, fructose, galactose, glucose, tyrosine, tryptophan, urea, aspartic acid, glutamine, cholesterol, α-ketoglutarate, isocitric acid, citric acid, lactic acid, and uric acid, purchased from Sigma-Aldrich, were used as standard substances in gas chromatographic quantification.
Apparatus
A cryogenic homogenizer SPEX SamplePrep (Freezer/Mills 6770) was used to prepare tissue homogenates. The extraction was carried out with a pellet mixer (VWR, UK). Metabolomic experiments were performed by direct infusion in a high resolution mass spectrometer QSTAR XL Hybrid system (Applied Biosystems, Foster City, CA, USA) using the electrospray ionization (ESI) source. Gas chromatography analysis were performed in a Trace GC ULTRA gas chromatograph coupled to an ion trap mass spectrometer detector ITQ900, both from Thermo Fisher Scientific (Bremen, Germany), using a Factor Four capillary column VF-5MS 30 m × 0.25 mm ID, with 0.25 μm of film thickness (Varian).
Animals and exposure protocol
M. musculus (inbred BALB/c strain) mice were obtained from Charles River Laboratory (Spain). Thirty-two mice of 7 weeks of age were fed ad libitum with conventional pellets containing an arsenic concentration <0.35 μg g−1. The animals were allowed to acclimate for 5 days with free access to food and water under controlled condition (temperature in the range of 25–30 °C and a 12-h light–dark cycle) prior to start exposure experiments. M. musculus mice were divided into two groups, one used as control and the other subjected to As(III) (in the form As2O3) exposure, using an oral administration of 100 μL of a solution containing 3 mg of As per kilogram of body weight per day during a total period of 12 days. The control mice were subjected to oral administration of 100 μL of ultrapure water with 0.9 % (w/v) NaCl per day for 12 days. The acute oral arsenite dose of 3 mg kg−1 bw/day was selected taking into account the LD50 for mice (42 mg kg−1), which was not exceed over experiment the overall exposure experiment (12 days).
Eight mice of each group were killed on the sixth day from the beginning of the experience and again on the last day of the exposure experiment to assess the effect of arsenic and the diet. Mice were individually anesthetized by isoflurane inhalation and exsanguinated by cardiac puncture, dissected using a ceramic scalpel, and finally, the organs were transferred rapidly to dry ice. Individual organs were excised, weighed in Eppendorf vials, cleaned with 0.9 % (w/v) NaCl solution in individual Petri dish, drained on a clean absorbent paper, frozen in liquid nitrogen, and stored at −80 °C until they were used for extracts preparation. Plasma collection was carried out by centrifugation (4,000×g, 30 min, 4 °C) after addition of heparin (ANTICLOT) as anticoagulant. Mice were handled according to the norms stipulated by the European Community. The investigation was performed after approval by the Ethical Committee of the University of Huelva (Spain).
Analytical procedures
Sample treatment for DI-ESI(±)-QTOF-MS analysis
First of all, individual organs were disrupted by cryogenic homogenization. Sample preparation of individual tissues for metabolomic analysis based on DI-ESI(±)-QTOF-MS was carried out in two-step. Firstly, polar metabolites were extracted with a mixture of (1:1, v/v) methanol/water by adding 500 μL to 50 mg of tissue in an Eppendorf tube followed by vigorous vortex shaking during 5 min. Then, the cells were disrupted using a pellet mixer (2 min) under low temperature, followed by centrifugation at 4,000×g for 10 min at 4 °C. The supernatant was carefully collected and transferred to another Eppendorf. The pellet was homogenized again, using a pellet mixer for 2 min with 100 μL of (1:1, v/v) methanol/water mixture, then it was centrifuged at the same conditions described above and the pellet was kept for further treatment. After that, both supernatants were combined (600 μL), and the resulting polar extract was taken to dryness under nitrogen stream and stored to −80 °C until analysis. The pellet was extracted with 500 μL of a mixture (2:1, v/v) chloroform/methanol, using a pellet mixer (2 min), to extract lipophilic metabolites and centrifuged at the same conditions described above. Finally, the resulting supernatant was taken to dryness under nitrogen stream and stored to −80 °C until analysis.
For DI-ESI(±)-QTOF-MS of plasma samples, proteins were removed from blood plasma by adding 400 μL of methanol/ethanol mixture (4:1, v/v) to 100 μL of plasma in an Eppendorf tube followed by vigorous vortex shaking for 5 min at room temperature and centrifugation at 4,000×g for 10 min at 4 °C. The supernatant was carefully collected avoiding contamination with the precipitated proteins, transferred to another Eppendorf tube, and the resulting supernatant was taken to dryness under nitrogen stream for storage at to −80 °C until analysis. The pellet was homogenized again, with 200 μL of a mixture of (2:1, v/v) chloroform/methanol, using a pellet mixer (2 min), to extract lipophilic metabolites and centrifuged (10,000×g at 4 °C for 10 min). Finally, the resulting supernatant was taken to dryness under nitrogen stream and stored to −80 °C until analysis.
The polar extracts were reconstituted to 200 μL of a mixture of methanol/water (4:1, v/v) and lipophilic extracts were reconstituted to 200 μL of (1:1) chloroform/water mixture before the analysis by ESI-MS. For data acquisitions from positive ionization, 0.1 % (v/v) formic acid was added to polar extract and 30 mM of ammonium acetate to lipophilic extract. In the case of negative ionization, intact extracts were directly infused to the mass spectrometer.
Sample treatment for GC-MS analysis
Sample preparation before GC-MS analysis should be performed carefully to ensure the reliability of metabolomic analysis. Plasma was thawed at 4 °C and vortex-mixed before use. For the extraction of metabolites from plasma, 100 μL of plasma samples was mixed with 400 μL of 1:1 methanol/ethanol mixture in an Eppendorf tube and vortexed for 5 min at room temperature, followed by centrifugation at 4,000×g for 10 min at 4 °C. The supernatant was transferred to another Eppendorf tube and dried under nitrogen stream. Plasma metabolites are not volatile and a previous derivatization step is mandatory. To this end, they were derivatized following a previously published procedure [44]. Briefly, all dried samples were derivatized with 50 μL of methoxylamine hydrochloride solution (20 mg mL−1 in pyridine) at 70 °C for 40 min for protection of carbonyl groups by methoximation, followed by treatment with 50 μL MSTFA with 1 % (w/v) of TMCS at 50 °C for 40 min for derivatization of primary amines and primary and secondary hydroxy groups in the case of MTSFA. In addition, TMCS aids in the derivatization of amides and secondary amines and hindered hydroxy groups, as a previously published elsewhere with some modifications [44]. Finally, the derivatized samples were vortex-mixed for 2 min before GC analysis, centrifuged at 4,000×g for 5 min and supernatant collected for analysis.
Analysis of samples by DI-ESI-QTOF-MS
Metabolomic experiments of liver, kidney, and plasma extracts from mice exposed to arsenic were performed by DIMS using an ESI. The parameters for hybrid analyzer QqQ-TOF system were optimized to obtain the higher sensitivity with minimal fragmentation of molecular ions, both in positive and negative ion modes. To acquire MS/MS spectra, nitrogen was used as collision gas (Table 1).
The extraction of metabolites from organs and plasma were carried out as described in the sample treatment section. Both extracts were analyzed in positive (ESI+) and negative (ESI−) ion modes resulting different profiles in a wide spectral range (m/z 50–1,100). Figure 1 shows the typical spectrum of mice plasma from control group (exposed to 0.9 % NaCl), after 6 days from the beginning of the experience, obtained by DI-ESI(±)-QTOF-MS. When these extracts were analyzed in positive ion mode (ESI+), metabolic profiles exhibited peaks in a wide range, including signals in the m/z interval 50–1,100. Both extracts show similar mass profiles, although several important differences can be remarked. In polar extracts, a large number of signals can be observed, with different intensities in the m/z range 50–400; in addition, several clusters of peaks at higher m/z values can also be remarked, Fig. 1a, probably associated to structurally related compounds, such as lyso-phosphatidylcholines (LPCs, m/z 400–600) and phosphatidylcholines (PCs, m/z 750–900). In lipophilic extracts (Fig 1b), peak clusters at m/z above 500 can also be observed, being remarkable that the number of peaks in these clusters and their intensities increase. Alternatively, the analysis of these extracts in negative mode (ESI−) gave completely different profiles (Fig 1c, d), which only exhibit signals with high sensitivity at m/z values under 500 in both extracts. On the other hand, high molecular mass metabolites (m/z 500–1,000) show a very low intensity, but the same manner as in ESI+, their intensity increase in the second extraction step. These results confirm the complementarily of the extraction procedure and molecule ionization mode to get a high diversity of signals.
Analysis of samples by GC-MS
Chromatography was performed on a Factor Four capillary column VF-5MS 30 m × 0.25 mm ID, with 0.25 μm of film thickness (Varian). The injector temperature was kept at 280 °C. Helium carrier gas was used at a constant flow rate of 1 mL/min. To acquire a good separation, the column temperature was initially maintained at 60 °C for 5 min, and then increased from 60 to 140 °C at a rate of 7 °C/min for 4 min. Then, the column temperature was increased to180°C at 5 °C/min for another 6 min. Finally, the temperature was increased to 280 °C at 5 °C/min, and held for 2 min. For mass spectrometry detection, ionization was carried out by electronic impact (EI) with a voltage of 70 eV, using full scan mode in the m/z range of 35–650, with an ion source temperature of 200 °C. Typical GC-MS total ion current chromatograms from control mice plasma (exposed to 0.9 % NaCl), after 6 days from the beginning of the experience are shown in Fig. 2. For the analysis, 1 μL of sample was injected in splitless mode. The identification of endogenous metabolites was based on comparison with the corresponding standards according to their retention times and mass spectra characteristics, complementarily, searching on NIST Mass Spectral Library (NIST 02) was used.
Data analysis
Markerview™ software (Applied Biosystems) was employed to filter the mass spectrometric results. Statistical data analysis (Partial least squares discriminant analysis (PLS-DA) were performed by means of statistical software packages SIMCA-P™ (version 11.5, published by UMetrics AB, Umeå, Sweden). PLS-DA is a partial least squares regression of a set Y of binary variables describing the categories of a categorical variable on a set X of predictor variables. It is a compromise between the usual discriminant analysis and a discriminant analysis on the significant principal components of the predictor variables [45]. The data were processed in order to find differences between mice groups submitted to different exposure time and to trace the metabolites altered by arsenic for later identification by their molecular mass and fragments in MS/MS experiments. In addition, altered metabolites were characterized using different databases of metabolomics based on DI-ESI-QqQ-TOF/MS, such as Human Metabolome Database (http://www.hmdb.ca), METLIN (http://metlin.scripps.edu), and Mass Bank (http://www.massbank.jp). In GC-MS analysis, identification of metabolites was performed using the NIST Mass Spectral Library (NIST 02).
Results
Metabolomic study of liver from Mus musculus mice under arsenic exposure by DI-ESI(±)-QTOF-MS
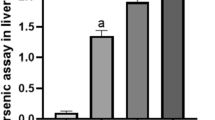
In order to discriminate between exposure groups, a PLS-DA was performed employing the intensities of the m/z signals in polar and lipophilic liver extracts, using positive and negative modes (Fig. 4). The models allow a good classification of the samples into two groups, which are showed by the respective scores plots (Fig. 3). To identify which variables were responsible for this separation, the variable influence on the projection (VIP) parameter was used. VIP is a weighted sum of squares of the PLS-DA weight, which indicates the importance of the variable to the whole model. VIP coefficients reflect the relative importance of each X variable for each X variate in the prediction model. VIP coefficients thus represent the importance of each X variable in fitting both the X- and Y-variates, since the Y-variates are predicted from the X-variates. VIP allows to classify the X-variables according to their explanatory power of Y. Predictors with large VIP, larger than 1, are the most relevant for explaining Y. Thus, it is possible to select variables with the most significant contribution in discriminating between metabolomic profiles corresponding to groups of exposure against controls. Only metabolites with VIP > 2 have been considered good biomarkers. Such as strict criterion was set because of the large number of variables or metabolites in the mass spectra (Fig. 1). The t test was performed in succession, and the variables without significant differences between the exposed mice and the controls (p > 0.01) were eliminated. Goodness of the model was measured through the R 2 Y and Q 2 values, provided by the software (indicative of class separation and predictive power of the model, respectively). These parameters are ranged between 0 and 1 and indicate the variance explained by the model for all the data analyzed (R 2), and this variance in a test set by cross-validation (Q 2). In particular, for all the analysis performed, the values of R 2 Y (cum) and Q 2 (cum) of the combined model are 0.90–0.99 and 0.8–0.95, respectively, indicating that a combination of datasets between groups provides the best classification and prediction. Mostly altered metabolites have been obtained in polar extracts from liver tissue (Table 2), but a number of metabolites were found in lipophilic extracts, which demonstrates the complementarity of using both extracts. Application of both ionization modes is also an important and critical point.
Metabolomic study of kidney from Mus musculus mice under arsenic exposure by DI-ESI(±)-QTOF-MS
The application of the metabolomic approach to kidney tissue, using MS signals in a wide m/z range (m/z 50–1100), allows discrimination between control and exposed mice (12th day) using polar metabolites with positive and negative acquisition modes (Fig. 4). The involvement of kidney in excretion and detoxification of water soluble metabolites and its relationship to blood plasma composition provides to these results an especial interest in health and biomedical fields. As it can be seen in Table 3, an important number of polar metabolites are altered under inorganic arsenic exposure.
Metabolomic study of plasma from Mus musculus mice under arsenic exposure by DI-ESI(±)-QTOF-MS
The application of PLS-DA to mass spectra signals from polar and lipophilic metabolites of plasma samples establish a slight separation between exposed and control mice for a wide range of peaks (m/z 50–1,100) using positive and negative acquisition mode (Fig. 5 ). In this case, the values of R 2 Y (cum) and Q 2 (cum) of the combined model are 0.90–0.99 and 0.80–0.90, respectively. Several polar metabolites are altered in plasma under inorganic arsenic exposure (see Table 4).
Quantification of altered metabolites in plasma from Mus musculus mice under arsenic exposure by GC-MS
GC-MS was applied to confirm and quantify some of the altered metabolites traced by DI-ESI(±)-QTOF-MS. Additionally, using GC-MS, it is possible to analyze others metabolites nonionized using ESI ionization source. For this purpose, three derivatizing reagents were used for plasma samples analysis in order to get as much metabolic information as possible. Plasma metabolic profiles of five mice plasma of each group of exposure were obtained with the method previously described in experimental section. The concentration of mice plasma metabolites are shown in Table 5.
Discussion
Perturbations in energy metabolism
Intermediate metabolites in energy metabolism such as glucose, glyceraldehide-3-phosphate, pyruvate, and citric acid are altered under inorganic arsenic exposure. The levels of these compounds decrease in liver and plasma according to results obtained by DI-ESI(±)-QTOF-MS (Tables 2 and 4). These results are confirmed by GC-MS analysis of plasma (Table 5), which shows decreased concentrations of lactic acid and alanine and increased concentrations of isocitric acid, α-ketoglutarate, glutamic acid, and citric acid. This fact can be related to perturbations in carbohydrate metabolism (Fig. 6A), and is consistent with the depletion of glucose, reported by Szinicz and Forth [46], as toxic effect of arsenic in rats. Other authors [47, 48] have also reported significant depletion of glucose in blood and liver of guinea pigs after arsenic exposure. The decreased level of pyruvate in liver, as end product of glycolysis cycle, supports this idea (Table 2), which is also borne by the formation of acetyl-CoA via pyruvate metabolism (Fig. 6A). The main function of acetyl-CoA is to convey carbon atoms within the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. As intermediate compound in the Krebs cycle, high levels of citric acid have been observed along arsenic exposure in liver, kidney, and plasma (see Tables 2, 3, and 4, respectively). The increased concentration of citric acid, isocitric acid, α-ketoglutarate, and glutamic acid observed in plasma confirm this hypothesis (see Table 5).
On the other hand, an increased presence of lipids (free fatty acids, triacylglycerols, diacylglycerols, and LPCs) was observed in liver and plasma (see Tables 2 and 4, respectively) as a result of apoptosis induced by arsenic (Fig. 7B). This fact has been observed in rat glioma and under effect of realgar undergoing apoptosis [33, 36]. In addition, increased levels of carnitine in liver (Table 2) as well as acetyl-carnitine in plasma (Table 4) are observed along the exposure time. Carnitine is required in living cells for the transport of fatty acids from the cytosol into the mitochondria during the breakdown of lipids for the generation of metabolic energy by β-oxidation. Therefore, this metabolite is also related to energy metabolism.
Perturbations in amino acid metabolism
Alterations of glucose and amino acid concentrations seem to be a common response to many toxins in several species and are likely indicative of a general response to xenobiotics exposure rather than specific biological response to a particular toxicant [49]. However, other metabolites altered under arsenic exposure were the aminoacids arginine, tryptophan, cysteine, glutamic acid, and methionine, some of them with antioxidant activity such as arginine [50] and tryptophan [51]. In the exposure experiment, decreased levels of arginine have been observed in liver, kidney, and plasma (see Table 2, 3, 4, and 5, respectively), and tryptophan in liver (see Table 2).
On the other hand, decreased levels of glutathione in liver and plasma and correlative increase in amino acids cysteine and glutamic acid (see Table 2, 4, and 5) can be observed. Glutathione can be biosynthesized in the body from the amino acids l-cysteine, l-glutamic acid, and glycine (Fig. 6B), and in reduced form, GSH, is an antioxidant compound that can protect cell from free radicals, which may play an important role against arsenic carcinogenesis, due to their activity as electron donors in arsenic methylation (Fig. 6C) [52]. Therefore, glutathione level decreases as a consequence of oxidative stress caused by arsenic and correlatively increases the presence of l-cysteine and l-glutamic acid to contribute to glutathione production. In addition, it is well known that iAsIII inhibits the activity of GSSG reductase enzyme [53].
In addition, methionine, involved in the biosynthesis of cysteine, carnitine, taurine, lecithin, phosphatidylcholine, and other phospholipids, increases in liver, kidney, and plasma (see Table 3, 4, and 5, respectively). The role of methionine in arsenic methylation is important due to the conversion of methionine into S-adenosylmethionine (SAM) by methionine adenosyltransferase. SAM donates a methyl group to arsenic to form methyl and dimethyl arsenic (Fig. 6D) [54], which also contributes to the rise in the concentration of methionine during arsenic exposure.
Degradation of membrane phospholipids
The increased levels of choline containing metabolites, such as choline and phosphorylcholine, were detected in liver and plasma principally after arsenic intake and have been associated with drug induced disruption of cell membrane [55]. One of the most important families of altered metabolites found in our study was phosphatidylcholines whose levels decrease in exposed mice. The majority of phospholipids in cellular membranes consist of glycerophospholipids such as phosphatidylcholine (PC), the most abundant phospholipids component in all the lipoprotein classes with levels ranging from 60 to 80 % of total phospholipids [56]. PC is involved in pathological mechanisms and crucial cellular functions such as signal transduction, apoptosis, necrosis, and protein sorting [57–59]. LPCs are produced by degradation of PC under enzymatic action of phospholipase A2, which releases one of the fatty acid groups (Fig. 6E). LPC is rapidly hydrolyzed to glycerophosphocholine and, finally, phosphorylcholine and choline.
The results show that intensities in MS spectra from different PC family compounds decreased under inorganic arsenic exposure in the m/z range of 700–850 in polar and lipophilic extracts using positive mode (ESI+) (Fig. 7). Secondly, LPC (m/z 450–600) increased in exposed mice in polar extract using positive mode of acquisition (Fig. 7). These results demonstrate breakdown of membrane phospholipids (cell apoptosis), which is corroborated by the increase in free fatty acids in the lipophilic extract from liver ESI+ and ESI− analysis (Fig. 7), such as pipecolic, arachidonic, oleic, linoleic, gamma-linolenic, docosahexanoic, piranaric, and lauric acids, as a consequence of PCs degradation (Fig. 6E).
Alterations in degradation choline pathway and methionine cycle
Degradation of phospholipids from cell membranes leads to accumulation of choline in plasma and tissues. As a consequence, perturbations in the pathway of choline degradation can be observed, which is shown by the increased levels of betaine aldehyde in kidney (Table 3). This metabolite is an intermediate compound in the oxidation of choline to betaine, reaction catalyzed by the enzyme choline monooxigenase. Betaine is a source of methyl groups required for methylation of inorganic arsenic and constitutes a detoxification mechanism for this element (Fig. 6D); for this reason, the betaine level increases in liver extract (Table 2). Therefore, the level of methionine increases in liver, kidney, and plasma of exposed mice, as previously mentioned, and correlatively the level of homocysteine, biosynthesized from methionine, that is recycled back to methionine completing the cycle.
In addition, changes in taurine levels in liver, kidney, and plasma can be observed (see Table 2, 4 and 5, respectively). Taurine has many important metabolic roles, such as conjugation of bile acids, antioxidation, osmoregulation, membrane stabilization, and modulation of calcium signaling. It also acts as an antioxidant and protects against toxicity of a number of substances (such as lead and cadmium) [60, 61]. Moreover, the opposite alteration of taurine and GSH has been related by GSH, which is a competitive metabolic product with taurine from cysteine. This fact suggests that the biosynthesis of GSH from cysteine was blocked, and the cysteine was diverted to produce taurine. The same results have been observed by other authors when realgar (As4S4) is administered to rats [33].
Induction of cell apoptosis and vascular risk
Perturbations in triglycerides and diglycerides levels in response to arsenic can also be observed (Fig. 7). Perturbations in triglycerides levels have previously been demonstrated in both bank voles under arsenic exposure [62] and rats under cadmium exposure [63]. In addition, increased biosynthesis of triglycerides and diacylglycerol has been found in apoptotic KB cells [64]. However, the mechanism for the relationship between increased lipids metabolites and cell apoptosis still remains unclear.
On the other hand, arsenic exposure has been correlated with a large number of cardiovascular diseases. In our study, we found slightly increased levels of di- and triglycerides in liver and plasma of exposed mice (see Table 2 and 5, respectively) that, together with the large amount of free fatty acid previously commented, suggest a situation of hyperlipidemia, which constitutes one of the most important vascular risk factors. Cardiovascular diseases such as hypertension, QT prolongation, peripheral arterial disease, atherosclerosis, impaired microcirculation, coronary heart disease, and stroke are well recognized in relation to dose and duration of arsenic exposure [65, 66]. Similar results have been obtained in renal tissue of exposed bank vole to cadmium [55].
Other metabolites
Other metabolites altered under arsenic exposure were also observed. Increased levels of xanthine and hypoxanthine were found in liver tissue from mice exposed to inorganic arsenic (see Table 2). Xanthine is a product generated in degradation pathway of purine. Xanthine is formed by oxidation of hypoxanthine by xanthine oxidase, an enzyme that generates reactive oxygen species [67]. This enzyme can further catalyze the oxidation of xanthine to uric acid [68]; for this reason, it is reasonable to expect that the observed altered levels of uric acid in kidney in arsenic exposed mice. In mammals, uric acid is the final oxidation (breakdown) product of purine metabolism and is excreted in urine [69]. For this reason, uric acid may be a marker of oxidative stress [70], and may have a potential therapeutic role as an antioxidant [69]. Thus, elevated levels of uric acid could be associated with a protective response caused by oxidative stress. In addition, increased concentration of uric acid is obtained in plasma by GC-MS (see Table 5). On the other hand, creatine is not an essential nutrient, as it is biosynthesis in the body from l-arginine, glycine, and l-methionine. It is reasonable the high levels of plasma creatine as a consequence of transmethylation perturbation due to increased choline levels (see Table 4). Moreover, creatine functions as part of the cell's energy shuttle from mitochondria to the cytosol. In addition, creatine is excreted as creatinine in the urine. The increased levels of creatinine in kidney support this idea (see Table 3).
Conclusions
Mass spectrometry has been proven to be an efficient technique to investigate the biochemical effects of trivalent inorganic arsenic. The time-dependent changes in endogenous metabolites by oral administration of arsenic were identified by PLS-DA method. Mass spectrometry analysis of polar and lipophilic metabolites of liver, kidney tissues, and plasma by DI-ESI(±)-QTOF-MS using positive and negative modes of acquisition highlighted a number of perturbations in the endogenous metabolites profiles, which could be explained to arsenic induced biochemical pathways alterations. In addition, complementarily of gas chromatography coupled to mass spectrometry with DI-ESI(±)-QTOF-MS has been demonstrated. The toxicity mechanism of arsenic still remains unclear, although enzymatic inhibition, impaired antioxidant metabolism, and oxidative stress, may play a role. In this work, the toxic effect of arsenic has been related in some metabolic pathways, such as energy metabolism (e.g., glycolysis, Krebs cycle), amino acid metabolism, choline metabolism, methionine cycle (transmethylation), purine metabolism, and degradation of membrane phospholipids (cell apoptosis). In addition, analysis of polar and lipophilic extracts using positive and negative mode (ESI+/ESI−) allows the study and characterization of a great number of possible biomarkers. Additionally, although DIMS had comparable classification and prediction capability to LC-MS, as reported by other authors. LC-MS method is necessary when comprehensive screening of biomarkers is required. For this reason, a complementary chromatography separation based on reverse phase and hydrophilic interaction chromatography using UPLC-ESI-QqQ-MS will be applied in the future. In this way, changes in the metabolome that were apparent after an exposure to 6 versus 12 days of exposure can be discussed, which would have been very useful from a biochemical point of view. In summary, mass spectrometry technique-based metabolomics approach can be employed to investigate the toxicological effects of different metals and other xenobiotics in the future using experiment exposure.
References
Vahter M (1999) Methylation of inorganic arsenic in different mammalian species and population groups. Sci Progress 82:69–88
Hirano S, Cui X, Li S, Kanno S, Kobayashi Y, Hayakawa T, Shraim A (2003) Difference in uptake and toxicity of trivalent and pentavalent inorganic arsenic in rat heart microvessel endothelial cells. Arch Toxicol 77:305–312. http://www.ncbi.nlm.nih.gov/pubmed/12799770
Geiszinger AE, Goessler W, Francesconi KA (2002) Identification of the new arsenic-containing betaine, trimethylarsoniopropionate, in tissues of a stranded sperm whale Physeter catodon. Marine Environ Res 53:37–50
Fattorini D, Regoli F (2004) Arsenic speciation in tissues of the mediterranean polychaete Sabella spallanzanii. Environ Toxicol Chem 23:1881–1887
Fattorini D, Notti A, Regoli F (2006) Characterization of arsenic content in marine organisms from temperate, tropical, and polar environments. Chem Ecol 22:405–414
Wnek SM, Jensen TJ, Severson PL, Futscher BW, Gandolfi AJ (2010) Monomethylarsonous acid produces irreversible events resulting in malignant transformation of a human bladder cell line following 12 weeks of low-level exposure. Toxicol Sci 116:44–57
Naranmandura H, Ogra Y, Iwata K, Lee J, Suzuki KT, Weinfeld M, Le XC (2009) Evidence for toxicity differences between inorganic arsenite and thioarsenicals in human bladder cancer cells. Toxicol Appl Pharmacol 238:133–140
Hirata S, Toshimitsu H, Aihara M (2006) Determination of arsenic species in marine samples by. HPLC-ICP-MS. Anal Sci 22:39–43
Petrick JS, Jagadish B, Mash EA, Aposhian HV (2001) Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Chem Res Toxicol 14:651–656
Thomas DH, Li J, Waters SB, Xing W, Adair BD, Drobna Z, Devesa V, Styblo M (2007) Arsenic (+3 oxidation state) methyltransferase and methylation of arsenicals. Exp Biol Med 232:3–13
Aposhian HV, Aposhian MM (2006) Arsenic toxicology: five questions. Chem Res Toxicol 19:1–15
Menzel DB, Hamadeh HK, Lee E et al (1999) Arsenic binding proteins from human lymphoblastoid cells. Toxicol Lett 105:89–101
Winski SL, Carter DE (1995) Interactions or rat red blood cell sulfhydryls with arsenate and arsenite. J Toxicol Environ Health 46:379–397
Lu M, Wang H, Li XF et al (2004) Evidence of hemoglobin binding to arsenic as a basis for the accumulation of arsenic in rat blood. Chem Res Toxicol 17:1733–1742
Ngu TT, Stillman MJ (2006) Arsenic binding to human metallothionein. J Am Chem Soc 128:12473–12483
Chang KN, Lee TC, Tam MF, Chen YC, Lee LW, Lee SY, Lin PJ, Huang NR (2003) Identification of galectin I and thioredoxin peroxidase II as two arsenic-binding proteins in Chinese hamster ovary cells. J Biochem 371:495–503
Kitchin KT (2001) Identification of galectin I and thioredoxin peroxidase II as two arsenic-binding proteins in Chinese hamster ovary cells. Toxicol Appl Pharm 172:249–261
Kitchin KT, Wallace K (2008) The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J Inorg Biochem 102:532–539
Gailer J, Ruprecht L, Reitmeir P, Benker B, Schramel P (2004) Mobilization of exogenous and endogenous selenium to bile after the intravenous administration of environmentally relevant doses of arsenite to rabbits. Appl Organomet Chem 18:670–675
Carew MW, Leslie EM (2010) Selenium-dependent and Independent Transport of Arsenic by the Human Multidrug Resistance Protein 2 (MRP2/ABCC2): Implications for the Mutual Detoxification of Arsenic and Selenium. Carcinogenesis 31:1450–1455
Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Del Razo LM, Thomas DJ (2003) Accumulation and metabolism of arsenic in mice after repeated administration of arsenate. Toxicol Appl Pharmacol 191:202–210
Carlson-Lynch H, Beck BD, Boardman PD (1994) Arsenic risk assessment. Environ Health Perspect 102:354–356
García-Chávez E, Santamaría A, Díaz-Barriga F, Mandeville P, Juárez BI, Jiménez-Capdeville ME (2003) Arsenite-induced formation of hydroxyl radical in the striatum of awake rats. Brain Res 976:82–89
Piao F, Ma N, Hiraku Y, Murata M, Oikawa S, Cheng F, Zhong L, Yamauchi T, Kawanishi S, Yokoyama K (2003) Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J Occup Health 47:445–449
Brown KG, Boyle KE, Chen CW, Gibb HJ (1989) A dose-response analysis of skin cancer from inorganic arsenic in drinking water. Risk Anal 9:519–528
Chen CJ, Chuang YC, You SL, Lin TM, Wu HY (1986) A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer 53:399–405
Warner ML, Moore LE, Smith MT, Kalman DA, Fanning E, Smith AH (1994) Increased micronuclei in exfoliated bladder cells of individuals who chronically ingest arsenic-contaminated water in Nevada. Cancer Epidemiol Biomarkers Prev 3:583–590
Szymanska-Chabowska A, AntonowiczJuchniewicz J, Andrzejak R (2007) The concentration of selected cancer markers (TPA, TPS, CYFRA 21-1, CEA) in workers occupationally exposed to arsenic (As) and some heavy metals (Pb, Cd) during a two-year observation study. Int J Occup Med Environ Health 20:229–239
Bowen BP, Northen TR (2010) Dealing with the unknown: metabolomics and metabolite atlases. J Am Soc Mass Spectrom 21:1471–1476
Fiehn O (2002) Metabolomics – the link between genotypes and phenotypes. Plant Mol Biol 48:155–171
Beger RD, Sun J, Schnackenberg LK (2010) Metabolomics approaches for discovering biomarkers of drug-induced hepatotoxicity and nephrotoxicity. Toxicol Appl Pharmacol 243:154–166
Nicholson JK, Lindon JC, Holmes E (1999) 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189
Wei L, Liao P, Wu H, Li X, Pei F, Li W, Wu Y (2009) Metabolic profiling studies on thetoxicological effects of realgar in rats by (1)H NMR spectroscopy. Toxicol Appl Pharmacol 234:314–325
García-Sevillano MA, Jara-Biedma R, González-Fernández M, García-Barrera T, Gómez-Ariza JL (2013) Metal interactions in mice under environmental stress. Biometals 5:855–860
Dunn WB (2008) Current trends and future requirements for the mass spectrometric investigation of microbial, mammalian and plant metabolomes. Phys Biol 5:1–11
Griffin JL, Blenkiron C, Valonen PK, Caldas C, Kauppinen RA (2006) High-Resolution Magic Angle Spinning (1)H NMR Spectroscopy and Reverse Transcription-PCR. Anal Chem 78:1546–1552
Lin L, Yu Q, Yan X, Hang W, Zheng J, Xing J, Huanga B (2010) Direct infusion mass spectrometry or liquid chromatography mass spectrometry for human metabonomics? A serum metabonomic study of kidney cancer. Analyst 135:2970
González-Domínguez R, García-Barrera T, Gómez-Ariza JL (2012) Metabolomic approach to Alzheimer’s disease diagnosis based on mass spectrometry. Chem Papers 66:829–835
Sun G, Yang K, Zhao Z, Guan S, Han X, Gross RW (2007) Shotgun metabolomics approach for the analysis of negatively charged water-soluble cellular metabolites from mouse heart tissue. Anal Chem 79:6629–6640
Favretto D, Cosmi E, Ragazzi E, Visentin S, Tucci M, Fais P, Cecchetto G, Zanardo V, Viel G, Ferrara SD (2012) Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem 402:1109–1121
Lin L, Yu Q, Yan X, Hang W, Zheng J, Xing J, Huang B (2010) A serum metabonomic study of kidney cancer. Analyst 135:2970–2978
Watson AD (2006) Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res 47:2101–2111
Sun G, Yang K, Zhao Z, Guan S, Han X, Gross RW (2007) Shotgun metabolomics approach for the analysis of negatively charged water-soluble cellular metabolites from mouse heart tissue. Anal Chem 79:6629–6640
Jiye A, Trygg J, Gullberg J, Johansson AI, Jonsson P, Antti H, Marklund SL, Moritz T (2005) Extraction and GC/MS Analysis of the Human Blood. Plasma Metabolome. Anal Chem 77:8086–8094
Pérez-Enciso M, Tenenhaus M (2003) Prediction of clinical outcome with microarray data: a partial least squares discriminant analysis (PLS-DA) approach. Human Genetics 112:581–592
Reichl FX, Szinicz L, Kreppel H, Forth W (1988) Effect of arsenic on carbohydrate metabolism after single or repeated injection in guinea pigs. Arch Toxicol 62:473–475
Reichl FX, Szinicz L, Kreppel H, Fichtl B, Forth W (1990) Effect of glucose in mice after acute experimental poisoning with arsenic trioxide (As2O3). Arch Toxicol 64:336–338
Szinicz W (1988) Effect of As2O3 on gluconeogenesis. Forth Arch Toxicol 61:444–449
Connor SC, Wu W, Sweatman BC, Manini J, Haselden JN, Crowther DJ, Waterfield DJ (2004) Effects of feeding and body weight loss on the 1H-NMR-based urine metabolic profiles of male Wistar Han Rats: Implications for biomarker discovery. Biomarkers 9:156–179
Dasgupta T, Hebbel RP, Kaul DK (2006) Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radical Biol Med 41:1771–1780
Watanabe S, Togashi S, Takahashi N, Fukui T (2002) L-Tryptophan as an antioxidant in human placenta extract. J Nutr Sci Vitaminol 48:36–39
Jin Y, Zhao F, Zhong Y, Yu X, Sun D, Liao Y, Lv X, Li G, Sun G (2010) Effects of exogenous GSH and methionine on methylation of inorganic arsenic in mice exposed to arsenite through drinking water. Environ Toxicol 25:361–366
Stýblo M, Thomas DJ (1995) In vitro inhibition of glutathione reductase by arsenotriglutathione. Biochem Pharmacol 49:971–977
Thomas DJ, Styblo M, Lin S (2001) The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol 176:127–144
Griffin JL, Mann CJ, Scott J, Shoulders CC, Nicholson JK (2001) Choline containing metabolites during cell transfection: an insight into magnetic resonance spectroscopy detectable changes. FEBS Lett 509:263–266
Skipski VP et al (1967) Lipid composition of human serum lipoproteins. Biochem J 104:340–352
Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharaju P (2001) Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J Lipid Res 42:663–672
Bashir S, Harma Y, Irshad M, Gupta SD, Dogra TD (2006) Arsenic-induced cell death in liver and brain of experimental rats. Basic Clin Pharmacol Toxicol 98:38–43
Zacarias A, Bolanowski D, Bhatnagar A (2002) Comparative measurements of multicomponent phospholipid mixtures by electrospray mass spectroscopy: relating ion intensity to concentration. Anal Biochem 308:152–159
Gürer H, Ozgünes H, Saygin E, Ercal E (2001) Antioxidant Effect of Taurine Against Lead-Induced Oxidative Stress. Arch Environ Cont Toxicol 41:397–402
Sinha M, Manna P, Sil PC (2008) Taurine protects antioxidant defense system in the erythrocytes of cadmium treated mice. BMB Reports 41:657–663
Griffin JL, Walker LA, Shore RF, Nicholson JK (2001) High-resolution magic angle spinning 1H-NMR spectroscopy studies on the renal biochemistry in the bank vole (Clethrionomys glareolus) and the effects of arsenic (As3+) toxicity. Xenobiotica 31:377–385
Griffin JL, Walker LA, Shore RF, Nicholson JK (2001) Metabolic profiling of chronic Cadmium exposure in the rat. Chem Res Toxicol 14:1428–1434
Engelmann J, Henke J, Willker W, Kutscher B, Nossner G, Engel J, Leibfritz D (1996) Early stage monitoring of miltefo- sien induced apoptosis in KB cells by mulktinuclear NMR. Anticancer Res 16:1429–1439
World Health Organization (2001) Arsenic and arsenic compounds. WHO, Geneva
States JC, Srivastava S, Chen Y, Barchowsky A (2009) Arsenic and Cardiovascular Disease. Toxicol Sci 107:312–323
Ardan T, Kovaceva J, Cejková J (2004) Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium. Acta Histochem 106:69–75
Harrison R (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33:774–797
Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA (2005) Uric acid and oxidative stress. Curr Pharm Des 11:4145–4151
Becker BF (1993) Towards the physiological function of uric acid. Free Radical Biol Med 14:615–631
Acknowledgments
This work was supported by the project CTM2012-38720-C03-01 from the Spanish Ministry of Economy and Competitiveness, and by projects P08-FQM-3554, P09-FQM-4659, P08-CVI-03829, and P08-RNM-00523 from the Regional Ministry of Economy, Innovation, Science and Employment (Andalusian Government, Spain). M.A. García Sevillano thanks the Spanish Ministry of Education for a PhD scholarship.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
García-Sevillano, M.A., Contreras-Acuña, M., García-Barrera, T. et al. Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal Bioanal Chem 406, 1455–1469 (2014). https://doi.org/10.1007/s00216-013-7564-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7564-z