Abstract
Phosphodiesterase (PDE) inhibitors are widely used because of their various pharmacological properties, and natural products are considered the most productive source of PDE inhibitors. In this work, a new ultrafiltration–high-performance liquid chromatography (HPLC)–diode-array detection–mass spectrometry based ligand screening was developed for the first screening of PDE inhibitors from Eucommia ulmoides bark, and then the target bioactive compounds were prepared by combination of stepwise preparative HPLC and high-speed countercurrent chromatography (HSCCC) methods. Experiments were conducted to optimize the parameters in ultrafiltration, stepwise preparative HPLC, and HSCCC to allow rapid and effective screening and isolation of active compounds from complex mixtures. Seven lignans with purity over 97 % were isolated and identified by their UV, electrospray ionization mass spectrometry, and NMR data as (+)-pinoresinol-4,4′-di-O-β-D-glucopyranoside (1), (+)-pinoresinol-4-O-β-D-glucopyranosyl(1 → 6)-β-D-glucopyranoside (2), (+)-medioresinol-4,4′-di-O-β-D-glucopyranoside (3), (+)-syringaresinol-4,4′-di-O- β-D-glucopyranoside (4), (−)-olivil-4′-O-β-D-glucopyranoside (5), (−)-olivil-4-O-β-D- glucopyranoside (6), and (+)-pinoresinol-4-O-β-D-glucopyranoside (7). Compound 2 was first isolated from the genus Eucommia. Lignan diglucopyranosides (compounds 1–4) shower a greater inhibitory effect than lignan monoglucopyranosides (compounds 5–7). The method developed could be widely applied for high-throughput screening and preparative isolation of PDE inhibitors from natural products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymes are attractive targets for drug discovery, and the process of finding new enzyme inhibitors is interesting and challenging [1]. A recent survey found that nearly half of all the marketed small-molecule drugs are enzyme inhibitors [2]. Phosphodiesterases (PDEs) are a class of enzymes which are categorized into 11 families on the basis of protein sequence, substrate specificity, intracellular location, tissue distribution, and mode of regulation [3–5]. PDEs are responsible for controlling cellular concentration of cyclic adenosine monophosphate or cyclic guanosine monophosphate because of their ability to cleave the phosphodiester bond in cyclic adenosine monophosphate or cyclic guanosine monophosphate to 5′-cyclic nucleotides [6]. PDE inhibitors are widely used for their various pharmacological properties, such as their cardiotonic, vasodilation, smooth muscle relaxant, antidepressant, antithrombotic, bronchodilation, anti-inflammatory, antioxidant, and enhancement of cognitive function properties, which has encouraged researchers to seek PDE inhibitors. Natural products have been used as a source of medicine throughout history and continued to be served as the basis for drug leads [5].
Eucommia ulmoides is one of the longest known and most used tonic herbs in Asia, and has been proved to possess a great therapeutic effect on reinforcing muscle and lung, lowering blood pressure, preventing miscarriages, improving the tone of the liver and kidney, increasing longevity, and so on [7–9]. The medicinal parts of E. ulmoides are the bark and leaf as mentioned in Pharmacopoeia of the People's Republic of China [10]. Much effort has been made to identify the bioactive components in E. ulmoides. Several types of components, including lignans (oligomers of Ar-C3 units), iridoids (a cyclopentane ring fused to a six-membered oxygen heterocycle), phenolic acids, and flavonoids have been isolated and identified from E. ulmoides [11–13], and lignans and iridoids are considered the main bioactive components [11]. About 70 components were found in E. ulmoides, 27 of which were lignans and 15 of which were iridoids [11, 14]. The content of lignans in E. ulmoides extract was in the order bark > leaf, and (+)-pinoresinol-4,4′-di-O-β-D-glucopyranoside was the main lignan in E. ulmoides bark, with a content of 0.1-0.55 % [15]. Further experiments revealed that Eucommia lignans have PDE and aldose reductase inhibiting activity and protective effects against hypertensive renal injury [14, 16–18]. However, most of the investigations on the pharmacological activities of Eucommia lignans were conducted on crude extracts or single isolated compounds, and it remained unclear which of the components in the crude extracts were active compounds and how each of these active components contributed to the pharmacological effects. It is widely accepted that the pharmacological effects of natural products may be produced by not only one or two components, and that there are a group of active compounds, some of which produce a synergistic effect or antagonistic action [19, 20]; therefore, the screening and identification of active compounds from crude extracts are very important. Moreover, the processes of modernization and globalization of natural products necessitated the identification of active compounds not only for the discovery of leading compounds but also for supplying suitable chemical markers for quality control. As such, it has become a challenging task for researchers to systematically elucidate the pharmacological profile of natural products.
Conventional bioassay-guided fractionation has been used to find many clinical drugs. However, it is a time-consuming, labor-intensive, and low-efficiency strategy [21], and it sometimes leads to the loss of activity during the isolation and purification process owing to dilution effects or decomposition. Moreover, much effort is devoted to the isolation of known compounds or compounds that are not of interest [22]. To avoid the above-mentioned problems, simple, rapid, and effective methods to screen and identify active compounds from complex mixtures are essential. Recent developments in high-performance liquid chromatography (HPLC)-based techniques, e.g., HPLC–diode-array detection (DAD), HPLC–tandem mass spectrometry (MS/MS), and HPLC–NMR spectroscopy, have made possible complete identification of numerous novel and interesting compounds online without prior isolation [23]. Furthermore, the combination of HPLC separation and biochemical detection methods can afford peaks in the chromatograms associated with bioactivity. As we reviewed before [22], a few online postcolumn assay formats for rapid screening and identification of enzyme inhibitors from natural products have been developed and applied successfully; however, there are some drawbacks to this method, such as more sophisticated instrument setup and lower sensitivity [24–26]. The recently developed ultrafiltration-based ligand screening has been widely applied for screening and analysis of active compounds from natural products because of its fast speed, easy, operation and high reliability [27–32].
As a preparative method, preparative HPLC has been widely used because of its target separation ability, great efficiency, and high recovery [33]. High-speed countercurrent chromatography (HSCCC) is a continuous liquid–liquid partition chromatography based on partitioning of compounds between two immiscible liquid phases without a support matrix, no irreversible adsorption, low risk of sample denaturation, total sample recovery, large load capacity, and low cost [34], and has been successfully applied to purify many bioactive compounds from natural products [35–37]. For some complex mixtures, neither preparative HPLC nor HSCCC can purify all the target active compounds with high purity in one-step separation; however, the combination of preparative HPLC and HSCCC was efficient to purify the target bioactive compounds with different polarities and contents because of their complementary and orthogonal properties [38].
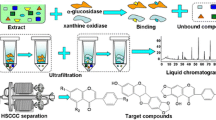
As part of our ongoing efforts to screen and isolate of active compounds from natural products [26, 32, 35], we presented a combination of preparative HPLC and HSCCC to separate PDE inhibitors from E. ulmoides bark guided by ultrafiltration-based ligand screening. The results yielded seven active compounds, (+)-pinoresinol-4,4′-di-O-β-D-glucopyranoside (1), (+)-pinoresinol-4-O-β-D- glucopyranosyl(1 → 6)-β-D-glucopyranoside (2), (+)-medioresinol-4,4′-di-O- β-D-glucopyranoside (3), (+)-syringaresinol-4,4′- di-O-β-D-glucopyranoside (4), (−)-olivil-4′-O-β-D-glucopyranoside (5) (−)-olivil-4-O-β-D-glucopyranoside (6), and (+)-pinoresinol-4-O-β-D-glucopyranoside (7) (Fig. 1). This is the first report on systematic screening and isolation of PDE inhibitors from E. ulmoides bark by preparative HPLC and HSCCC guided by ultrafiltration experiments, and compound 2 is first reported from the genus Eucommia.
Chemical structures of seven active lignans isolated from Eucommia ulmoides bark. PDG (+)-pinoresinol-4,4′-di-O-β-D-glucopyranoside, PGG (+)-pinoresinol-4-O-β-D-glucopyranosyl(1 → 6)-β-D-glucopyranoside, MDG (+)-medioresinol-4,4′-di-O-β-D-glucopyranoside, SDG (+)-syringaresinol-4,4′-di-O-β-D-glucopyranoside, PG (+)-pinoresinol-4-O-β-D-glucopyranoside, OG1 (−)-olivil-4′-O-β-D-glucopyranoside, OG2 (−)-olivil-4-O-β-D-glucopyranoside
Experimental
Chemicals and reagents
PDE 3′,5′-cyclic nucleotide activator deficient from bovine heart (more than 0.1 U/mg) was purchased from Sigma-Aldrich. (St Louis, MO, USA). Petroleum ether (60–90 °C), ethyl acetate, n-butanol, methanol, ethanol, tris(hydroxymethyl)aminomethane (Tris) buffer, and HCl for extraction, fractionation, ultrafiltration, and isolation were all of analytical grade and were purchased from Chemical Reagent Factory of Hunan Normal University (Hunan, China), and were used without further purification. Acetonitrile and formic acid used for HPLC were of chromatographic grade (Merck, Darmatadt, Germany). All aqueous solutions were prepared with pure water produced by a Milli-Q water (18.2 MΩ) system (Millipore, Bedford, MA, USA). D101 macroporous resin was purchased from the Chemical Plant of Nankai University (Tianjin, China), and was cross-linked polystyrene copolymer.
The E. ulmoides bark was collected from Zhangjiajie, Hunan province, China, in 2010, and was identified by one of us (Mi-Jun Peng). A voucher specimen (EUBZJJ2010) was deposited at the School of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, China.
Preparation of lignan-enriched fraction
Fresh E. ulmoides bark was dried at 55 °C for 3 days before being ground. About 400 g of the powder was decocted by 75 % ethanol at 95 °C three times (each for 2 h). All the filtrates were combined and concentrated to dryness under reduced pressure by rotary evaporation at 60 °C to give 48.3 g of syrup. A mass of 48 g of syrup was then suspended in water and submitted to liquid–liquid fractionation using ethyl acetate and n-butanol with increasing polarities, which produced ethyl acetate (9.2 g) and n-butanol (5.7 g) fractions. The n-butanol fraction was subsequently suspended in water and subjected to D101 column chromatography (20.0 cm × 100 cm, containing 3.0 kg D101 macroporous resin) to enrich lignans. First, water was used to elute the resin until the eluate was nearly colorless, then increasing concentrations of ethanol were used to give two main fractions (A and B): fraction A (0.6 g)—15 % ethanol elution, which contained some compounds without lignans; fraction B (2.1 g)—40 % ethanol elution, which contained lignans. Fraction B was stored at 4 °C for further preparative HPLC and HSCCC experiments. Figure 2 summarizes the procedure for screening and identification of active lignans from E. ulmoides bark.
Ultrafiltration–HPLC–DAD–MS
The ultrafiltration-based ligand screening consisted of three steps: incubation, ultrafiltration, and analysis. A 3-ml volume of PDE (0.5 mg/ml, dissolved in 0.1 mol/l Tris buffer, pH 6.8) was added to 3 ml of lignan-enriched fraction (2.0 mg/ml, dissolved in 0.1 mol/l Tris buffer, pH 6.8) and then the mixture was incubated at 37 °C for 1.0 h. After incubation, the mixture were ultrafiltrated with a model 5417R centrifuge (Eppendorf, Hamburg, Germany) with Nanosep MF centrifugal devices (10 kDa or less; Pall, Ann Arbor, MI, USA) at 8,000 rpm, 4 °C for 20 min to separate the unbound low-mass molecules from the ligand–PDE complexes and PDE. The ultrafiltrates was filtered through a 0.45-μm membrane, and 20 μl of the solution was injected directly into the HPLC-DAD-MS system for analysis.
HPLC-DAD-MS was performed with an AcquityTM UPLC system (Waters, Milford, MA, USA) with a cooling autosampler and column oven allowing temperature control of the analytical column. The target compounds were separated by a reversed-phase SunFire™ C18 column (250 mm × 4.6-mm inner diameter, 5 μm; Waters, Milford, MA, USA). The mobile phase consisted of 0.1 % formic acid in water (solvent A) and 0.1 % formic acid in acetonitrile (solvent B). The program was as follows: 0–10.0 min, 18.0 % solvent B; 10.0-35.0 min, 18.0-25.0 % solvent B. The flow rate was 0.8 ml/min and the temperature was set at 20 °C. Spectra were recorded from 200 to 400 nm (peak width 0.2 min and data rate 1.25 s-1), and the chromatogram was acquired at 277 nm. Triple-quadrupole tandem mass-spectrometric detection was performed with a Micromass® Quattro micro™ API mass spectrometer (Waters, Milford, MA, USA) with an electrospray ionization (ESI) interface. The ESI source was set in negative ionization mode. The following settings were applied to the instrument: capillary voltage, 3.00 kV; cone voltage, 40.0 V; extractor voltage, 3.00 V; source temperature, 120 °C; desolvation temperature, 400 °C; desolvation gas flow rate, 750 l/h; cone gas flow rate, 50 l/h, dwell time, 0.05 s. Nitrogen was used as the desolvation and cone gas. Mass detection was performed in full-scan mode for m/z in the range from 150 to 800. All data were acquired and processed by MassLynx™ NT 4.1 including QuanLynx™ (Waters, Milford, MA, USA).
Preparative HPLC
Preparative HPLC experiments were performed using a self-assembled instrument, which was composed of a P3000 delivery pump, a 2PB00C sample injection pump, a UV3000 variable-wavelength detector with detection monitored at 277 nm, and an SCJS-3000 ChemStation. A preparative dynamic axial compression (DAC) column (500 mm × 80-mm inner diameter) packed with 5-μm octadecylsilane (Fuji, Japan) was used for the preparative separation. The mobile phase consisted of a mixture of 0.1 % formic acid in water (solvent A) and 0.1 % formic acid in ethanol (solvent B). The program was as follows: 0–28 min, 20 % solvent B, 45 ml/min; 28–45 min, 25 % solvent B, 45 ml/min; 45–60 min, 25 % solvent B, 55 ml/min. The sample injection volume for each run was 10 ml and contained 2.0 g of lignan-enriched fraction. The peak fractions (fraction B1, 320.9 mg, and fraction B2, 230.5 mg) were collected manually according to the chromatogram. After the run, the collected fractions were evaporated to dryness in a vacuum, and small portions of water were added occasionally to prevent acid glycoside hydrolysis. The residues were lyophilized, analyzed by analytical HPLC, and purified further by HSCCC.
HSCCC separation
HSCCC apparatus
The preparative HSCCC was performed using a model TBE-300B HSCCC system (Shanghai Tauto Biotechnique, Shanghai, China). The apparatus consisted of an upright coil J-type planet centrifuge with three multilayered coils connected in series (diameter of tube, 1.6 mm, total capacity 260 ml) and a 20-ml manual sample loop. The rotation speed was adjustable, ranging from 0 to 1,000 rpm. The HSCCC system was equipped with a TBP-1002 pump, a TBD-2000 UV detector, an HX-1050 constant-temperature regulator (Beijing Boyikang Lab Implement, Beijing, China), which was used to control the separation temperature, and a WH V4.0 workstation (Shanghai Wuhao Information Technology, Shanghai, China). In the separation process, the temperature of the separation columns was maintained at 25 °C, and the effluents were analyzed at 277 nm.
Selection of the solvent system
A series of solvent systems were evaluated by HPLC according to the partition coefficients. About 1.0 mg of sample was added to a test tube, and then 2 ml of each phase of the equilibrated two-phase solvent system was added and thoroughly mixed. The test tube was rigorously shaken for several minutes and left to stand at room temperature until equilibrium was attained. Then 20 μl of the upper phase and 20 μl of the lower phase were analyzed by HPLC at 277 nm. The partition coefficient (K) is defined as A upper/A lower, where A upper and A lower are the HPLC peak areas of the compounds under study in the upper and lower phases, respectively. Suitable values for HSCCC are 0.5 ≤ K ≤ 2.0.
Preparation of solvent system and sample solution
Two solvent systems composed of ethyl acetate–ethanol–water (2:1:3, v/v/v) and petroleum ether–ethyl acetate–methanol–water (2:5:2:5, v/v/v/v) were used for HSCCC separation. The preparation of each two-phase solvent system was performed in a separation funnel according to the volume ratios and the system was thoroughly equilibrated after the funnel had been shaken at room temperature. The upper phase and the lower phase were then separated and degassed by ultrasonication for 30 min prior to use. For HSCCC separation of fraction B1, the sample solution was prepared by dissolving 320.0 mg of fraction B1 in 20-ml solvent mixtures of the lower phase and the upper phase (1:1, v/v) of ethyl acetate–ethanol—water (2:1:3, v/v/v), and the sample solution of fraction B2 for HSCCC separation was prepared by dissolving 230.0 mg of sample in 20-ml solvent mixtures of the lower phase and upper phase (1:1, v/v) of petroleum ether–ethyl acetate–methanol–water (2:5:2:5, v/v/v/v).
HSCCC separation procedure
Each HSCCC separation was performed as follows in the whole separation. The column was first entirely filled with the upper phase as the stationary phase. Subsequently, the lower phase was pumped into the inlet of the column as the mobile phase at a flow rate of 1.2 ml/min, and the apparatus run at 850 rpm. After hydrodynamic equilibrium had been reached, samples were subjected to HSCCC. The effluent was continuously monitored at 277 nm and the peak fractions were collected manually according to the chromatographic profile. After the target compounds had been eluted, the centrifuge was stopped and the stationary phase was pumped out with pressured nitrogen and collected in a graduated cylinder to measure the retention volume.
Analysis and identification of target compounds
The isolated active compounds were recrystallized with methanol, analyzed by HPLC, and identified by UV, MS, and NMR spectra. NMR experiments were performed with an INOVA-400 NMR spectrometer (Varian, USA). The reference compound tetramethylsilane was used as an internal standard for the determination of chemical shifts.
PDE assay
PDE inhibitory assays were performed according to a previously described two-step radioisotopic procedure [39]. The PDE inhibitory activity was expressed as the half-maximal inhibitory concentration (IC50).
Results and discussion
Optimization of HPLC-DAD-MS analysis
The HPLC analysis of all compounds in natural products is a crucial and challenging task. To the best of our knowledge, lignans in E. ulmoides bark have similar skeletal structures with hydroxyl groups [18]. Since acid is known to produce better resolution for compounds with hydroxyl groups by reducing the peak tailing, acid must be added to the mobile phase [26]. In the course of optimizing the separation conditions, the mobile phase (methanol–water or acetonitrile–water containing different concentrations of formic acid), gradient program (gradient time, gradient shape, and initial composition of the mobile phase), column temperature, and detection wavelength (relatively higher absorption) were investigated. It was found that acetonitrile provided a higher peak resolution and a shorter analysis time than methanol. Moreover, ion signal enhancement in the ESI-MS spectrum was observed when acetonitrile was used, indicating the significance of analyte–solvent interactions during ion generation. Therefore, the acetonitrile–formic acid–water mobile phase was found to be the most appropriate mobile phase, and the final results showed that the best resolution, shortest analysis time, and lowest pressure variations were achieved when a gradient elution mode composed of 0.1 % formic acid in water (solvent A) and 0.1 % formic acid in acetonitrile (solvent B) was programmed as follows: 0–10.0 min, 18.0 % solvent B; 10.0-35.0 min, 18.0-25.0 % solvent B (Fig. 3, chromatogram a). The flow rate was 0.8 ml/min, and the column temperature was set at 25 °C. Spectra were recorded from 190 to 400 nm, and 277 nm was selected as the detection wavelength.
HPLC chromatograms of the lignan-enriched fraction of E. ulmoides bark before (a) and after (b) interaction with phosphodiesterase (PDE). The HPLC conditions were as follows: reversed-phase SunFireTM C18 column (250 mm × 4.6-mm inner diameter, 5 μm); mobile phase consisting of solvent A (0.1 % formic acid in water) and solvent B (0.1 % formic acid in acetonitrile), which was programmed as follows: 0–10.0 min, 18.0 % solvent B, 10.0-35.0 min, 18.0-25.0 % solvent B; flow rate, 0.8 ml/min; UV wavelength, 277 nm; column temperature, 20 °C
Both negative and positive ions were applied in the ESI-MS experiment to analyze compounds. In ESI-MS, gas phase ions are produced, and the MS response of target compounds is largely associated with their ionization and evaporation as well as their interactions with the electrolytes and solvents coexisting in the charged droplets generated at the electrospray tip [40]. The results indicated that the negative ion mode had cleaner ion chromatography, better resolution, and lower background noise in the ESI-MS spectrum than the positive ion mode, which is likely due to the contribution of hydroxyl groups in the compounds. The mobile phase with formic acid as an additive could sometimes enhance the ion signal in negative ion mode, probably because the formation of analyte–formate adduct ions followed by leaving of the neutral formic acid in the gas phase facilitated production of the analyte ions [41]. Therefore, negative ion mode was selected.
Ultrafiltration screening of PDE inhibitors from E. ulmoides bark
The ultrafiltration–HPLC–DAD–MS method can be used for rapid screening of bioactive compounds from complex mixtures, particularly for natural products with a minimum of sample preparation [27–32]. Deyama et al. showed [18] lignans in E. ulmoides have a PDE-inhibiting effect. When the lignan-enriched fraction of E. ulmoides bark was incubated with PDE, active lignans could bind with PDE, and then unbound small molecules could be separated from ligand–PDE complexes or PDE by use of an ultrafiltration membrane. On the basis of this, the ultrafiltration screening method can analyze unbound compounds directly by comparing the HPLC peak areas before and after ultrafiltration: the peak areas of bound compounds will decrease or disappear, whereas for unbound compounds, the peak areas are seldom changed because they freely pass through the filter membrane. The incubation pH, temperature, and time were optimized for higher PDE activity and more compounds binding with PDE. The previous reports indicated that PDE was stabler over a wide range of acidic pH values at higher temperature [42]. The incubation pH and temperature were then set at 6.8 and 37 °C, respectively. The incubation time was estimated in the range from 0.5 to 3.0 h; ultimately, 1.0 h was chosen on account of more bound compounds. Chromatogram b in Fig. 3 shows the ultrafiltration–HPLC analysis of E. ulmoides bark. The peak areas of seven compounds (1–7) reduced; therefore, it could be concluded that compounds 1–7 can bind with PDE. The retention times and UV and MS/MS data of the active compounds are presented in Table 1.
The degree of activity of seven compounds in E. ulmoides bark could be defined as follows: degree of activity = (A b – A a)/A b, where A b and A a are the concentrations of each compound before and after ultrafiltration screening, respectively. The results demonstrate that compound 3 possessed the greatest degree of activity (48.1 %) followed by 1 (42.4 %), 2 (32.7 %), 4 (25.7 %), 7 (24.9 %), 5 (18.0 %), and 6 (8.3 %), which might be caused by the structures.
Preparative HPLC separation
From the HPLC-UV-MS experiment, we can conclude that the molecular weight and partition coefficient between target active compounds and some impurities were relatively close to each other; therefore, this increased the difficulty to separate them. One-step separation was not enough to obtain preferably active compounds with high purity; thus, we combined preparative HPLC and HSCCC.
Preparative HPLC was applied as a powerful technology for the purification of natural products [33] because of its high efficiency, selectivity, online detection, and automatic control. The DAC column performed well with good efficiency and repeatability, which was considered as the optimum selection for preparative HPLC, because the DAC column was axially compressed under constant pressure to maintain a stable bed, and then the packing materials were kept homogenous during the process of repetitive scale-up separation. Gradient elution with ethanol as the organic solvent was selected because it could give satisfactory separation and was economically acceptable. However, it is obvious that the resolution in preparative HPLC decreased compared with that in analytical HPLC, and then isolation of compounds with similar structures and polarities was difficult and challenging. In the first preparative HPLC separation step, the process throughput is more important rather than the target purity, and then the objective was to simplify complex samples into fractions; hence, fast elution was selected using a short column, and a mobile phase with high elution efficiency and high sample loading was recommended. The preparative HPLC procedure realizes online detection of target compounds and automatic control to guarantee standardization, and it is an important industrially applied separation process for the isolation and purification of pharmaceuticals.
In this work, the mobile phase, flow rate, and sample loading amount were optimized. With increase of the content of organic solvent, the flow rate, and the sample loading amount, the purification efficiency of the compounds decreased. Finally, 0.1 % formic acid in water (solvent A) and 0.1 % formic acid in ethanol (solvent B) were used as the mobile phase in stepwise preparative HPLC. The program was set as follows: 0–28 min, 20 % solvent B, 45 ml/min; 28–45 min, 25 % solvent B, 45 ml/min; 45–60 min, 25 % solvent B, 55 ml/min. The sample injection volume for each run was 10 ml and contained 2.0 g sample. Figure 4a shows the stepwise preparative HPLC chromatogram of the lignan-enriched fraction, which yielded 329.4 mg of fraction B1 (mainly containing compounds 1–3; Fig. 4b) and 236.7 mg of fraction B2 (mainly containing compounds 4–7; Fig. 4c). To obtain pure compounds, fractions B1 and B2 were subjected to HSCCC for further purification.
The stepwise preparative HPLC chromatogram of the lignan-enriched fraction of E. ulmoides bark (a), and the analytical HPLC chromatograms of fraction B1 (b) and fraction B2 (c). The preparative HPLC conditions were as follows: reversed-phase C18 column (500 mm × 80-mm inner diameter, 5 μm); mobile phase consisting of solvent A (0.1 % formic acid in water) and solvent B (0.1 % formic acid in ethanol), which was programmed as follows: 0–28 min, 20 % solvent B, 45 ml/min, 28–45 min, 25 % solvent B, 45 ml/min, 45–60 min, 25 % B, 55 ml/min; UV wavelength, 277 nm. The sample injection volume for each run was 10 ml and contained 2.0 g samples. The analytical HPLC parameters were the same as described in the legend for Fig. 3
Preparative HSCCC separation
An appropriate two-phase solvent system plays an important role in successful separation of target compounds in HSCCC. The partition coefficient (K) is the most important parameter in selection of the solvent system, and should be close to 1 to obtain an efficient separation and a suitable run time. If it is much smaller than 1, the compounds will be eluted close to each other near the solvent front, which may result in loss of peak resolution; if K is much greater than 1, the compounds will be eluted in excessively broad peaks, and this may lead to extended elution times [35]. Moreover, the settling time of the two-phase solvent system should be less than 30 s, and satisfactory retention of the stationary phase should also be considered.
The seven target compounds are lignan derivatives considering their two maximum absorption bands at about 226 and 277 nm from the biogenetic point of view, and the relatively shorter retention time could be interpreted as resulting from attached saccharide structures. Compounds 1–3 had a characteristic fragment ion [M-H-162-162]- in the MS/MS spectra, and it was ascribed to the presence of two hexose units. Lignans with greater polarity can be dissolved in water, methanol, and ethanol; therefore, two types of solvent systems based on ethyl acetate–n-butanol–water (2:1:3, 1:1:2, 1:2:3, v/v/v) and ethyl acetate–ethanol–water (4:1:5, 3:2:5, 2:1:3, v/v/v) were used to conduct coefficient tests. The K values in the ethyl acetate–n-butanol–water system were much bigger than 1, indicating broad peaks and long separation time. The ethyl acetate–ethanol–water (2:1:3, v/v/v) system gave acceptable K values for compounds 1 (0.97), 2 (0.81), and 3 (1.72). Compounds 4–7 had smaller polarities than compounds 1–3, and the MS/MS spectra showed the presence of only one hexose unit ([M-H-162]-) for compounds 5–7. Therefore, various solvent systems based on n-hexane–ethyl acetate–methanol–water were used in partition coefficient tests. From comparison of different ratios, 2:5:2:5 (v/v/v/v) n-hexane–ethyl acetate–methanol–water gave suitable partition coefficients for compounds 4 (0.42), 5 (0.86), 6 (1.21), and 7 (2.33).
The rotation speed of the separation column and the flow rate of the mobile phase were also investigated because they can affect the retention of the stationary phase. Different rotation speeds (500, 600, 700, 800, 850, and 1,000 rpm) and flow rates (1.0, 1.2, 1.5, 2.0, and 2.5 ml/min) were examined. The retention of the stationary phase is one of the most important parameters in HSCCC. Successful separation in HSCCC largely depends on the amount of stationary phase retained in the column. In general, the higher the retention of the stationary phase, the better the peak resolution. It was clear that high rotation speed can increase the retention of the stationary phase. High flow rate was unfavorable to the retention of the stationary phase, but a low flow rate can produce good separation, but a longer time and more mobile phase will be needed, and the chromatographic peaks are extended. The result showed that when the flow rate was 1.2 ml/min and the rotation speed was 850 rpm, appropriate retention percentage of the stationary phase and good separation results could be obtained.
Under these optimum conditions, three pure compounds, compounds 1 (62.5 mg, collected during 240–286 min), 2 (2.9 mg, collected during 210–225 min), and 3 (37.2 mg, collected during 325–365 min), were obtained from fraction B1 using a solvent system composed of ethyl acetate–ethanol–water (2:1:3, v/v/v) (Fig. 5a). Similarly, four peaks were collected and concentrated from fraction B2 to yield 54.5 mg of compound 4 (collected during 125–155 min), 6.4 mg of compound 5 (collected during 161–172 min), 10.1 mg of compound 6 (collected during 190–215 min), and 9.8 mg of compound 7 (collected during 285–320 min) using petroleum ether–ethyl acetate–methanol–water (2:5:2:5, v/v/v/v) (Fig. 5b).
HSCCC chromatograms of fraction B1 (a) and fraction B2 (b). (a) Two-phase solvent system: ethyl acetate–ethanol–water (2:1:3, v/v/v). (b) Two-phase solvent system: petroleum ether–ethyl acetate–methanol–water (2:5:2:5, v/v/v/v). The other HSCCC conditions were all the same as follows: stationary phase, upper phase; mobile phase, lower phase; rotation speed, 850 rpm; injection volume, 20 ml; detection wavelength, 277 nm; flow rate, 1.2 ml/min; separation temperature, 25 °C. Compound 1 PDG, compound 2 PGG, compound 3 MDG, compound 4 SDG, compound 5 OG1, compound 6 OG2, compound 7 PG
In addition, all seven compounds were recrystallized and their purities were over 97.0 % by HPLC peak area percentage.
Identification of isolated PDE inhibitors
The structural identification of isolated PDE inhibitors was performed by UV, MS, and 1H NMR analysis (Table 1) and comparison with published data. Compounds 1–7 were finally characterized as (+)-pinoresinol-4,4′-di-O-β-D-glucopyranoside, (+)-pinoresinol-4-O-β-D-glucopyranosyl(1 → 6)-β-D-glucopyranoside, (+)-medioresinol-4,4′-di-O-β-D-glucopyranoside, (+)-syringaresinol-4,4′-di-O-β-D-glucopyranoside, (−)-olivil-4′-O-β-D-glucopyranoside, (−)-olivil-4-O-β-D-glucopyranoside, and (+)-pinoresinol-4-O-β-D-glucopyranoside, respectively [43–45], and compound 2 was first isolated from the genus Eucommia.
PDE inhibitory assay
The PDE inhibitory activities of isolated compounds 1–7 were investigated in a concentration range of 0.02-200 μmol/l. Compounds 1–4 and 7 exhibited effective PDE inhibitory activities with IC50 values of 63.5, 89.4, 42.1, 110.6, and 123.8 μmol/l, respectively, and compounds 5 and 6 had weak PDE inhibitory activities of 200 μmol/l, which were in accordance with the ultrafiltration binding assay. The lignan diglucopyranosides (compounds 1–4) exhibited a greater inhibitory effect than the lignan monoglucopyranosides (compounds 5–7), which was consistent with the findings in a previous report [18].
Conclusions
Seven lignans with PDE-inhibiting activity were successively isolated and purified from E. ulmoides bark by combination of preparative HPLC and HSCCC methods under the guidance of ultrafiltration-based ligand screening. The ultrafiltration method can screen active compounds from complex mixtures. There are too many active compounds with different polarities and contents in crude extracts, and it is comparatively difficult to purify all the compounds in a single run by only preparative HPLC or HSCCC. It is therefore important to combine preparative HPLC and HSCCC because of their complementary and orthogonal properties. Therefore, the method described was proposed as a good strategy to systematically screen and purify active compounds from crude extracts.
References
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Wang X, Li KF, Adams E, Van Schepdael A (2011) Matrix metalloproteinase inhibitors: a review on bioanalytical methods, pharmacokinetics and metabolism. Curr Drug Metabol 12:395–410
Omori K, Kotera J (2007) Overview of PDEs and their regulation. Circ Res 100:309–327
Schenk T, Breel GJ, Koevoets P, van den Berg S, Hogenboom AC, Irth H, Tjaden UR, van den Greef J (2003) Screening of natural products extracts for the presence of phosphodiesterase inhibitors using liquid chromatography coupled online to parallel biochemical detection and chemical characterization. J Biomol Screen 8:421–429
Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M (2010) A review of the herbal phosphodiesterase inhibitors: future perspective of new drugs. Cytokine 49:123–129
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520
Hong ND, Rho YS, Won DH, Kim NJ, Cho BS (1987) Studies on the anti-diabetic activity of Eucommia ulmoides Oliver. Korean J Pharmacog 18:112–117
Horii Y, Tanida M, Shen J, Hirata T, Kawamura N, Wada A, Nagai K (2010) Effects of Eucommia leaf extracts on autonomic nerves, body temperature, lipolysis, food intake, and body weight. Neurosci Lett 479:181–186
Park SA, Choi MS, Jung UJ, Kim MJ, Kim DJ, Park HM, Park YB, Lee MK (2006) Eucommia ulmoides Oliver leaf extract increases endogenous antioxidant activity in type 2 diabetic mice. J Med Food 9:474–479
National Commission of Chinese Pharmacopoeia (2010) Pharmacopoeia of the People's Republic of China, vol 1. China Medical Science and Technology Press, Beijing, P 154
Deyama T, Nishibe S, Nakazawa Y (2001) Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin 22:1057–1070
Takamura C, Hirata T, Ueda T, Ono M, Miyashita H, Ikeda T, Nohara T (2007) Iridoids from the green leaves of Eucommia ulmoides. J Nat Prod 70:1312–1316
Takamura C, Hirata T, Yamaguchi Y, Ono M, Miyashita H, Ikeda T, Nohara T (2007) Studies on the chemical constituents of green leaves of Eucommia ulmoides Oliv. J Nat Med 61:220–221
Luo LF, Wu WH, Zhou YJ, Yan J, Yang GP, Ouyang DS (2010) Antihypertensive effect of Eucommia ulmoides Oliv extracts in spontanesously hypertensive rats. J Ethnopharmacol 129:238–243
Sha ZF, Sun WJ (1986) High performance liquid chromatography of pinoresinol diglucoside in Eucommia ulmoides Oliv bark. Acta Pharmaceut Sin 21:708–711
Li L, Yan J, Hu K, Gu J, Wang JJ, Deng XL, Li H, Jing X, Li ZY, Ye QF, Ouyang DS (2012) Protective effect of Eucommia lignans against hypertensive renal injury by inhibiting expression of aldose reductase. J Ethnopharmacol 139:454–461
Gu J, Wang JJ, Yan J, Cui CF, Wu WH, Li L, Wang ZS, Yu M, Gao N, Liu L, Ouyang DS (2011) Effect of lignans extracted from Eucommia ulmoides and aldose reductase inhibitor epalrestat on hypertensive vascular remodeling. J Ethnopharmacol 133:6–13
Deyama T, Nishibe S, Kitagawa S, Ogihara Y, Takeda T, Ohmoto T, Nikaido T, Sankawa U (1988) Inhibition of adenosine 3',5'-cyclic monophosphate phosphodiesterase by lignin glucosides of Eucommia bark. Chem Pharm Bull 36:435–439
Wen XD, Qi LW, Chen J, Song Y, Yi L, Yang XW, Li P (2007) Analysis of interaction property of bioactive components in Danggui Buxue decoction with protein by microdialysis coupled with HPLC-DAD-MS. J Chromatogr B 852:598–604
Ye G, Li YZ, Li YY, Guo HZ, Guo DA (2003) SPE-HPLC method for the determination and pharmacokinetic studies on pqeoniflorin in rat serum after oral administration of traditional Chinese medicinal preparation Guan-Xin-Er-Hao decoction. J Pharm Biomed Anal 33:521–527
Robards K (2003) Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J Chrom A 1000:657–691
Shi SY, Zhou HH, Zhang YP, Jiang XY, Chen XQ, Huang KL (2009) Coupling HPLC to on-line, post-column (bio)chemical assays for high-resolution screening of bioactive compounds from complex mixtures. Trends Anal Chem 28:865–877
Roldán C, de la Torre A, Mota S, Morales-Soto A, Menédez J, Segura-Carretero A (2013) Identification of active compounds in vegetal extracts based on correlation between activity and HPLC-MS data. Food Chem 136:392–399
Li YJ, Chen J, Li Y, Li Q, Zheng YF, Fu Y, Li P (2011) Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chrom A 1218:8181–8191
Dapkevicius A, van Beek TA, Niederländer HAG (2011) Evaluation and comparison of two improved techniques for the on-line detection of antioxidants in liquid chromatography eluates. J Chrom A 912:73–82
Zhang YP, Shi SY, Xiong X, Chen XQ, Peng MJ (2012) Comparative evaluation of three methods based on high-performance liquid chromatography analysis combined with a 2,2'-diphenyl-1-picrylhydrazyl assay for the rapid screening of antioxidants from Pueraria lobata flowers. Anal Bioanal Chem 402:2965–2976
Kunihiro K (2011) Simplified extraction of tetracycline antibiotics from milk using a centrifugal ultrafiltration device. Food Chem 126:687–690
Yang ZZ, Zhang YF, Sun LJ, Wang Y, Gao XM, Cheng YY (2012) An ultrafiltration high-performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterizing tyrosinase inhibitors from mulberry leaves. Anal Chim Acta 719:87–95
Qian ZM, Qin SJ, Li P, Li HJ, Li P, Wen XD (2008) Binding study of Flos Lonicerae Japonicae with bovine serum albumin using centrifugal ultrafiltration and liquid chromatography. Biomed Chromatogr 22:202–206
Chen XP, Xia YQ, Lu Y, Liang JY (2011) Screening of permeable compounds in Flos Lonicerae Japonicae with liposome using filtration and HPLC. J Pharm Biomed Anal 54:406–410
Luo YD, Wu SS, Li XY, Li P (2010) LC-ESI-MS-MS determination of rat plasma protein binding of major flavonoids of Flos Lonicerae Japonicae by centrifugal ultrafiltration. Chromatographia 72:71–77
Zhang YP, Peng MJ, Liu LL, Shi SY, Peng S (2012) Screening, identification and potential interaction of active compounds from Eucommia ulmodies leaves binding with bovine serum albumin. J Agric Food Chem 60:3119–3125
Lo TC, Nian HC, Chiu KH, Wang AY, Wu BZ (2012) Rapid and efficient purification of chrysophanol in Rheum Palmatum LINN by supercritical fluid extraction coupled with preparative liquid chromatography in tandem. J Chromatogr B 893–894:101–106
Ito Y (2005) Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chrom A 1065:145–168
Shi SY, Ma YJ, Zhang YP, Liu LL, Liu Q, Peng MJ, Xiong X (2012) Systematic separation and purification of 18 antioxidants from Pueraria lobata flower using HSCCC target-guided by DPPH-HPLC experiment. Sep Purif Technol 89:225–233
Schroder M, Vetter W (2011) High-speed counter-current chromatographic separation of phytosterols. Anal Bioanal Chem 400:3615–3623
Yoon KD, Chin YW, Yang MH, Kim J (2011) Separation of anti-ulcer flavonoids from Artemisia extracts by high-speed countercurrent chromatography. Food Chem 129:679–683
DeAmicis C, Edwards NA, Giles MB, Harris GH, Hewitson P, Janaway L, Ignatova S (2011) Comparison of preparative reversed phased liquid chromatography and countercurrent chromatography for the kilogram scale purification of crude spinetoram insecticide. J Chrom A 1218:6122–6127
Yamazaki T, Anraku T, Matsuzawa S (2011) Ibudilast, a mixed PDE3/4 inhibitor, causes a selective and nitric oxide/cGMP-independent relaxation of the intracranial verbrobasilar artery. Eur J Pharmacol 650:605–611
Kebarle P, Verkerk UH (2009) Electrospray: from ions in solution to ions in the gas phase, what we know now. Mass Spectrum Rev 28:898–917
Sun L, Li HK, Willson K, Breidinger S, Rizk ML, Wenning L, Woolf EJ (2012) Ultrasensitive liquid chromatography-tandem mass spectrometric methodologies for quantification of five HIV-1 integrase inhibitors in plasma for a microdose clinical trial. Anal Chem 84:8614–8621
Noguchi S, Shimura G, Kimura K, Samejima H (1976) Production of 5'-mononucleotides using immobilized 5'-phosphodiesterase and 5'-AMP deaminase. Appl Biochem Biotechnol 2:105–118
Abe F, Yamauchi T, Wan ASC (1988) Lignans related to olivil from Cerbera (Cerbera. VI). Chem Pharm Bull 36:795–799
Deyama T (1983) The constituents of Eucommia ulmoides Oliv. I. Isolation of (+)-medioresinol di-O-β-D-glucopyranoside. Chem Pharm Bull 31:2993–2997
Katsuzaki H, Osawa T, Kawakishi S (1994) Chemistry and antioxidative activity of lignan glucosides in sesame seed. In: Ho C-T, Osawa T, Huang M-T, Rosen RT (eds) Food phytochemicals for cancer prevention II. American Chemical Society, Washington, pp 275–280
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21275163, 21005089), the Science and Technology Program of Hunan Province, China (2012FJ2006), the Freedom Explore Program of Central South University (201012200015), the Shenghua Yuying project of Central South University, the Open Fund of the Key Laboratory of Hunan Forest Products and Chemical Industry Engineering, and the Aid Program for Science and Technology Innovative Research Team (Chemicals of Forestry Resources and Development of Forest Products) in Higher Educational Institutions of Hunan Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, SY., Peng, MJ., Zhang, YP. et al. Combination of preparative HPLC and HSCCC methods to separate phosphodiesterase inhibitors from Eucommia ulmoides bark guided by ultrafiltration-based ligand screening. Anal Bioanal Chem 405, 4213–4223 (2013). https://doi.org/10.1007/s00216-013-6806-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6806-4