Abstract
Effect-directed analysis (EDA)-based strategies have been increasingly used in order to identify the causative link between adverse (eco-)toxic effects and chemical contaminants. In this study, we report the development and use of an EDA approach to identify endocrine-disrupting chemicals (EDCs) in a multi-contaminated river sediment. The battery of in vitro reporter cell-based bioassays, measuring estrogenic, (anti)androgenic, dioxin-like, and pregnane X receptor (PXR)-like activities, revealed multi-contamination profiles. To isolate active compounds of a wide polarity range, we established a multi-step fractionation procedure combining: (1) a primary fractionation step using normal phase-based solid-phase extraction (SPE), validated with a mixture of 12 non-polar to polar standard EDCs; (2) a secondary fractionation using reversed-phase-based high-performance liquid chromatography (RP-HPLC) calibrated with 33 standard EDCs; and (3) a purification step using a recombinant estrogen receptor (ER) affinity column. In vitro SPE and HPLC profiles revealed that ER and PXR activities were mainly due to polar to mid-polar compounds, while dioxin-like and anti-androgenic activities were in the less polar fractions. The overall procedure allowed final isolation and identification of new environmental PXR (e.g., di-iso-octylphthalate) and ER (e.g., 2,4-di-tert-butylphenol and 2,6-di-tert-butyl-α-methoxy-p-cresol) ligands by using gas chromatography coupled with mass spectrometry with full-scan mode acquisition in mid-polar fractions. In vitro biological activity of these chemicals was further confirmed using commercial standards, with di-iso-octylphthalate identified for the first time as a potent hPXR environmental agonist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic systems are the receptacle for natural and anthropogenic chemical contaminants, including endocrine-disrupting chemicals (EDCs), that may trigger adverse effects on wildlife and humans [1]. With respect to the implementation of the European Water Framework Directive, which demands a good ecological status by 2015, an increasing expectation to analyze and monitor hazardous chemicals has emerged during the last decade [2]. However, environmental monitoring based on chemical analysis of priority pollutants often fails to identify effective chemicals due to the great diversity of environmentally occurring contaminants which impedes accurate chemical risk assessment. Hence, development of novel strategies for the identification of pollutants that indeed trigger adverse effects could tackle these challenges.
Effect-directed analysis (EDA) aims at identifying biologically active chemicals by using fractionation procedures that reduce the complexity of environmental matrices and allow the progressive isolation of chemicals detected by bioassays [3]. In EDA assessment of EDC-mediated activities, the fractionation procedure often consists of a multiple-step approach using, for instance, a combination of solid-phase extraction (SPE) and high-performance liquid chromatography (HPLC) [4, 5]. However, the majority of existing fractionation methods for sediment extract was mainly developed to study a single class of compounds (i.e., non-polar or polar compounds [3, 6]) and only few studies have dealt with multiple biological endpoints [7, 8]. One common feature of these few studies is the co-occurrence of several biological activities in the same final fractions or group of fractions. Hence, there is still a need to improve and validate fractionation strategies allowing both (1) the separation of chemicals exerting a wide range of polarities (i.e., polar, semi-polar, and non-polar chemicals) and (2) the isolation of active compounds in the final fractions.
To date, EDA has been successfully used for the identification of EDCs able to interfere with hormonal and xenobiotic receptors, such as estrogenic [6, 9], androgenic [4], anti-androgenic [4, 10], and dioxin-like chemicals [11]. Over and above these EDC families, many environmental chemicals can bind to other nuclear receptors that play a critical role in the regulation of the endocrine system and metabolism. For instance, recent studies have shown the occurrence of human pregnane X receptor (hPXR) ligands in river sediment, surface water river, and STP effluents [12–14]. This receptor is known to be activated by a wide range of environmental pollutants including pharmaceuticals and personal care products [12, 15–17] and has been proposed as a xenosensor for the detection of a broad range of environmental chemicals presenting different physicochemical properties (e.g., polarity and solubility). In addition, because hPXR plays a crucial role in the xenobiotic detoxification and the clearance of endogenous hormones [18], abnormal modulation of its activity could lead to an adverse effect. Therefore, the identification of environmental pollutants that can activate this receptor may enhance knowledge on environmental risk.
By using a battery of complementary in vitro bioassays, we previously reported the occurrence of complex toxicity profiles, including PXR-like activity, in a multi-impacted French river [13] where ecotoxicological impacts on fish were evidenced [19]. We showed that bioanalytical characterization based on a mass balance analysis derived from targeted chemical analysis provided only partial information on the identity of chemicals detected by the bioassays. The aim of the present study was thus to implement an EDA approach based on multi-steps fractionation in order to isolate and identify active chemicals occurring in this sediment. This protocol, which has been validated using a broad range of standards, uses normal phase SPE as a first fractionation step, followed by a second fractionation step using reversed-phase-based high-performance liquid chromatography (RP-HPLC). Finally, we focused on the chemical identification of hPXR and human estrogen receptor (hER) ligands. For this latter purpose, an affinity column based on immobilized recombinant hERα was used to purify estrogen receptor (ER) ligands in active fractions [20].
Materials and methods
Chemicals and reagents
Chemicals used as standards for analytical methods (Table 1) were purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France), LGC standard (Molsheim, France), and Cluzeau (Sainte Foy La Grande, France). Acetonitrile (ACN), dichloromethane (DCM), isooctane, and methanol (MeOH; HPLC reagent grade, Scharlau, Spain) were purchased from ICS (Belin Beliet, France). Luciferin and dimethylsulfoxide (DMSO) were purchased from Sigma Aldrich (Saint-Quentin-Fallavier, France).
Study site and sample preparation
The study site is located on the Réveillon River, a small French stream subjected to mixed anthropogenic pressure (i.e., agricultural and urban; GPS coordinates: N 48°34′00″; E 2°32′09″). Sediment was sampled in April 2009 with a grab, sieved (mesh size, 1 mm), freeze-dried, homogenized, and stored in amber glass bottles at −20 °C before analysis.
Sediment was extracted using an accelerated solvent extraction systems (ASE® 350, Dionex, France) equipped with a 10-ml stainless extraction cell vessel. Five grams of freeze-dried sediment were placed in an extraction cell and thoroughly mixed with sand in order to fill the volume of the extraction cell. A mixture of MeOH/DCM (50:50, v/v) was used for the extraction. The operating conditions were the following: extraction temperature, 75 °C; extraction pressure, 1,500 PSI; preheating period, 5 min; static extraction, 5 min; number of extraction cycles, three; final extraction volume, 25 ml; flush volume, 60 % of the cell volume; and nitrogen purge, 60 s. These extraction conditions were previously determined as suitable for the extraction of a broad range of chemicals in terms of both physico-chemical properties and biological activities [21]. The extracts were then evaporated to residues using an EZ-2® solvent evaporator system (Genevac, UK) and dissolved in MeOH/DCM for storage. For in vitro bioassays, aliquots of MeOH/DCM extract were dried to a few microliters and dissolved in DMSO. For fractionation experiments, sediment extracts were dissolved in heptane.
Fractionation strategy
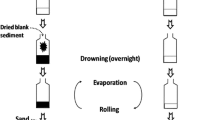
Fractionation of sediment extracts were performed using a sequential procedure based on normal phase-SPE (NP-SPE), RP-HPLC, and recombinant ER-based affinity column (Fig. 1). NP-SPE and RP-HPLC fractionations have been calibrated for the isolation of chemicals exerting a broad range of polarity. This calibration is detailed in the results and discussion section.
Polarity-based fractionation
NP-SPE fractionation was assessed on sediment extract dissolved in 1 ml of heptane, using silica cartridges (500 mg–6 cm3; Supelco, Saint-Quentin-Fallavier, France) previously conditioned with 5 ml of heptane. Sequential elution was performed using heptane (10 ml), heptane/DCM (50:50, v/v; 10 ml), ethyl-acetate (10 ml), and MeOH/H2O (50:50, v/v; 10 ml) leading to four fractions (F1 to F4) of increasing polarity. The obtained fractions were then evaporated to dryness under nitrogen flow (F1, F2, and F3) or using the EZ-2 evaporator (F4) and then dissolved in 1.5 ml ACN. Additionally, the influence of a purification step using an alumina-silica column on the biological activity of F1–F4 fractions was also assessed. This step allows the elimination of polar to semi-polar compounds and is dedicated to the analysis of non-polar chemicals, such as polycyclic aromatic hydrocarbons (PAHs), polychlorobiphenyls (PCBs), and organochlorinated pesticides (OCPs) [22].
The second fractionation step was performed using an RP-HPLC system equipped with a C18 column (Poursuit C18, 5 μm, 250 × 4.6 I.D.; Agilent Technologies, Les Ulis, France), by using an H2O/ACN gradient at a starting ratio of 80:20 (v/v). The run was performed at 25 °C at a flow rate of 1 ml/min with the following gradient program: 0–10 (80:20), 60 (55:45), 100–120 (0:100), and 120.01–125 min (80:20). In the present study, F2 and F3 fractions were fractionated, leading to 40 fractions of 3 ml each (F2.1 to F2.40 and F3.1 to F3.40). The collected fractions were evaporated to dryness using the EZ-2 solvent evaporator system and then dissolved in 200 μl of ACN for storage. Aliquots of this ACN extract were then transferred to DMSO for biological analysis.
ER binding affinity-based fractionation
An hERα affinity column was used to selectively isolate human ERα ligands in HPLC fractions exerting estrogenic activity (Fig. 1), as previously described [20]. All the experiments were carried out at 4 °C. The recombinant histidine-hERα was first immobilized on an Ni-NTA agarose phase (Quiagen, France) in a polytetrafluoroethylene column. In order to eliminate impurities, immobilized receptors were then washed three times with 500 μl of washing buffer (20 mM Tris-HCl, pH 7.5, 300 mM NaCl, 10 % glycerol, 0.1 mg/ml BSA, and 10 mM imidazole). Then, the test sample was diluted (1/100) in washing buffer and loaded on the column. After three washes, hERα ligands were eluted in four fractions (E1 to E4) using an elution buffer (20 mM Tris–HCl, pH 7.5, 300 mM NaCl, 0.1 mg/ml, BSA, and 200 mM imidazole), and the remaining material was eluted using ethanol. Fractions corresponding to flow-through (FT) and washes (L1 to L3) were also collected and processed for further (bio)analysis. The resulting fractions were then concentrated and purified in order to suppress any potential matrix effect in the in vitro bioassays due to buffer components. For this purpose, fractions were first concentrated to 10 μl, redissolved in heptane and then purified on silica cartridges using the NP-SPE fractionation procedure as described above.
Biological analysis and bio-TEQ calculation
Procedures for routine cell culture and sample in vitro testing have been previously described [12]. In brief, MELN [23], MDA-kb2 [24], HG5LN-HPXR [16], and PLHC-1 [25] cell lines were used to assess estrogenic, (anti)-androgenic, PXR-like, and dioxin-like activities, respectively. For the luciferase assay in MELN, MDA-kb2, and HG5LN-hPXR bioassays, cells were seeded in opaque 96-well plates at 105 cells/well. After 24 h, the cells were exposed in triplicate to a serial dilution of crude extract or fractions and incubated for 16 h. The solvent (DMSO) final concentration was always 0.5 % (v/v) in the culture medium. Luciferase activity was assessed by addition of d-luciferin (0.3 mM) and measurement on a microtiter plate luminometer (MicroBeta, Wallac, Perkin-Elmer). For the EROD assay, PLHC-1 cells were seeded in transparent 96-well plates at 5.104 cells/well. After 24 h, the cells were exposed to test samples for 4 and 24 h in order to distinguish between persistent and readily metabolized chemicals. After the addition of 5 μM 7-ethoxyresorufin, EROD activity was assessed on a spectrofluorimeter at excitation/emission wavelengths of 400/460 nm (Saphire2, Tecan, Lyon, France).
Dose-response curves from bioassays were modeled using the Microsoft Excel™ macro RegTox 7.0.3 (freely available at http://www.normalesup.org/~vindimian/fr_index.html). This was used to calculate biological toxic equivalents (bio-TEQ), which were defined as the ratio of the EC20 of the reference compounds to those of the sample extracts in a given assay, as detailed previously [13].
Chemical analyses and chem-TEQ calculation
Targeted chemical analyses in sediment and mass balance analysis
Chemical analyses of steroids, alkylphenols, bisphenol A (BPA), PAHs, PCBs, and OCPs are listed in Table 1. Chemical toxic equivalents (chem-TEQ) were then determined according to the following equation: chem-TEQ = ΣC i × TEF i , where C i is the concentration of compound i in the extract or fraction and TEF i is the toxic equivalent factor (TEF) defined as the ratio of the EC20 of the reference compound to the EC20 of compound i. All TEF values used in this study are provided in Table S1 (Electronic supplementary material (ESM)). They have been determined in each individual assay by testing a range of concentrations (up to 10 μM) of individual chemicals.
Chemical identification of unknown compounds isolated in RP-HPLC fractions
Gas chromatography coupled with mass spectrometry (GC-MS) was used to identify the unknown compounds in active fractions. Analyses were assessed using a GC (Agilent 6890, France) equipped with a HP-5MS fused silica capillary column, coupled to a MS (Agilent 5973) and an autosampler-injector (Agilent 7683). Helium was used as carrier gas at a constant flow rate of 1.3 ml/min. Sample aliquots of 1 μl were injected in splitless mode at an injector temperature of 250 °C. The oven temperature was increased from 50 to 300 °C at a rate of 4 °C/min and held for 6.5 min. The mass range scanned was 50–500 m/z using electron impact ionization at 70 eV.
Results and discussion
Calibration of the NP-SPE and RP-HPLC fractionations
NP-SPE fractionation procedure has been first calibrated for the isolation of organic compounds by using a mixture of 12 active EDCs (E1, E3, 4-tert-octylphenol, BPA, 2,4′DDT, fenofibrate, triphenyl phosphate, clotrimazole, dibenzo[a,h]anthracen, benzo[k]fluranthen, PCB126, and TCDD), which covered a large range of hydrophobicity (i.e., log K OW comprised between 2 and 7). Several different cartridges (silica, alumina, Oasis HLB, C18) were first tested for the isolation of active chemicals (data not shown), and silica cartridges were identified as allowing the best recoveries (Table 2). The established calibration procedure showed a weak matrix effect and good extraction recoveries (i.e., higher than 70 %) for the tested chemicals, as well as an overall weak matrix effect, except for clotrimazole with 63 % of recovery in the spiked blank sediment extract (Table 2).
The elution gradient used for RP-HPLC fractionation was calibrated using a set of 33 chemicals belonging to different organic contaminant families and exerting a broad range of hydrophobicity (log K OW between 0 and 7) (Table S1 in the ESM). The retention time of standard chemicals was correlated to their log K OW (R 2=0.893) (Fig. 2). The RP-HPLC elution profile of these standard EDCs after an NP-SPE pre-fractionation step was then established and is reported in Table 3. These data helped to validate the established fractionation method for the separation of a wide range of EDCs, prior to its application to environmental samples.
Biological and chemical analyses of sediment crude extracts
Estrogenic, anti-androgenic, PXR- and dioxin-like activities were detected in the crude extracts of the Réveillon sediment. Dose response curves allowed bio-TEQ values to be established for each of these activities (Fig. 3). Overall, the bio-TEQ values were quite similar to those previously reported at the same site in sediment sampled in 2005 (data in Fig. 3), suggesting that this river is subject to a constantly high contamination level.
Dose–response curves of crude extract of Réveillon sediment for a estrogenic, b anti-androgenic, c PXR-like, d BaP-like, and e TCDD-like activities. Results are expressed as a percentage of maximal activity induced by a 10-nM 17β-estradiol, b 0.1-nM DHT, c 3-μM SR12813, and d, e 1-nM TCDD. Asterisks, values from Kinani et al. [13] obtained in sediment sampled at the same site in 2005
The targeted chemical analyses revealed the presence of trace levels of natural but not synthetic hormones (E2, testosterone, and progesterone), as well as detectable levels of miscellaneous organic contaminants, such as OCPs, PAHs, PCBs, PBDEs, alkyphenols, and BPA. However, based on a mass balance calculation, the chemical analyses explained only a small part of the activities detected using bioassays (Table S2 in the ESM). These observations are overall in line with those previously reported for the same site [13]. Therefore, fractionation of the crude extract was implemented in order to isolate and identify the active chemicals responsible for the biological activities.
Biological and chemical analyses of NP-SPE fractions
A primary fractionation step was performed in order to obtain an initial characterization of the physico-chemical properties of active ligands. Figure 4 shows that in vitro activities were differently distributed between the four fractions, with anti-androgenic and dioxin-like activities mainly detected in F1 and F2, the less polar fractions, while ER and PXR ligands were essentially in mid-polar to polar fractions (F2 and F3).
Overall, comparison of biological activities between crude and F1–F4 pool extracts suggested limited loss of active chemicals associated with this SPE procedure (Fig. 4). In addition, for most of the activities, the sum of the individual fractions’ activities was equal to those of crude and pool extracts, suggesting an additive effect of active chemicals. Some exceptions were however noted. A significant difference was noted for anti-androgenic activity, which was higher in the crude extract than in pooled fractions suggesting a loss of anti-androgenic chemicals at this stage. For estrogenic activity, NP-SPE fractionation may have highlighted an inhibitory mixture effect in crude and pooled extracts since the sum of E2-EQ in individual F2 and F3 fractions was higher than that measured in global extracts (Fig. 4). Matrix interference might explain such an anti-estrogenic effect as matrix components are isolated from the active chemicals in the fractionation process.
The purification on an alumina-silica micro-column led to the elimination of polar organic contaminants and provided further information on bioactive chemicals. This purification step led to the loss of estrogenic and PXR-like activities while anti-androgenic and dioxin-like activities persisted (Fig. 5). This confirmed that ER and PXR activators were polar compounds whereas AR antagonists and dioxin-like chemicals were mainly non-polar chemicals. Some OCPs, known as anti-androgenic chemicals [10, 26] and AhR active compounds [27], may have contributed to such responses in F1 and F2, respectively. Interestingly, elimination of dioxin-like activity in F3 revealed the occurrence in the sediment extract of polar AhR ligands other than classical dioxin-like chemicals, such as PCBs, PAHs, and PCDD/Fs. These results were in accordance with recent studies reporting the occurrence of such polar AhR ligands in sediment [7, 28, 29].
Finally, targeted chemical analyses on these fractions confirmed the distribution of PAHs, PCBs and OCPs in F1, nonylphenol in F2, and BPA and E2 in F3 (data not shown). These chemical patterns closely correlated with the distribution of biological activities although the major contributors remain to be identified, especially in F2 and F3 where estrogenic and PXR-like activities were poorly explained by target chemicals. To this end, RP-HPLC fractionation was performed on these fractions.
RP-HPLC fractionation of F2 and F3 primary fractions
Firstly, in order to check potential loss of active compounds at this stage, RP-HPLC fractions were pooled and tested in each bioassay and the resulting activity was compared with that of parent F2 or F3 fractions. As seen in Fig. 6, no alteration of PXR-like, anti-androgenic, and dioxin-like activities could be noted. However estrogenic activity in pooled HPLC fractions was about twofold lower than in the initial F3 and F2 fractions (Fig. 6a). These results suggested a loss of active chemicals associated with the hyper-fractionation procedure. Similar observations have been previously reported in other studies. For instance, Brack et al. [30] noted a strong decrease in EROD activity after fractionation by RP-HPLC whereas calibration of the procedure showed recovery higher than 70 %. These authors attributed this loss to irreversible adsorption of organic matter to the stationary phase of the HPLC column and/or to solvent exchange to a polar solvent such as ACN.
The in vitro RP-HPLC profiles of F2 and F3 fractions are presented in Table 4. These profiles highlighted significant dioxin-like activities in two groups of fractions, i.e., F2.24–34 and F3.22–30. This activity was mediated by readily metabolized compounds, such as PAH, since it was almost undetectable after 24 h. Apart from dioxin-like activity, this fractionation step allowed good isolation of ER and PXR activities in a small number of individual fractions. Active fractions for these latter activities were then isolated and further investigated by GC-MS to identify the responsible compounds as described below.
Isolation and identification of PXR ligands
In order to identify PXR activators, the fractions F2.34, F2.35, and F2.36 were injected into GC-MS in full-scan mode acquisition. One major peak was detected in F2.35 while no peak was detected in both F2.34 and F2.36. Comparison with the NIST98 mass spectra database allowed identification of di-isooctylphtalate (DIOP) as a potential PXR ligand (Fig. 7).
It is noteworthy that background contamination of samples by laboratory plastics and equipment is a well-known confounding factor when analyzing phthalates in environmental samples. In the present study, since all procedural blanks were found negative for this compound (Fig. 7), we assumed that DIOP was present in the initial sediment extract and did not come from background contamination by experimental procedure.
Several phthalates have been described as PXR ligands. By using the same cell line as in the present study, Mnif et al. [17] showed that bis(2-ethylhexyl)-phtalate, dibutyl-phtalate, and benzylbutyl-phthalate were able to activate this receptor. Milnes et al. [31] also showed that diethyl-, benzylbutyl-, bis(2-ethylhexyl)-, dicyclo-hexyl-, dibutyl-, n-dipentyl-, n-dipropyl-, and di-n-hexyl-phtalate were PXR activators in transiently transfected COS-7 cells. To our knowledge, hPXR activation by DIOP is reported here for the first time. To confirm its potency, we exposed HG5LN-hPXR and HG5LN cell lines to a range of concentrations of this chemical. A full dose-response curve was obtained in the HG5LN-hPXR cell line, yielding an SR-EF (SR12813 equivalent factor) value of 0.03. The absence of modulation of luciferase activity in HG5LN cells that does not express hPXR (Fig. 8), confirmed DIOP as a specific activator of the hPXR. On the basis of its SR-EF, DIOP could be ranked amongst the most potent activators of hPXR as compared with the miscellaneous chemicals previously tested in the same cell line [12, 16, 17].
RP-HPLC fractionation of F3 allowed the isolation of PXR-like activity in fractions F3.28 to F3.34 (Table 4). These fractions were injected in GC-MS in full-scan mode but no peak was detected. Pre-fractionation calibration showed that the polar F3 pre-fraction may contain chemicals with log K OW values of less than 3, such as steroids, BPA, and parabens (Tables 2 and 3). Therefore, it is likely that GC-MS was not suitable to identify such compounds due to their potential high polarity and/or low volatility properties. Several studies have reported that GC-MS offers a useful first approach for chemical identification due to the reproducibility of the mass spectra in electron impact ionization and the existence of a mass spectra databases [32]. However, the same authors highlighted that such a tool remains limited to the assessment of non polar and volatile chemicals. Hence, liquid chromatography coupled to high-resolution mass spectrometry (e.g., HR-LC-MS, LC-TOF, and LC-LIT-MS) or hydrid techniques (Q-TOF, LTQ-FT-Orbitrap®) will be required to identify hPXR activators in F3. The use of these techniques is increasingly reported for the identification of unknown polar compounds [33, 34].
Identification of ER ligands using an ERα affinity column
The strongest estrogenic activity was detected in F2.30, which exerted 36 % of the maximal response, but also to a lesser extent in F2.31 and F2.32 (Table 4). All of these fractions also possessed dioxin-like activity. In order to isolate ER ligands from AhR ligands, we used an hERα affinity column, which has been successfully used for the isolation of estrogenic activity in environmental matrices [20] or in infant formulas [35]. The three active fractions (F2.30–2.32) were pooled, deposited on the hERα affinity column. Fractions corresponding to FT, washing (L1–L3) and elution (E1–E4) steps were then collected and tested for biological activities. Analysis of bioassays showed that estrogenic activity was only detected in the elution fraction E2 while dioxin-like activity was detected in FT and thus not retained by the column (data not shown). This elution fraction was then injected into GC-MS in full scan mode, allowing the identification of 2,4-di-tert-butylphenol (2,4-t-BP) and 2,6-di-tert-butyl-α-methoxy-para-cresol (DTB) as major compounds (Fig. 9). Alkylphenols have been widely described as endocrine disruptors through their capacity to bind to and activate the ER. Tollefsen et al. [36] have recently described the capacity of 2,4-t-BP to bind to the rainbow trout ER while Sun et al. [37] demonstrated that 4-t-butylphenol activates rat and human ER. To our knowledge, the estrogenic effect of DTB has not been described previously. Butyl-hydroxy-toluene (i.e., BHT or 2,6-di-tert-butyl-para-cresol) and p-cresol, which are structurally closed, were described as human ER ligands [38, 39]. The potency of 2,4-t-BP and DTB to induce luciferase activity in MELN cells has been further confirmed using pure chemicals (Fig. 10).
Estrogenic activity was also detected in F3.6–8, F3.15–16, and F3.18 (Table 4). SPE-HPLC calibration showed the elution of standards n-propylparaben in F3.15, n-butylparaben in F3.18 and BPA and βE2 in F3.16 (Table 3). Kinani et al. reported the occurrence of n-propylparaben, n-butylparaben, BPA, and βE2 in this river sediment [13]. In the present study, targeted chemical analyses confirmed the occurrence of BPA and β-E2 in F3.16 (data not shown). As already observed in crude extract, BPA weakly contributed to estrogenic response in F3.16 whereas β-E2 explained 50 % of the estrogenic activity in this fraction (data not shown).
Other biological activities
Surprisingly, no anti-androgenic activity could be detected in any of the RP-HPLC fractions (data not shown). Nevertheless, the pool of the 40 fractions exerted significant anti-androgenic activity, which was similar to that of the initial F2 (Fig. 6b). This suggested that overall anti-androgenic activity was due to many different chemicals that were distributed over many different fractions, hence impeding their detection in individual fractions. This demonstrated that fractionation strategy remains a critical task when several biological activities are to be considered. To date, only few studies have dealt with EDA an approach using more than two biological endpoints [7, 8, 40]. These studies showed that a reduced number of fractions (3 to 20) often led to the co-occurrence of several biological activities in the same fraction, hence rendering difficult the specific identification of the active chemicals. In the present case study, further investigations, for instance using normal phase-based HPLC fractionation step, will be necessary in order to identify anti-androgenic chemicals.
Regarding dioxin-like activity, it was distributed between many successive fractions yielding a cloudy signal rather than clear isolated peaks. Such a pattern could be explained by either the occurrence of numerous AhR activators that were eluted in different fractions or the occurrence of few AhR ligands with an overlapping elution profile in these fractions. This may suggest that the established RP-HPLC protocol was not adapted to the isolation of AhR ligands. However, Luebcke-von Varel et al. [7] using calibrated NP-HPLC observed a similar display of active fractions.
Conclusions
Our study demonstrated the usefulness of the combined use of multi-receptors and multi-step fractionation approaches to reveal a broad range of EDCs in complex environmental samples. Here, our approach allowed the successful isolation and identification of novel hPXR and ER ligands. In addition, the NR affinity column appears as a useful tool for the final purification of active estrogenic chemicals in case of multiple biological activities. One significant result was the identification of di-iso-octyl-phtalate as a strong inducer of hPXR.
Nevertheless, our results also revealed some limitations of the method as observed with the loss of anti-androgenic activity or the spreading of the dioxin-like activity in the fractions. The fractionation strategy should be considered as a dynamic process that should be modulated for the isolation of active chemicals. Moreover, the use of an NR affinity column on the primary fractions would also provide a powerful alternative to the isolation and identification of new EDCs such as AR or hPXR ligands [41]. Overall, several fractionation tools can be used alone or in combination for the isolation of a broad range of active chemicals in order to enhance knowledge on the occurrence of EDCs in situ.
References
Sumpter JP (2005) Endocrine disrupters in the aquatic environment: an overview. Acta Hydrochim Hydrobiol 33:9–16
Devier MH, Mazellier P, Ait-Aissa S, Budzinski H (2011) New challenges in environmental analytical chemistry: identification of toxic compounds in complex mixtures. C R Chim 14:766–779
Brack W (2003) Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures? Anal Bioanal Chem 377:397–407
Hill EM, Evans KL, Horwood J, Rostkowski P, Oladapo FO, Gibson R, Shears JA, Tyler CR (2010) Profiles and some initial identification of (anti)androgenic compounds in fish exposed to wastewater treatment works effluents. Environ Sci Technol 44:1137–1143
Thomas KV, Hurst MR, Matthiessen P, Waldock MJ (2001) Characterization of estrogenic compounds in water samples collected from United Kingdom estuaries. Environ Toxicol Chem 20:2165–2170
Houtman CJ, Booij P, Jover E, del Rio DP, Swart K, van Velzen M, Vreuls R, Legler J, Brouwer A, Lamoree MH (2006) Estrogenic and dioxin-like compounds in sediment from Zierikzee harbour identified with CALUX assay-directed fractionation combined with one and two dimensional gas chromatography analyses. Chemosphere 65:2244–2252
Luebcke-von Varel U, Machala M, Ciganek M, Neca J, Pencikova K, Palkova L, Vondracek J, Loeffler I, Streck G, Reifferscheid G, Flueckiger-Isler S, Weiss JM, Lamoree M, Brack W (2011) Polar compounds dominate in vitro effects of sediment extracts. Environ Sci Technol 45:2384–2390
Schmitt C, Streck G, Lamoree M, Leonards P, Brack W, de Deckere E (2010) Effect directed analysis of riverine sediments—the usefulness of Potamopyrgus antipodarum for in vivo effect confirmation of endocrine disruption. Aquat Toxicol 101:237–243
Houtman CJ, Cenijn PH, Hamers T, Lamoree MH, Legler J, Murk AJ, Brouwer A (2004) Toxicological profiling of sediments using in vitro bioassays, with emphasis on endocrine disruption. Environ Toxicol Chem 23:32–40
Weiss JM, Hamers T, Thomas KV, van der Linden S, Leonards PEG, Lamoree MH (2009) Masking effect of anti-androgens on androgenic activity in European river sediment unveiled by effect-directed analysis. Anal Bioanal Chem 394:1385–1397
Brack W, Schirmer K (2003) Effect-directed identification of oxygen and sulfur heterocycles as major polycyclic aromatic cytochrome P4501A–inducers in a contaminated sediment. Environ Sci Technol 37:3062–3070
Creusot N, Kinani S, Balaguer P, Tapie N, LeMenach K, Maillot-Marechal E, Porcher JM, Budzinski H, Ait-Aissa S (2010) Evaluation of an hPXR reporter gene assay for the detection of aquatic emerging pollutants: screening of chemicals and application to water samples. Anal Bioanal Chem 396:569–583
Kinani S, Bouchonnet S, Creusot N, Bourcier S, Balaguer P, Porcher JM, Ait-Aissa S (2010) Bioanalytical characterisation of multiple endocrine- and dioxin-like activities in sediments from reference and impacted small rivers. Environ Pollut 158:74–83
Mnif W, Dagnino S, Escande A, Pillon A, Fenet H, Gomez E, Casellas C, Duchesne MJ, Hernandez-Raquet G, Cavailles V, Balaguer P, Bartegi A (2010) Biological analysis of endocrine-disrupting compounds in Tunisian sewage treatment plants. Arch Environ Cont Toxicol 59:1–12
Kretschmer XC, Baldwin WS (2005) CAR and PXR: xenosensors of endocrine disrupters? Chem-Biol Interact 155:111–128
Lemaire G, Mnif W, Pascussi JM, Pillon A, Rabenoelina F, Fenet H, Gomez E, Casellas C, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P (2006) Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol Sci 91:501–509
Mnif W, Pascussi JM, Pillon A, Escande A, Bartegi A, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P (2007) Estrogens and antiestrogens activate hPXR. Toxicol Lett 170:19–29
Orans J, Teotico DG, Redinbo MR (2005) The nuclear xenobiotic receptor pregnane X receptor: recent insights and new challenges. Mol Endocrinol 19:2891–2900
Sanchez W, Ait-Aissa S, Palluel O, Ditche J-M, Porcher J-M (2007) Preliminary investigation of multi-biomarker responses in three-spined stickleback (Gasterosteus aculeatus L.) sampled in contaminated streams. Ecotoxicology 16:279–287
Pillon A, Boussioux AM, Escande A, Ait-Aissa S, Gomez E, Fenet H, Ruff M, Moras D, Vignon F, Duchesne MJ, Casellas C, Nicolas JC, Balaguer P (2005) Binding of estrogenic compounds to recombinant estrogen receptor-α: application to environmental analysis. Environ Health Persp 113:278–284
Creusot N (2011) Contribution de l’approche effect directed analysis à l’identification de perturbateurs endocriniens dans les milieux aquatiques. Thesis University of Bordeaux I, Bordeaux, p 288
Cailleaud K, Forget-Leray J, Souissi S, Hilde D, LeMenach K, Budzinski H (2007) Seasonal variations of hydrophobic organic contaminant concentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoida, copepoda). Part 1: PCBs and PAHs. Chemosphere 70:270–280
Balaguer P, Francois F, Comunale F, Fenet H, Boussioux AM, Pons M, Nicolas JC, Casellas C (1999) Reporter cell lines to study the estrogenic effects of xenoestrogens. Sci Tot Environ 233:47–56
Wilson VS, Bobseine K, Lambright CR, Gray LE (2002) A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci 66:69–81
Louiz I, Kinani S, Gouze ME, Ben-Attia M, Menif D, Bouchonnet S, Porcher JM, Ben-Hassine OK, Ait-Aissa S (2008) Monitoring of dioxin-like, estrogenic and anti-androgenic activities in sediments of the bizerta lagoon (Tunisia) by means of in vitro cell-based bioassays: contribution of low concentrations of polynuclear aromatic hydrocarbons (PAHs). Sci Tot Environ 402:318–329
Aït-Aïssa S, Laskowski S, Laville N, Porcher JM, Brion F (2010) Anti-androgenic activities of environmental pesticides in the MDA-kb2 reporter cell line. Toxicol in Vitro 24:1979–1985
Kojima H, Takeuchi S, Nagai T (2010) Endocrine-disrupting potential of pesticides via nuclear receptors and aryl hydrocarbon receptor. J Health Sci 56:374–386
Koh CH, Khim JS, Villeneuve DL, Kannan K, Johnson BG, Giesy JP (2005) Instrumental and bioanalytical measures of dioxin-like and estrogenic compounds and activities associated with sediment from the Korean coast. Ecotoxicol Environ Saf 61:366–379
Song MY, Xu Y, Jiang QT, Lam PKS, O’Toole DK, Giesy JP, Jiang GB (2006) Measurement of estrogenic activity in sediments from Haihe and Dagu River, China. Environ Int 32:676–681
Brack W, Schirmer K, Erdinger L, Hollert H (2005) Effect-directed analysis of mutagens and ethoxyresorufin-O-deethylase inducers in aquatic sediments. Environ Toxicol Chem 24:2445–2458
Milnes MR, Garcia A, Grossman E, Grun F, Shiotsugu J, Tabb MM, Kawashima Y, Katsu Y, Watanabe H, Iguchi T, Blumberg B (2008) Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ Health Persp 116:880–885
Schymanski EL, Bataineh M, Goss KU, Brack W (2009) Integrated analytical and computer tools for structure elucidation in effect-directed analysis. Trends Anal Chem 28:550–561
Krauss M, Singer H, Hollender J (2010) LC-high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal Bioanal Chem 397:943–951
Petrovic M, Barceló D (2007) LC-MS for identifying photodegradation products of pharmaceuticals in the environment. Trends Anal Chem 26:486–493
Riu A, Balaguer P, Perdu E, Pandelova M, Piccinelli R, Gustafsson JA, Leclercq C, Schramm KW, Dagnino S, Debrauwer L, Cravedi JP, Zalko D (2008) Characterisation of bioactive compounds in infant formulas using immobilised recombinant estrogen receptor-[alpha] affinity columns. Food Cheml Toxicol 46:3268–3278
Tollefsen K-E, Nilsen J-A (2008) Binding of alkylphenols and alkylated non-phenolics to rainbow trout (Oncorhynchus mykiss) hepatic estrogen receptors. Ecotoxicol Environ Saf 69:163–172
Sun H, Xu X-L, Qu J-H, Hong X, Wang Y-B, Xu L-C, Wang X-R (2008) 4-alkylphenols and related chemicals show similar effect on the function of human and rat estrogen receptor [alpha] in reporter gene assay. Chemosphere 71:582–588
Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, Takatori S, Kitagawa Y, Hori S, Utsumi H (2000) Estrogenic activities of 517 chemicals by yeast two-hybrid assay. J Health Sci 46:282–298
Wada H, Tarumi H, Imazato S, Narimatsu M, Ebisu S (2004) In vitro estrogenicity of resin composites. J Dent Res 83:222–226
Smital T, Terzic S, Zaja R, Senta I, Pivcevic B, Popovic M, Mikac I, Tollefsen KE, Thomas KV, Ahel M (2010) Assessment of toxicological profiles of the municipal wastewater effluents using chemical analyses and bioassays. Ecotoxicol Environ Saf 74:844–851
Dagnino S, Bellet V, Grimaldi M, Riu A, Aït-Aïssa S, Cavaillès V, Fenet H, Balaguer P (2012) Affinity purification using recombinant PXR as a tool to characterize environmental ligands. Environ Toxicol. doi:10.1002/tox.20787
Tapie N (2006) Contamination des écosystèmes aquatiques par les PCB et PBDE: application à l’estuaire de la Gironde. Thesis University of Bordeaux I, Bordeaux
Tapie N, Dévier MH, Soulier C, Creusot N, Le Menach K, Aït-Aïssa S, Vrana B, Budzinski H (2011) Passive samplers for chemical substance monitoring and associated toxicity assessment in water. Wat Sci Technol 63:2418–2426
Togola A, Budzinski H (2007) Analytical development for analysis of pharmaceuticals in water samples by SPE and GC-MS. Anal Bioanal Chem 388:627–635
Cailleaud K, Forget-Leray J, Souissi S, Lardy S, Augagneur S, Budzinski H (2007) Seasonal variation of hydrophobic organic contaminant concentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoid, copepod). Part 2: alkylphenol-polyethoxylates. Chemosphere 70:281–287
Acknowledgments
This study was supported by the French Ministry of Ecology (P190-ECOPI) and by a doctoral fellowship to NC (contract No. CIFRE703/2008). The authors wish to thank Dr. Marina Grimaldi for her help with the purification on the hERα column and two anonymous reviewers for helping to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Creusot, N., Budzinski, H., Balaguer, P. et al. Effect-directed analysis of endocrine-disrupting compounds in multi-contaminated sediment: identification of novel ligands of estrogen and pregnane X receptors. Anal Bioanal Chem 405, 2553–2566 (2013). https://doi.org/10.1007/s00216-013-6708-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6708-5