Abstract
The fixed constant (Fc) region of IgG is subject to changes in glycosylation state in response to diseases. On the basis of sera from 75 healthy controls, 75 pancreatitis (PT) patients, and 75 pancreatic adenocarcinoma (PAC) patients, we analyzed six fucosylated glycoforms of IgG2 (G0F, G1F, G2F, G0FN, G1FN, and G2FN), by matrix-assisted laser desorption/ionization–Fourier-transform ion cyclotron resonance mass spectrometry (MALDI–FTICR MS), to evaluate their use as biomarkers for pancreatic diseases. Compared with healthy controls, significant increases in agalactosylated glycoforms and decreases in galactosylated glycoforms were observed for PT and PAC patients. Logistic regression analysis suggested that truncation of the sugar chain was prone to occur in PT and, especially, PAC patients. After participants were stratified by sex and age, receiver operating characteristic curve analysis revealed good overall sensitivity and specificity for discrimination of PAC and PT patients from healthy controls. A combination of G0F and galactosylation also had acceptable power for differentiating PAC patients from PT patients.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human immunoglobulin G (IgG) is a glycoprotein containing asparagine-linked glycan at Asn-297 on the CH2 domains of the Fc region. The oligosaccharide moiety of IgG consists of a series of biantennary complex-type structures. IgG functions rely on interactions of the Fc region with other proteins and depend on appropriate Fc-attached glycosylation [1,2]. For instance, addition of a fucose residue to the core N-acetylglucosamine contributes to reduced activity of antibody-dependent cell-mediated cytotoxicity [3,4]. These findings indicate that changes of IgG Fc-glycosylation may serve as potential markers for clinical diagnosis.
Pancreatic cancer is a highly lethal disease, with five-year survival less than 5 %. The vast majority of the patients with pancreatic cancer have progressed to an advanced stage at diagnosis, making the prognosis very poor. Although early detection and multimodal treatment have been investigated, pancreatic cancer is the fourth and fifth leading cause of cancer deaths among women and men, respectively, in developed countries, and its occurrence has been increasing in China for the last decade [5]. The most common type of pancreatic cancer, accounting for >85 %, is pancreatic adenocarcinoma (PAC), which develops within the exocrine region of the pancreas. Known risk factors for development of PAC include cigarette smoking, a high-fat diet, diabetes mellitus, and chronic pancreatitis (PT). Chronic PT is long-term inflammation of the pancreas. Patients with chronic PT have a markedly increased risk of PAC compared with the general population [6]. It has long been assumed that chronic inflammation has the potential to evolve toward neoplasia, which may be an early event in the development of malignancy, with genetic changes as a later manifestation of the prolonged inflammatory process [7].
Starting from this assumption, we focused on changes of the Fc-glycosylation of IgG, an important immunological and inflammatory mediator. Glycosylation differences among healthy individuals and patients with PT and PAC were determined by use of a matrix-assisted laser desorption/ionization–Fourier-transform ion cyclotron resonance mass spectrometry (MALDI–FTICR MS)-based profiling procedure in combination with statistical analysis. Our intention was to provide important data on the characteristics of Fc-glycosylation in the two pancreatic diseases, and to identify and characterize Fc-glycosylation as a useful marker for disease discrimination. The objective of this study was to provide a non-invasive, potential approach for differentiation between healthy individuals and PT and PAC patients.
Materials and methods
Ethics statement
This study was approved by the Ethics Review Boards at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The participants gave the informed consent.
Study cohort
Sex-matched healthy individuals, and PT and PAC patients (n = 75 of each) were studied (Table 1). Healthy controls were from Caoxian Gongfei Hospital (Shandong Province, China). Evaluation of clinical hematochemical data was performed in the hospital on the basis of standard clinical practices. Records were reviewed to ensure the participants were in relatively healthy condition and devoid of clinical abnormalities. PT and PAC patients were from the Peking Union Medical College Hospital. Sera were collected from 2008 to 2010 for PT patients and from 2008 to 2012 for PAC patients. For PAC patients, disease was confirmed by histological diagnosis. The PT patients consisted of 15 acute and 22 chronic inflammation cases, and 38 “acute-or-chronic” cases (merely labeled “pancreatitis” in the available records). Clinical diagnoses were performed by qualified physicians in the hospital. Because of the possibility that some clinical abnormalities (believed to be barely related or unrelated to the complications of PT and PAC) were confounding factors associated with changes in Fc-glycosylation, clinical records were reviewed carefully, especially for elderly patients, who are susceptible to such disorders as autoimmune diseases. All samples were the sera remaining after clinical laboratory examination. Venous blood is collected by use of BD Vacutainer blood collection red tubes in accordance with standard clinical procedure. The supernatant sera were collected and stored at −80 °C until use.
MALDI–FTICR MS-based profiling
IgG separation and tryptic digestion were performed as described elsewhere [8], except that the protein-digestion procedure was performed in a 96-well transparent plate. Briefly, 1 μL serum was separated on 12 % SDS–PAGE gel. The gel was moderately stained by use of Coomassie G-250 for approximately 30 min, and the band containing IgG was excised and destained in a solution of 25 mmol L−1 ammonium bicarbonate in 50 % acetonitrile, followed by dehydration in acetonitrile. After removing the acetonitrile, 40 μL 10 ng μL−1 trypsin (modified sequencing grade; Roche Diagnostics) in 25 mmol L−1 ammonium bicarbonate was added. IgG digestion was immediately performed at 37 °C for approximately 12 h. The resulting IgG digests were subjected to reversed-phase solid-phase extraction on Empore C18 disks (3 M Corporation, St Paul, MN, USA) for glycopeptide enrichment. Trifluoroacetic acid was excluded from this procedure because the acidic conditions, for example 0.1 % trifluoroacetic acid, can lead to desialylation of sialylated glycopeptides and thus effect data accuracy (for example, the desialylation of G1FS or G2FS can result in an increase of G1F or G2F, respectively). The disk was cut into pieces (ca. 2.0 mm × 2.0 mm) and stored in 20 % acetonitrile after C18 conditioning. One piece of the disk was added into a plate well (one serum sample) and incubated in IgG digest for 2 h at room temperature for adequate binding. The piece of disk was then transferred to another plate, followed by washing three times with 180 μL deionized water. The piece was then carefully transferred into a 0.6-mL tube by use of clean tweezers, and 10 μL 20 % acetonitrile solution was added. The tube was centrifuged at 5,000 g for 5 min to ensure that the piece of disk was sufficiently covered with 20 % acetonitrile solution and that bubbles that might be present between the C18 disk surface and liquid were removed. The total incubation time for a piece of disk in 20 % acetonitrile was 1 h at room temperature. The supernatant was collected and stored at −80 °C until use.
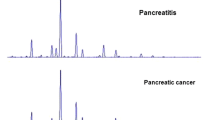
MALDI–FTICR MS-based IgG glycopeptide profiling was performed by use of a 9.4 Tesla apex-ultra hybrid Qh-FTICR mass spectrometer (Bruker Daltonics, Billerica, MA, USA) as described elsewhere [8], with minor modification. Only 0.1–0.2 μL supernatant was spotted on the AnchorChip 600/384 plate (Bruker Daltonics), air-dried at room temperature, and layered by use of 0.1–0.2 μL solution of 0.33 g L−1 cyano-4-hydroxycinnamic acid in methanol–acetone–water (3:2:1, v/v). No more than 50 scans were accumulated from one sample spot on the MALDI plate, with 50 laser shots per scan. The spectra of all samples were acquired within 60 h. A representative mass spectrum is shown in Fig. 1a, and theoretical masses and measured masses for the six peptides of interest (G0F, G1F, G2F, G0FN, G1FN, and G2FN, identified on the basis of their measured masses) are also listed in Fig. 1a, with mass error of less than 30 ppm. Quality-control (QC) samples were used to evaluate intraday and interday repeatability throughout the experimental procedure. A QC sample was subjected to parallel analysis after every 10 test samples throughout the procedure. The QC sample was a batch of identical serum aliquots from one volunteer which was stored at −80 °C until use. Coefficients of variation for the QC sample were below 10 % (8.5 ± 2.5 %, n = 22), suggesting acceptable intraday and interday reproducibility for the entire IgG Fc N-linked glycosylation profiling procedure in this study.
(a) MALDI–FTICR MS profiling of six fucosylated IgG2 Fc glycopeptides. Both experimental and theoretical masses are listed. Glycan symbols were constructed by use of the standard Consortium for Functional Glycomics nomenclature, i.e.: blue square, N-acetylglucosamine; red triangle, fucose; green circle, mannose; yellow circle, galactose. P represents the amino acid sequence (i.e. EEQFNSTFR) (b) Box plots of the 11 glycosylation variables included in this study. Significant differences among the three states (healthy controls, PT, and PAC) were determined by one-way ANOVA post-hoc test, with the p-values adjusted by Bonferroni correction method. Bar, median; box, interquartile range (IQR); whisker, range of measurements within 1.5 × IQR; dot, measurement outside the range of the whiskers; *p < 0.05; **p < 0.01; ***p < 0.001
Data handling
The study focused on six fucosylated Fc-glycoforms, G0F, G1F, G2F, G0FN, G1FN, and G2FN, for two reasons. First, the fucosylated glycopeptides from IgG1 and IgG3 (or IgG4; the glycopeptide masses of IgG3 and IgG4 are exactly the same) usually have identical masses with the non-fucosylated ones from IgG3 (or IgG4) and IgG2, respectively [9]. Second, compared with IgG1, much less is known about the Fc-glycosylation of IgG2.
Data handing was based on that in a previous study [8]. Besides the glycoforms mentioned, four glycosylation features, including galactosylation, agalactosylation, monogalactosylation, and digalactosylation, were analyzed in this study. The calculation formulas were: galactosylation = (G1F + G1FN)/2 + G2F + G2FN; agalactosylation = G0F + G0FN; monogalactosylation = G1F + G1FN; digalactosylation = G2F + G2FN. We also included the ratio of G1FN to G1FN + G1F, denoted the G1FN ratio.
Statistical analysis
Data with skewed distribution were log10-transformed for conversion into normal distribution. For statistical description of glycosylation levels, the 11 variables mentioned above were shown by use of boxplots (Fig. 1b). The significance of differences was tested by one-way analysis of variance (ANOVA) post-hoc tests, and p-values were adjusted by use of the Bonferroni correction method.
For estimation of odd ratios (OR) and 95 % confidence intervals of glycosylation variables we performed binary logistic regression using the stepwise forward method. Before this regression procedure, the glycosylation levels were set as categorical variables. For the variables in controls, values of the quartiles (Q1, Q2, and Q3), minimum (min), and maximum (max) as the cut points were first calculated. On the basis of these values, the values 1, 2, 3, 4, 5, and 6 were assigned to the glycosylation levels, if the values of the levels were < min, [min, Q1], (Q1, Q2], (Q2, Q3], (Q3, max], or > max, respectively.
The age correlation of Fc-glycosylation for each state was demonstrated by linear regression of glycosylation levels with age as independent variable (Fig. 2). Because of the observed sex and age dependencies of IgG2 Fc-glycosylation (Fig. 2), stratification of both sex and age (60 years as cut-off point) was performed; the demographics are shown in Table 2.
(a) For female subjects the level of G1F in PT and PAC patients was significantly different (**p < 0.01). (b) For males, G1F levels for the three states were not significantly different. (c) The scatter plot (level vs age), constructed from the level of galactosylation with age as independent variable, with estimates of the three linear regression curves for 75 healthy controls (black line; p = 0.013), 75 patients with PT (blue line; p = 0.003), and 75 patients with PAC (red line; p = 0.065)
Area under the curve (AUC) values were determined by receiver operating characteristic (ROC) analysis using SPSS software, after the participants had been stratified by sex and age. Sensitivity and specificity were calculated as the maximum sum of the two values using the generated coordinate points of the ROC curve.
All statistical tests were two-tailed. A p-value of less than 0.05 is reported as statistical significance.
Results and discussion
Fc-glycosylation changes in PT and PAC
We first determined differences between Fc-glycosylation levels among healthy controls and patients with PT and PAC. The log10-transformed glycosylation levels of G0F, G1F, G2F, G0FN, G1FN, G2FN, galactosylation (gal), agalactosylation (agal), monogalactosylation (m.gal), digalactosylation (digal), and G1FNratio with statistical significance (p < 0.05, except for G1F) are shown in Fig. 1b. The ANOVA post-hoc tests, with p-value adjustment by Bonferroni correction, indicated that most of variables changed significantly in pancreatic disease patients compared with healthy controls (Fig. 1b). As shown in Fig. 1b, the levels of G2F, G1FN, G2FN, m.gal, digal, and G1FNratio decrease significantly in the order healthy controls > PT > PAC, whereas levels of G0F and G0FN, with the two outer arms lacking a galactose residue or gal (G2F, G1FN, and G2FN) and agal (G0F plus G0FN) change in the opposite direction, i.e. healthy controls < PT < PAC.
Student’s t-test of results from acute (n = 15) and chronic PT (n = 22) revealed no statistical significance for all 11 variables (the p-values were: G0F, 0.266; G1F, 0.270; G2F, 0.188; G0FN, 0.081; G1FN, 0.854; G2FN, 0.853; galactosylation, 0.238; agalactosylation, 0.174; monogalactosylation, 0.360; digalactosylation, 0.266; and G1FNratio, 0.513), indicating that the two separate inflammatory diseases seem to have similar glycosylation profiles.
Compared with healthy controls, levels of galactosylated glycoforms were significantly lower, and those of the agalactosylated glycoforms higher, in PT and PAC patients. Our results suggest a greater change of Fc-glycosylation for PAC patients compared with PT patients, and that inflammation in PAC may be different and more severe than that in PT.
Truncation of sugar chain is prone to occur in PT and especially PAC
On the basis of the changes observed in the patterns of Fc-glycosylation in PT and PAC patients, logistic regression analysis was used to estimate OR values of the glycosylation variables. As shown in Table 3, in the regression model for controls and PAC (the control group was set as the reference category), the coefficient is significantly negative for digalactosylation (OR = 0.29, 95 % CI = 0.19–0.43), indicating that digalactosylation is prone to decrease in PAC patients. In the regression model for controls and PT patients (the control group was set as the reference category), the coefficients are negative for G2F (OR = 0.46, 95 % CI = 0.31–0.68) and G1FNratio (OR = 0.48, 95 % CI = 0.29–0.78). In the regression model for PT and PAC patients (PT was set as the reference category), the G0F (OR = 1.78, 95 % CI = 1.26–2.53) is prone to increase in PAC.
Change in Fc-glycosylation is sex and age-dependent
The effects of sex and age on IgG2 Fc-glycosylation were demonstrated, respectively, by ANOVA post-hoc tests after sex stratification and by the linear regression of glycosylation level with age as an independent variable. As shown in Fig. 1b, the level of G1F has no statistic significance among the three states. For female subjects, however, the difference in the level of G1F between PT and PAC patients was significant (Bonferroni corrected p-value <0.01) (Fig. 2a) whereas no such significant difference was observed for male subjects (Fig. 2b). Figure 2c shows the scatter plot of galactosylation (level vs. age) for 75 controls, 75 patients with PT, and 75 patients with PAC, with respective Spearman correlation coefficients (rho values) of −0.293 (p = 0.011), −0.444 (p < 0.0001), and −0.224 (p = 0.053). The three linear regression curves were also estimated for controls (p = 0.013) and PT (p = 0.003) and PAC (p = 0.065) patients. These results indicate there is a need to perform sex and age stratification especially when better elucidation of disease-associated change in Fc-glycosylation is required. The sex and age dependence of Fc-glycosylation indicated that some subpopulations (differing in sex and age) have different patterns of Fc-glycosylation change as a result of diseases [8]. Therefore, stratification was performed to refine elucidation of the diagnostic potential of IgG2 Fc-glycosylation.
Fc-glycosylation has the potential for disease discrimination
To demonstrate the sex and age-related diagnostic potential of IgG2 Fc-glycosylation, a ROC analysis was first used to determine the AUC value for each variable after the participants had been split by sex and age (two age groups, using 60 years as cut-off point, as shown in Table 2). G0F, G2F, galactosylation, agalactosylation, and digalactosylation (each with AUC > 0.90) have good potential for discrimination between healthy controls and PAC patients (for females aged >60 years and males ≤60 years). The ROC curves are exemplified by agalactosylation for females aged >60 years (Fig. 3a) and males aged ≤60 years (Fig. 3b).
Next, we tried to test all possible types of combination of variables for the ROC analysis. As shown in Fig. 4, the combination of digalactosylation and G1FNratio provides 94.7 % sensitivity and 100.0 % specificity, with an AUC value of 0.99, for discrimination between PAC patients and healthy controls (for females aged >60 years) (Fig. 4a). Likewise, the combination of G2F, galactosylation, and G1FNratio provides good sensitivity (90.9 %) and specificity (90.9 %) for discrimination between PT patients and healthy controls (for females aged >60 years) (Fig. 4b). It should be noted that the high AUC value of 0.97 for the combination of these three different variables may be partially because of overfitting, and an evaluation study with a large sample size is necessary to confirm these findings.
(a) ROC curve for a combination of digalactosylation and G1FNratio for discrimination between controls and PAC patients (females >60 years). (b) ROC curve for a combination of G2F, galactosylation, and G1FNratio for discrimination between controls and PT patients (females >60 years). (c) ROC curve for a combination of G0F and galactosylation for discrimination between PT and PAC patients (females ≤60 years). The AUC value, sensitivity, and specificity are listed for each ROC curve
The combination of G0F plus galactosylation provides 66.7 % sensitivity and 88.2 % specificity for discrimination between PAC and PT patients (for females aged ≤60 years) (Fig. 4c). This performance is comparable with that of carbohydrate antigen 19–9 (CA 19–9), a clinical marker widely used for diagnosis of pancreatic cancer which is elevated in 80 % of pancreatic cancer patients but also in 20 % of chronic PT patients [10].
Abbreviations
- ANOVA:
-
One-way analysis of variance
- AUC:
-
Area under receiver operating characteristic curve
- MALDI–FTICR MS:
-
Matrix-assisted laser desorption/ionization–Fourier-transform ion cyclotron resonance mass spectrometry
- MS:
-
Mass spectrometry
- PAC:
-
Pancreatic adenocarcinoma
- PT:
-
Pancreatitis
- ROC:
-
Receiver operating characteristic
References
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50
Chan AC, Carter PJ (2010) Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 10:301–316
Hodoniczky J, Zheng YZ, James DC (2005) Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 21:1644–1652
Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE (1999) Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 17:176–180
Jemal A, Bray F, Center MM, Ferlay J, Ward E et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG et al (1993) Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 328:1433–1437
Farrow B, Evers BM (2002) Inflammation and the development of pancreatic cancer. Surg Oncol 10:153–169
Chen G, Wang Y, Qiu L, Qin X, Liu H et al (2012) Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteomics 75:2824–2834
Selman MH, McDonnell LA, Palmblad M, Ruhaak LR, Deelder AM et al (2010) Immunoglobulin G glycopeptide profiling by matrix-assisted laser desorption ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem 82:1073–1081
Nouts A, Levy P, Voitot H, Bernades P (1998) Diagnostic value of serum Ca 19–9 antigen in chronic pancreatitis and pancreatic adenocarcinoma. Gastroenterol Clin Biol 22:152–159
Acknowledgments
This study was supported by grant no. 21075137 from the National Natural Science Foundation of China and by the Research Fund for the Doctoral Program of Higher Education (grant no. 20121106110023) (to Z. Li).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Li, H., Qiu, L. et al. Change of fucosylated IgG2 Fc-glycoforms in pancreatitis and pancreatic adenocarcinoma: a promising disease-classification model. Anal Bioanal Chem 406, 267–273 (2014). https://doi.org/10.1007/s00216-013-7439-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7439-3