Abstract
We report the development and demonstration of an assay that distinguishes the pharmacological effects of two widely used antiplatelet therapies, aspirin (COX-1 inhibitor) and clopidogrel (P2Y12 inhibitor). Whole blood is perfused through a low-volume microfluidic device in contact with a well-characterized (ellipsometry, atomic force microscopy) acid-soluble type I collagen surface. Whole human blood treated in vitro with a P2Y12 inhibitor 2-methylthioadenosine 5′-monophosphate triethylammonium salt (2-MeSAMP) extended the time to the start of platelet recruitment, i.e., platelet binding to the collagen surface. Treatment with 2-MeSAMP also slowed the rate of aggregate buildup, with an overall reduced average platelet aggregate area after 8 min of constant blood flow. A far smaller effect was observed for in vitro treatment with aspirin, for which the rate of change of surface coverage is indistinguishable from controls. In whole blood obtained from patients under treatment with dual-antiplatelet therapy (aspirin and clopidogrel), a significant extension of time to platelet recruitment was observed along with a slowed rate of aggregate buildup and an average aggregate size approximately half that of control measurements. Differentiation of the pharmacological effects of these two well-targeted antiplatelet pathways suggests a role for this assay in determining the antiplatelet effects of these and related new therapeutics in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Platelets are the body’s first line of defense to vascular injury, preventing blood loss by adhering and aggregating at sites of exposed vascular matrix proteins [1]. In pathophysiological states such as ruptured atherosclerotic plaques, platelets are recruited to surfaces, leading to arterial thrombosis resulting in clinical events such as myocardial infarction and stroke [2]. Platelet adhesion is mediated by the interaction of platelet glycoprotein (GP)Ib-IX-V with subendothelial von Willebrand factor (vWF) under high-fluid-shear conditions and by platelet integrin α2β1 and immunoglobulin GPVI binding to collagen under low-shear conditions [3]. The adherent platelets recruit additional platelets into a growing thrombus by secreting autocrine factors, adenosine diphosphate (ADP), and thromboxane (Tx) A2, all potent platelet aggregation mediators. The final common pathway allowing platelet cross-linking and aggregation is expression and conformational change in the fibrinogen receptor GPIIb-IIIa [4].
Aspirin [5] and clopidogrel [6] are the two most commonly used antiplatelet agents. Aspirin irreversibly inhibits the enzyme cyclooxygenase-1 (COX-1), which is responsible for platelet TxA2 generation from arachidonic acid (AA) [5]. Clopidogrel, a prodrug, blocks the ADP receptor P2Y12 [6], with its active metabolite inhibiting platelet activation. Both aspirin [7] and clopidogrel [8] have been shown in clinical trials to be effective agents in reducing myocardial infarction mortality; when clopidogrel is added to low-dose aspirin therapy, a modest infarction mortality risk reduction is observed [9, 10]. Nevertheless, a significant proportion of dual-antiplatelet-treated patients continue to experience recurrent ischemic events.
To address the nonuniform efficacy of current antiplatelet drugs, new therapeutics are being actively explored primarily because of the redundancy of pathways leading to platelet aggregation [1]. 2-Methylthioadenosine 5′-monophosphate triethylammonium salt (2-MeSAMP), a compound that antagonizes P2Y12 in vitro, and other similar antagonists are being investigated as the next-generation P2Y12 inhibitors owing to better pharmacokinetic availability [11, 12]. While the mechanism of action of these agents is reasonably well understood, their precise effect on thrombus formation under arterial shear hemodynamic conditions is not well known relative to existing therapies. This knowledge gap provides an opportunity for integrated systems that measure platelet adhesion and aggregation of whole blood under arterial shear and physiological hemodynamic conditions to provide valuable information on drug efficacy and assessment of thrombotic risk [13, 14].
Parallel-plate flow chambers have been in use for over 30 years. They mimic closely physiological conditions [15] by using arterial flow rates and whole blood to access platelet thrombus formation processes [16, 17] over shear-dependent thrombogenic surfaces such as collagen [18]. While these flow chamber assays have great potential as clinical screening tools, the preparation and characterization of the thrombogenic surfaces have been poorly standardized [19].
The present study focuses firstly on the characterization of collagen deposition to provide a thrombogenic surface in a well-described parallel-plate flow chamber [13]. Second, the effect platelet inhibitors (the P2Y12 inhibitor clopidogrel and the COX-1 inhibitor aspirin) have on dynamic thrombus formation is described using real-time platelet kinetic accumulation parameters over the well-characterized collagen surfaces under physiological hemodynamic conditions at arterial shear rates (1,500 s−1). This new platelet–collagen interaction assay monitors the antagonistic effect of antiplatelet drugs via their inhibition of platelet function that leads to aggregate formation.
Materials and methods
Optimization of collagen coating
Characterization of collagen deposition was performed using high-resolution atomic force microscopy (AFM) to construct a topological map of the surface and ellipsometry to determine coverage thickness uniformity and reproducibility. Briefly, bovine acid-soluble collagen type I (C8919, Sigma-Aldrich, St. Louis, MO) protein dissolved in phosphate-buffered saline (PBS) over a range of concentrations was adsorbed for at least 2 h at 24 °C on a SiO2-coated silicon wafer. Layer thickness was evaluated with a DeltaPsi2 spectroscopic ellipsometer by averaging nine measurement points on each sample over an area of 1 cm2. AFM studies were performed with a commercial instrument (Dimension 3100 AFM with Nanoscope IIIa controller equipped with a phase imaging extender, Digital Instruments). Collagen deposition on glass coverslips was visualized at varying concentrations in PBS by fluorescent microscopy using fluorescein isothiocyanate (FITC)-labeled monoclonal anti-type I collagen antibody (C2456, Sigma-Aldrich).
Microfluidic chamber
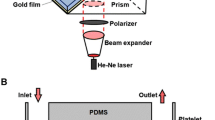
Microfluidic parallel-plate flow chambers, described previously [13], were assembled from 6-mm-thick poly(methyl methacrylate) top plates with an inlet and outlet; a laser-patterned, double-sided pressure-sensitive adhesive intermediate layer (50 μm thick; Ar-CARE 8890, Adhesives Research) to define the flow path; and a standard glass microscope coverslip as the bottom plate (Fig. 1b). The assembled device flow path was coated by adsorption from 200 μg/mL bovine acid-soluble collagen type I (C8919, Sigma-Aldrich) protein diluted in PBS overnight at 4 °C. This was followed by blocking with 1 % bovine serum albumin (BSA; Sigma-Aldrich) for 1 h at room temperature and rinsing by gentle perfusion with PBS buffer prior to the flow assay.
Device schematic. a Schematic of assay system with mounted microfluidic parallel-plate flow chamber illustrating blood perfusion with a syringe pump, Zeiss epifluorescence inverted microscope enclosed in a 37 °C chamber fitted with a liquid-cooled Quantix-57 CCD camera connected to PC with MetaMorph software for image capture. b Microfluidic parallel-plate flow chamber with a 6-mm-thick poly(methyl methacrylate) top plate containing an inlet (I) and outlet (O); a laser-patterned, double-sided pressure-sensitive adhesive intermediate layer 50 μm thick × 2 mm wide defines the flow path (FP); and a standard glass microscope coverslip which is the bottom plate. c Microfluidic parallel-plate flow chamber mounted in the stage area of the inverted microscope. d Image storage by MetaMorph software on a PC computer following capture using the Quantix-57 CCD camera from whole blood perfused through the microfluidic parallel-plate flow chamber using a Harvard syringe pump
Blood flow in microfluidic device and image acquisition
Blood was perfused through the flow chamber using a Harvard syringe pump (model PhD 2000, Harvard Apparatus, UK) at 75 μL/min, giving a fluid shear rate of 1,500 s−1 (60 dyn/cm2), corresponding to physiologically relevant arterial shear rates, for 8 min. Whole blood samples were labeled with 1 μM DiOC6 (Invitrogen, Carlsbad, CA) fluorescent dye, which localizes to the endoplasmic reticulum and mitochondria of platelets. Flow chambers were mounted on an epifluorescence inverted microscope (Zeiss Axiovert-200; Fig. 1a, c). Images were captured at one frame per second using a liquid-cooled Quantix-57 CCD camera (Photometrics, Tucson, AZ) with output processed by MetaMorph software (version 7.5, Molecular Devices Ltd., UK). Illumination was from a mercury lamp and a FITC filter set (excitation filter S490, emission filter S528; Chroma Technology, Bellows Falls, VT). A ×63 objective lens was used to record platelet interactions with the collagen matrix protein (Fig. 1a, d).
Image analysis
Acquired images were analyzed for the surface area covered by labeled adhered platelets using a previously described algorithm [13] designed in LabVIEW (National Instruments, Austin, TX), primarily based on the fluorescent intensity of platelets relative to the background. The algorithm assigns pixels with intensities above a predetermined threshold a numeric value of 1 and those below it a value of 0. A percent surface coverage value as a function of time was calculated over 8 min for each image from the number of pixels that are occupied normalized to the total number of available pixels in the image. Rates of aggregate formation at various surface coverages were calculated as follows:
Mean aggregate-covered areas were also calculated for the final image following perfusion for 8 min using ImageJ software [20].
Inhibitor preparation
2-Methylthioadenosine 5′-monophosphate triethylammonium salt, an antagonist of the P2Y12 receptor, was purchased from Sigma-Aldrich (M1434) and dissolved in PBS. Cyclooxygenase inhibitor acetylsalicylic acid (ASA) from Tocris Biosciences (4092, UK) was dissolved in 10 % dimethyl sulfoxide (DMSO) prior to use and added to 1 μM DiOC6 fluorescently labeled whole blood for a final concentration of 0.01 % DMSO. Inhibitor incubation times in whole blood were 15 min for 2-MeSAMP (50 μM) and 30 min for ASA (20 μM) at 37 °C prior to perfusion through the flow chamber.
Blood sample collection and patient population
Blood was obtained from healthy individuals who had not taken medication within the previous 10 days. Cardiovascular patients on dual-antiplatelet therapy (aspirin and clopidogrel) were recruited from the Clinical Research Centre at Beaumont Hospital, Dublin, Ireland. Blood was drawn from the antecubital vein using a 19-gauge needle into a clean polypropylene syringe containing the anticoagulant Hirudin (final concentration, 300 ATU). The first five milliliters of blood collected was routinely discarded. Blood samples were used within 1 h of phlebotomy and kept at room temperature with gentle rocking. All experiments carried out in this study were approved by the Medical Research Ethics Committees at the Royal College of Surgeons and Beaumont Hospital and comply fully with the Declaration of Helsinki.
Aggregations
A previously described [21] modification of light transmission aggregometry uses a 96-well plate and measures light absorbance after the addition of soluble agonists to platelet-rich plasma (PRP). This method enables assessment of the effects of multiple platelet agonists and multiple concentrations in a single experiment. Platelet aggregation in response to AA and ADP was assessed in the patients taking aspirin and clopidogrel to confirm drug compliance. In brief, 180 μL of platelet-rich plasma was added to the wells of a 96-well plate containing the agonist AA at concentrations of 500, 375, 188, 93.8, 46.9, 23.4, 11.8, and 5.86 μg/mL and ADP concentrations of 20, 10, 5, 2.5, 1.25, 0.625, 0.3, and 0.15 μM. Control wells containing saline buffer and platelet-rich and platelet-poor plasma were also prepared. Light transmission was measured on a plate reader (Perkin Elmer, Wellesley, MA) using a 572-nm filter following 1 mm orbital shaking for 15 min. The results were converted to percentage platelet aggregation based on the platelet-rich and platelet-poor plasma absorbance values, representing 0 and 100 % aggregation, respectively. The percentage aggregation for each agonist concentration was plotted in the form of a dose–response curve.
Statistical analysis
All data are presented as the mean ± standard error of the mean. An unpaired Student’s t test was used to detect statistically significant differences between the groups using Prism 4.0 software (GraphPad InStat 3). Values of P <0.05 were considered significant.
Results
Characterization of acid-soluble collagen type I protein deposition
Collagen is a highly conserved protein across species with bovine collagen type I having an expect value (E) of 0 (indicating a highly significant match not occurring by chance) when aligned with the human protein Refseq sequence database using NCBI BLAST p. Various bovine acid-soluble collagen type I protein concentrations were used to form uniform, homogenous collagen protein surfaces within the parallel-plate flow chamber. The resulting surfaces were characterized for uniformity and reproducibility. Firstly, the deposition of acid-soluble collagen type I on oxide-coated Si wafer pieces was carried out as described in “Materials and methods.” SiO2-coated silicon substrates, the surfaces of which have very similar chemical composition to glass, were chosen for ellipsometry and AFM measurements because of their superior flatness and smoothness relative to ordinary glass slides or plates and because the well-defined oxide layer thickness in combination with the large refractive index discontinuity at the oxide/silicon interface yields more precise ellipsometry measurements for protein layers than ellipsometry on glass [22]. Ellipsometry was used to characterize the homogeneity of the collagen protein layer thickness at multiple points on a given sample.
Figure 2a shows the results of ellipsometric measurements of collagen layer thickness vs. the concentration of the collagen adsorption solution, all samples having the same incubation time of 2 h. The results show that increasing the protein concentration from 10 to 200 μg/mL results in a progressively thicker protein film. To confirm homogeneity of the collagen layer, surface ellipsometry measurements were carried out over nine points on a large area (1 cm2) for each collagen concentration prepared. Surface collagen coverage reaches a plateau with a concentration of 200 μg/mL and a thickness of 27 Å (2.7 nm), indicating the formation of a protein monolayer, considering an individual triple helix in collagen type I as approximately 2 nm in diameter [23]. The nine measurements show a maximum variation of 10 % thickness for adsorption from a collagen solution of 200 μg/mL, confirming the homogeneity of the coating across a relatively large sample area (five times the area defined by the flow path in our parallel-plate flow device). For collagen concentrations above 200 μg/mL, the protein thickness begins to decrease, attributed to pH changes (see “Discussion”).
Reproducible collagen thrombogenic surface. a Ellipsometry of acid-soluble collagen (ASC) type I protein adsorbed on SiO2-covered Si wafers as a function of collagen concentration; adsorption time is 2 h at room temperature. Error bars represent the standard deviation of nine points measured for each sample. b Atomic force microscopy images over an area of 25 × 25 μm2 for 0 and 200 μg/mL ASC type I on SiO2 wafers, 2-h adsorption at room temperature. c Fluorescence microscopy of BSA and 200 μg/mL ASC type I adsorbed onto glass overnight at 4 °C, followed by staining with FITC-labeled anti-type I collagen antibody
Next, collagen deposits were studied using AFM to construct a topological map of the collagen protein surface, characterizing surface morphology at the nanometer scale; collagen was adsorbed for 2 h onto oxidized Si wafers over the 1- to 1,000-μg/mL concentration range. Figure 2b shows a 625-μm2 AFM image prepared with the adsorbed 200 μg/mL collagen: the surface is uniformly coated compared to the control (uncoated) sample.
Finally, collagen layers were deposited onto glass surfaces using collagen concentrations ranging from 1 to 1,000 μg/mL to permit testing of the protein’s biochemical functionality by detection with fluorescently labeled anti-collagen type I antibody. The antibody reacts with the native (helical) forms of collagen type I, but is not reactive to thermally denatured collagen [24]. Collagen consists of peptide chains which assemble into triple helices [23] with natural fibrillar collagens, types I and II, supporting greater thrombus formation [25]. The images in Fig. 2c show uniform fluorescent emission in a sample prepared with 200 μg/mL collagen type I, indicating uniform protein adsorbed on the surface in its native helical conformation. These fluorescent microscopy results are consistent with those obtained by ellipsometry and AFM, which indicated that 200 μg/mL of collagen provides a uniform distribution of protein. Overall, from multiple characterization methods, the adsorption of collagen onto glass (or similar surfaces) at 200 μg/mL for a duration of at least 2 h at room temperature yields a uniform monolayer of native helical collagen protein; such layers were therefore selected for use in all parallel-plate flow chamber experiments described below. Our results are in agreement with those of Hansen et al. [26] who demonstrated the utility and potential advantages of using acid-soluble collagen in platelet adhesion flow assays compared to insoluble collagen preparations. In Hansen’s study, the formation of reconstituted protein fibers with acid-soluble collagen at the solid/liquid interface during thin film collagen deposition onto glass showed greater homogeneity in the resulting fiber size and surface coverage as well as greater functionality in vWF adsorption and platelet adhesion compared with other traditional insoluble collagen substrates.
Quantitative measure of platelet aggregate formation from whole blood using a collagen-coated microfluidic parallel-plate flow chamber
In order to evaluate the pharmacological effect of antiplatelet agents, we used a microfluidic flow chamber system to quantitatively analyze platelet aggregate formation at physiological arterial shear rates. Hirudin-anticoagulated whole blood was perfused over the well-characterized collagen-coated microfluidic chamber surfaces at 75 μL/min corresponding to a shear rate of 1,500 s−1, with platelet aggregate formation observed continuously using video microscopy. Images acquired using whole blood from healthy unmedicated donors to monitor platelet recruitment, platelet coverage, and average aggregate area are represented in Fig. 3a. For each flow run, images were acquired at one frame per second over 8 min (480 images). Images were post-processed using LabVIEW software to mask regions covered by thrombus by applying a threshold function (see “Image analysis” above and image masking example in Fig. 3b). A percent surface coverage value was calculated for each image from the number of pixels occupied by platelets normalized to the total number of available pixels in the image; the resulting coverages are plotted against time for one such measurement (Fig. 3c). The time of initial platelet recruitment is defined as when surface coverage reaches 2.5 %; beyond this, a rapid increase in platelet surface coverage is observed (Fig. 2c). For normal anticoagulated but otherwise untreated whole blood, the time to reach 2.5 % platelet surface coverage, corresponding to the beginning of stable adhesion, was 33 s (Table 1) after initiation of flow. This time delay is believed to correspond to the time to adsorb sufficient vWF (present in the blood) to allow initial recruitment of platelets for adhesion [3].
Parameter measurements of platelet surface aggregate formation in real time over a collagen thrombogenic surface. a Representative images of 1-μM DiOC6 fluorescently labeled platelets accumulating on 200 μg/mL acid-soluble collagen type I following perfusion at a shear rate of 1,500 s−1 for the indicated times. b Representative image showing the result of using a masking algorithm in LabVIEW to define the regions covered and not covered by DiOC6 fluorescently-labeled platelets. c Semi-logarithmic graph of percentage surface coverage vs. time used to calculate time to initial platelet recruitment at 2.5 % coverage and rate of aggregate formation for percentage coverage values of 5, 10, and 15 % (n = 12)
The times to reach 2.5, 5, 10, and 15 % platelet coverage (Table 1) are indicative of the rate of early events leading to thrombus or aggregate formation. The rate of aggregate formation as indicated by the change in platelet coverage of the surface in normal whole blood at 2.5, 5, 10, and 15 % surface coverage was calculated to be nearly independent of time at ~0.1 % per second (Table 1). This rate measures the ability of adherent platelets to recruit additional platelets into a growing thrombus by secreting autocrine factors such as ADP and TxA2. The average aggregate area (26 ± 2 μm2 for normal blood; see Figs. 4c, e and 5c) was also calculated for the last image acquired in a flow run to give an indication of individual thrombus size and stability, which is a function of fibrinogen receptor GPIIb-IIIa activation and platelet cross-linking. Taken together, these parameters were used to characterize aggregate formation effects when P2Y12 and COX-1 pathways are inhibited in platelets.
Time series of platelet surface aggregation images and derived parameters on a collagen thrombogenic surface with P2Y12 or COX-1 inhibition. a Representative images of 1-μM DiOC6 fluorescently labeled platelets accumulating on 200 μg/mL acid-soluble collagen type I following perfusion at a shear rate of 1,500 s−1 for the indicated times for untreated, aspirin-treated (20 μM), and 2-MeSAMP-treated (50 μM) whole blood. b Percentage platelet surface coverage vs. log time on 200 μg/mL acid-soluble type I collagen for controls (black line) and aspirin-treated (20 μM, gray line) whole blood (n = 5). c Average platelet aggregate area after 8 min of whole blood perfusion at 1,500 s−1 over 200 μg/mL acid-soluble collagen type I surface for untreated controls (ctrl) and aspirin (ASA, 20 μM, n = 5). d Percentage platelet surface coverage vs. log time on 200 μg/mL acid-soluble type I collagen for controls (black line) and 50 μM 2-MeSAMP-treated (gray line) whole blood (n = 10). e Average platelet aggregate area after 8 min of whole blood perfusion at 1,500 s−1 over 200 μg/mL acid-soluble collagen type I surface for untreated control (ctrl) and 2-MeSAMP (50 μM, n = 10). Values represent the means ± standard errors of measurement analyzed using unpaired Student’s t test. *P < 0.05 (relative to controls)
Time series of platelet surface aggregation images and derived parameters on a collagen thrombogenic surface for cardiovascular disease (CVD) patients receiving aspirin and clopidogrel. a Representative images of 1-μM DiOC6 fluorescently labeled platelets accumulating for the indicated times on 200 μg/mL acid-soluble collagen type I at a shear rate of 1,500 s−1 for untreated healthy controls and CVD patients taking both aspirin and clopidogrel. b Percentage platelet surface coverage vs. time of platelet accumulation on 200 μg/mL acid-soluble type I collagen for untreated healthy controls (n = 12, black line) and CVD patients (n = 9, gray line) taking aspirin and clopidogrel. c Average platelet aggregate area after 8 min of whole blood perfusion at 1,500 s−1 over 200 μg/mL acid-soluble collagen type I surface for healthy controls (n = 12) and CVD patients (n = 9) taking aspirin and clopidogrel. Various concentrations of arachidonic acid (d) and adenosine diphosphate (ADP)-induced platelet aggregation (e), measured using light transmission aggregometry for PRP from CVD patients (gray line) receiving aspirin and clopidogrel and untreated PRP controls (black line), are shown. Values represent the means ± standard errors of measurement analyzed using unpaired Student’s t test. *P < 0.05 (relative to controls)
P2Y12 inhibition shows a marked reduction in parameters of aggregate formation compared to COX-1 inhibition
Using the protocol and derived parameters described above, we tested the effect of in vitro treatment of whole blood from healthy donors with aspirin (COX-1 antagonist) and 2-MeSAMP (P2Y12 antagonist) compared to control (anticoagulated but otherwise untreated) whole blood (Fig. 4a). Treatment with 2-MeSAMP (50 μM; Fig. 4d), but not aspirin (20 μM; Fig. 4b), increased the time to platelet recruitment (56 s for 2-MeSAMP vs. 33 s for the control and 32 s for ASA; see Table 1) as well as reduced the rate of aggregate formation at ≥5 % surface coverage level: 0.04 % per second for 2-MeSAMP compared to 0.1 % per second for both control and ASA (see Table 1). This causes a shift to longer times for the platelet surface coverage plot and a reduced slope of coverage vs. time for 2-MeSAMP (Fig. 4d) compared to the untreated control and ASA-treated whole blood (Fig. 4b). This indicates that P2Y12, but not COX-1, inhibition results in delayed platelet recruitment on a collagen thrombogenic surface for flowing blood at a shear rate of 1,500 s−1.
At shear rates over 1,500 s−1, the adsorbed vWF is thought to be a dominant driver of platelet recruitment and aggregate formation on collagen surfaces [27]. The results here suggest that COX-1 inhibition does not significantly impair platelet recruitment onto adsorbed vWF (at arterial shear rates), but P2Y12 inhibition does. The average aggregate area following perfusion for 8 min was significantly decreased using 2-MeSAMP (6.2 ± 1.4 μm2 for 2-MeSAMP; Fig. 4e) relative to the control (18 ± 6 μm2), which may be indicative of thrombus size and stability. The average aggregate area was reduced with aspirin treatment, but not as significantly as 2-MeSAMP treatment (14 ± 6 μm2 for ASA vs. 27 ± 8 μm2 for the control; Fig. 4c).
Changes in parameters of aggregate formation in patients on dual-antiplatelet therapy: in vivo effects of aspirin and clopidogrel
In order to test whether our assay system distinguishes in vivo pharmacological effects, we obtained whole blood from cardiovascular patients (demographics, Table 2) on combination aspirin and clopidogrel therapy. To confirm that patients were compliant with their medication regimens, platelet aggregation was assessed in response to the agonists AA and ADP using light transmission aggregometry. Aggregation in response to AA (Fig. 5d) at various concentrations was below 10 % for the patient group; in response to ADP (Fig. 5e) at the indicated concentrations, this did not exceed 40 % for the patient group. Both results confirm patient drug compliance.
Blood from these patients was assayed in the dynamic assay. As demonstrated in the representative flow runs in Fig. 5a, in vivo-treated whole blood from patients displayed a pronounced difference for platelet recruitment and rate of aggregate formation on a collagen surface relative to healthy donor controls (Fig. 5b, graphical representation). Time to platelet recruitment was greatly increased in patients on dual-antiplatelet therapy (165 s) vs. control healthy individuals (30 s; see Table 3). A decreased rate of aggregate formation was also measured for patients on dual-antiplatelet therapy: 0.02 % per second at 5, 10, and 15 % coverage vs. 0.1 % per second at 5, 10, and 15 % coverage for healthy controls (Table 3). Furthermore, the average aggregate area following perfusion for 8 min was also significantly smaller in the patients on dual-antiplatelet therapy (17 ± 3 μm2) vs. controls (26 ± 2 μm2; Fig. 5c). Overall, there were very clear reductions in the pathological aggregate formation parameters in clinical whole blood samples from patients taking aspirin and clopidogrel. Compared to in vitro-treated samples, the differences in the measured parameters were enhanced, most likely due to synergistic antiplatelet effects of the two pharmaceutical agents in vivo.
Discussion
Clinical trial data have shown both aspirin [7] and clopidogrel [8] to be effective agents in reducing mortality in patients with cardiovascular disease, and when the two are combined, a greater risk reduction occurs [9, 10]. While the mechanism of action of these drugs is reasonably well understood [1], their precise effect on aggregate formation under arterial shear conditions is not well understood. We tested the effects of these agents in vitro and in vivo using the microfluidic parallel-plate flow chamber [13, 14] as described above (Fig. 1) to address this knowledge gap.
Flow chamber methods, including microfluidic implementations like those reported here, enable investigations using whole blood to assess platelet physiological adhesion and activation in a well-defined shear environment that includes interactions with protein surfaces [16, 17]. It is important to remember that the mechanism of aggregate formation can be influenced by blood flow properties [15] as well as the details of the thrombogenic surface [27]. As new microfluidic platform technologies emerge, standardization of vascular matrices that mediate aggregate formation is crucial to allow a comparison of the physiological adhesive function of platelets across studies [19].
We utilized multiple measurement technologies to develop and confirm a standardized method for collagen coating conditions suitable for microfluidic parallel-plate flow chamber assays. Acid-soluble collagen type I was used here as the thrombogenic surface to coat the microfluidic chamber surfaces, and the ellipsometry results (Fig. 2a) show that saturation thickness was optimal with collagen adsorption from a 200-μg/mL solution in PBS for 2 h; increasing the collagen protein concentration above 200 μg/mL resulted in a reduction in the protein layer thickness. This could be due to the maximum solubility of collagen in PBS of approximately 200 μg/mL: increasing collagen concentration beyond its solubility may result in collagen fiber aggregates and precipitate formation in solution, with less collagen available for surface adsorption.
AFM (Fig. 2b) revealed that good topological uniformity of collagen surfaces was achieved at concentrations of 200 μg/mL in PBS for adsorption on glass-like SiO2. It has previously been reported that collagen in acidic solutions results in variable coating efficacies, with coating amounts consistently lower than that of collagen adsorbed from PBS solutions [28]. This same study reported that neutralizing the acid collagen solution facilitated the formation of collagen fibrils, which bind well to vWF. Others report that the formation of reconstituted protein fibers with acid-soluble collagen (pH 7.4) at the solid/liquid interface during thin film collagen deposition onto glass is greater compared with other traditional insoluble collagen substrates [26]. Our findings support these studies: diluting our stock acetic acid collagen solution fourfold with PBS, from 1 mg/mL to 200 μg/mL, increased collagen coating efficiency: collagen in acetic acid solution at 1 mg/mL exhibited limited surface coating. Finally, the collagen layer’s biochemical functionality and uniformity were confirmed by detection using an FITC-labeled anti-collagen antibody that is reactive only with the native (helical) forms of collagen.
The major advantages of using collagen as a matrix are principally its ability to differentiate the antiplatelet effects of multiple agents without the need for exogenous drug-specific agonists and its ability to induce aggregate formation at shear rates equivalent to those reported in arterioles, i.e., 1,500 s−1 in this work. The advantage of using microchip-based flow chamber systems to analyze platelet thrombus formation over collagen surfaces has clearly been demonstrated using flow pressure changes within the device that measure occlusion [29]. While thrombus formation is a useful assessment of platelet function, it does not assess the dynamic nature of platelet function through tracking of early events in platelet recruitment and adhesion processes as measured by our assay. Our goal was to produce the dynamics of collagen-induced platelet aggregate formation in our devices equivalent to those typical of collagen exposed in damaged vessels or ruptured atherosclerotic plaques. This approach has advantages over other in vitro platelet assays in correcting for fluid dynamic factors, enabling platelet–surface interactions and allowing timed measurements of aggregate formation events and their rates. These events can be segregated into (1) initial platelet capture from whole blood by vWF that depends on vWF–collagen interactions; (2) platelet–collagen binding and activation, leading to further platelet recruitment; and (3) aggregate growth. Integrating results from the individual steps that lead to aggregate formation and growth into a single assay that predicts therapeutic benefit is desirable for patients receiving antiplatelet therapy as well as for the assessment of novel inhibitors.
We showed above that in vitro treatment of whole blood with a P2Y12 inhibitor resulted in a slower rate of platelet recruitment and aggregate buildup on a collagen surface compared to controls (Fig. 4d), with no similar effect for exogenous aspirin-treated blood samples (Fig. 4b). Measuring surface coverage by fluorescent labeling of platelets in this assay may underestimate the effect of antiplatelet therapy, particularly aspirin, as thrombus volume is not fully accounted for. A recent study by Mendolicchio et al. [30] using a P2Y12 inhibitor resulted in reduced platelet thrombus volume, but found no correlation with vasodilator-stimulated phosphoprotein phosphorylation, a measure of P2Y12 inhibition. This discordance reflects the need for more physiological and standardized hemodynamic assays to monitor platelet function. Aspirin in that study had little effect on thrombus volume, in agreement with our data (Fig. 4b).
In order to account for thrombus volume, the average aggregate area was also calculated for the last image acquired in each flow run (after 8 min of perfusion) to give an indication of individual aggregate size and stability. This revealed a significant decrease in average aggregate area for P2Y12 inhibition and a lesser decrease with in vitro aspirin (Fig. 4c, e). The lack of a detectable aspirin-induced effect may be due to the shear rates used in these experiments: at 1,500 s−1, vWF is thought to be a dominant driver of platelet recruitment and aggregate formation to collagen. Our results suggest that aspirin does not prevent vWF and collagen platelet receptor effects at this shear rate compared with P2Y12 inhibition. Previous reports have indicated that P2Y12 antagonists reduce thrombus formation independent of shear [31], while COX-1 inhibition, e.g., aspirin effects, depends on shear rates [32]. Together with our data, this suggests that P2Y12 inhibition more efficiently extends platelet recruitment time and reduces aggregate growth rates and overall aggregate area at arterial shear compared to aspirin. The assay of Hosokawa et al. [33] mentioned above results in diminished pressure changes (thrombus growth) in patients receiving dual-antiplatelet therapy (aspirin and/or thienopyridine), whereas aspirin monotherapy patients showed a lesser effect on the pressure changes when whole blood was perfused over a collagen surface. The study concludes that aspirin monotherapy patients have high residual platelet thrombogenicity that is predominantly dependent on ADP. These results are in agreement with those presented here.
More interestingly, in vivo effects of these inhibitors were detected by this assay. Patients taking aspirin and clopidogrel exhibit delayed rates of platelet recruitment and aggregate buildup on collagen compared to controls that were qualitatively in significant excess of the delay in aggregate formation measured for either in vitro clopidogrel or aspirin, together with a reduction in average aggregate area qualitatively similar to the in vitro effect of ASA (Fig. 5). Importantly, this demonstrates the utility and robustness of this assay in determining the antiplatelet effects of these two agents in a clinical setting and suggests the potential to discern non-response to clopidogrel even in patients on dual-antiplatelet therapy. Furthermore, combining this assay method with thrombus volume measurements [30] may prove useful for comparing the effects of new clinical antiplatelet agents to determine their potential to reduce thrombotic risk.
In conclusion, the present study characterizes a novel alternative assay method to measure the inhibition of human platelet function following antiplatelet therapy. 2-MeSAMP and aspirin produce their pharmacological effects through two distinct mechanisms, both of which produce measureable results in the recorded assay parameters; this assay detects the antagonistic effect of these drugs as well as their distinctive ability to inhibit platelet function that leads to aggregate formation.
References
Jackson SP (2011) Arterial thrombosis—insidious, unpredictable and deadly. Nat Med 17:1423–1436
Falk E (1985) Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation 71:699–708
Ruggeri ZM, Mendolicchio GL (2007) Adhesion mechanisms in platelet function. Circ Res 100:1673–1685
Furie B, Furie BC (2008) Mechanisms of thrombus formation. N Engl J Med 359:938–949
Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C (2005) Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 353:2373–2383
Patrono C (2009) The P2Y12 receptor: no active metabolite, no party. Nat Rev Cardiol 6:271–272
Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373:1849–1860
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK (2001) Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345:494–502
Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS, COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group (2005) Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366:1607–1621
Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE et al (2006) Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 354:1706–1717
Freedman JE, Hylek EM (2009) Clopidogrel, genetics, and drug responsiveness. N Engl J Med 360:411–413
Small DS, Farid NA, Payne CD, Weerakkody GJ, Li YG, Brandt JT, Salazar DE, Winters KJ (2008) Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 48:475–484
Kent NJ, Basabe-Desmonts L, Meade G, MacCraith BD, Corcoran BG, Kenny D, Ricco AJ (2010) Microfluidic device to study arterial shear-mediated platelet-surface interactions in whole blood: reduced sample volumes and well-characterised protein surfaces. Biomed Microdevices 12:987–1000
Lincoln B, Ricco AJ, Kent NJ, Basabe-Desmonts L, Lee LP, MacCraith BD, Kenny D, Meade G (2010) Integrated system investigating shear-mediated platelet interactions with von Willebrand factor using microliters of whole blood. Anal Biochem 405:174–183
O’Brien S, Kent N, Lucitt M, Ricco AJ, McAtamney C, Kenny D, Meade G (2012) Effective hydrodynamic shaping of sample streams in a microfluidic parallel-plate flow-assay device: matching whole blood dynamic viscosity. IEEE Trans Biomed Eng 59:374–382
Zwaginga JJ, Nash G, King MR, Heemskerk JWM, Frojmovic M, Hoylaerts MF, Sakariassen KS (2006) Flow-based assays for global assessment of hemostasis. Part 1: Biorheologic considerations. J Thromb Haemost 4:2486–2487
Zwaginga JJ, Sakariassen KS, Nash G, King MR, Heemskerk JW, Frojmovic M, Hoylaerts MF (2006) Flow-based assays for global assessment of hemostasis. Part 2: Current methods and considerations for the future. J Thromb Haemost 4:2716–2717
Brass LF, Zhu L, Stalker TJ (2005) Minding the gaps to promote thrombus growth and stability. J Clin Invest 115:3385–3392
Heemskerk JW, Sakariassen KS, Zwaginga JJ, Brass LF, Jackson SP, Farndale RW (2011) Collagen surfaces to measure thrombus formation under flow: possibilities for standardization. J Thromb Haemost 9:856–858
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Moran N, Kiernan A, Dunne E, Edwards RJ, Shields DC, Kenny D (2006) Monitoring modulators of platelet aggregation in a microtiter plate assay. Anal Biochem 357:77–84
Toscano A, Santore MM (2006) Fibrinogen adsorption on three silica-based surfaces: conformation and kinetics. Langmuir 22:2588–2597
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78:929–958
Mayne R (1988) Preparation and applications of monoclonal antibodies to different collagen types. Clin Biochem 21:111–115
Munnix IC, Gilio K, Siljander PR, Raynal N, Feijge MAH, Hackeng TM et al (2008) Collagen-mimetic peptides mediate flow-dependent thrombus formation by high- or low-affinity binding of integrin alpha2beta1 and glycoprotein VI. J Thromb Haemost 6:2132–2142
Hansen RR, Tipnis AA, White-Adams TC, Di Paola JA, Neeves KB (2011) Characterization of collagen thin films for von Willebrand factor binding and platelet adhesion. Langmuir 27:13648–13658
Sakariassen KS, Hanson SR, Cadroy Y (2001) Methods and models to evaluate shear-dependent and surface reactivity-dependent antithrombotic efficacy. Thromb Res 104:149–174
Mendelboum Raviv S, Szekeres-Csiki K, Jenei A, Nagy J, Shenkman B, Savion N, Harsfalvi J (2012) Coating conditions matter to collagen matrix formation regarding von Willebrand factor and platelet binding. Thromb Res 129:e29–e35
Hosokawa K, Ohnishi T, Fukasawa M, Kondo T, Sameshima H, Koide T, Tanaka KA, Maruyama I (2012) A microchip flow-chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc Res 83:154–161
Mendolicchio GL, Zavalloni D, Bacci M, Corrada E, Marconi M, Lodigiani C, Presbitero P, Rota L, Ruggeri ZM (2011) Variable effect of P2Y12 inhibition on platelet thrombus volume in flowing blood. J Thromb Haemost 9:373–382
Roald HE, Barstad RM, Kierulf P, Skjørten F, Dickinson JP, Kieffer G, Sakariassen KS (1994) Clopidogrel—a platelet inhibitor which inhibits thrombogenesis in non-anticoagulated human blood independently of the blood flow conditions. Thromb Haemost 71:655–662
Barstad RM, Orvim U, Hamers MJ, Tjonnfjord GE, Brosstad FR, Sakariassen KS (1996) Reduced effect of aspirin on thrombus formation at high shear and disturbed laminar blood flow. Thromb Haemost 75:827–832
Hosokawa K, Ohnishi T, Sameshima H, Miura N, Ito T, Koide T, Maruyama I (2007) Analysing responses to aspirin and clopidogrel by measuring platelet thrombus formation under arterial flow conditions. Thromb Haemost 98:1316–1322
Acknowledgments
The authors gratefully acknowledge the help of Ms. Gail Plunkett who assisted with patient recruitment. This material is based upon research supported by Science Foundation Ireland under grant no. 05/CE3/B754.
Disclosure and conflict of interests
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucitt, M.B., O’Brien, S., Cowman, J. et al. Assaying the efficacy of dual-antiplatelet therapy: use of a controlled-shear-rate microfluidic device with a well-defined collagen surface to track dynamic platelet adhesion. Anal Bioanal Chem 405, 4823–4834 (2013). https://doi.org/10.1007/s00216-013-6897-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6897-y