Abstract
Hydrophilic interaction chromatography (HILIC) coupled with inductively coupled plasma mass spectrometry (ICP-MS) was optimized for speciation analysis of gadolinium-based contrast agents in environmental samples, in particular surface river waters and plants. Surface water samples from the Teltow channel, near Berlin, were investigated over a distance of 5 km downstream from the influx of a wastewater treatment plant. The total concentration of gadolinium increased significantly from 50 to 990 ng L−1 due to the influx of the contrast agents. After complete mixing with the river water, the concentration remained constant over a distance of at least 4 km. Two main substances [Dotarem® (Gd-DOTA) and Gadovist® (Gd-BT-DO3A)] have been identified in the river water using standards. A gadolinium-based contrast agent, possibly Gd-DOTA (Dotarem®), was also detected in water plant samples taken from the Teltow channel. Therefore, uptake of contrast agents [Gadovist® (Gd-BTDO3A), Magnevist® (Gd-DTPA), Omniscan® (Gd-DTPA-BMA), Dotarem® (Gd-DOTA), and Multihance® (Gd-BOPTA)] by plants was investigated in a model experiment using Lepidium sativum (cress plants). HILIC–ICP-MS was used for identification of different contrast agents, and a first approach for quantification using aqueous standard solutions was tested. For speciation analysis, all investigated contrast agents could be extracted from the plant tissues with a recovery of about 54 % for Multihance® (Gd-BOPTA) up to 106 % for Gadovist® (Gd-BT-DO3A). These experiments demonstrate that all contrast agents investigated are transported from the roots to the leaves where the highest content was measured.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadolinium-based contrast agents are frequently used in the field of magnetic resonance imaging (MRI) to enhance the contrast of the image. The effect is due to the presence of seven unpaired electrons in the 4f shell of gadolinium (III), which results in a high magnetic momentum decreasing the spin relaxation time (T1) of near water protons and thus increasing the MRI signal intensity.
Since free Gadolinium (Gd3+) is highly toxic, the contrast agents are most often applied in a chelated form for instance by use of compounds with a polyaminocarboxylic acid structure. It has been shown that upon chelation, the toxicity in mice decreased from a lethal dose (where 50 % of the animals would die, LD50) of 0.35 mmol/kg (GdCl3, i.v.) to 10.6 mmol/kg (Gd-DOTA) [1] together with the positive side effect of a much better water solubility.
The first commercially available gadolinium-based contrast agent was Magnevist® (Gd-DTPA) which was first tested in a clinical application in 1984 [2]. Nowadays, nine different gadolinium-based contrast agents are accepted for medical applications in humans in Germany [3]. These agents can be subdivided into two main classes, macrocyclic complexes (trade names are Dotarem®, ProHance®, Gadovist®) and linear (open chain) complexes (trade names are Magnevist®, Omniscan®, OptiMARK®, MultiHance®, Primovist®, Vasovist®). For the investigations described in this paper, five different gadolinium-based contrast agents were used (see Electronic Supplementary Material, Fig. S1).
Today, in about 25 % of all MRI applications, contrast agents are used [4, 5]. For one application, about 1.2 g of such a contrast agent is needed for an average person with a body weight of about 65 kg [6]. Normally, the contrast agents are excreted after a few hours in a nonmetabolised form [3]. Most of the complexes are not retained in the wastewater treatment plant (WWTP) and might be released into the surface waters of rivers or lakes [7–9]. This is the reason for a positive gadolinium anomaly in rivers and lakes of cities [6, 8–19] first described by Bau et al. [7]. An estimate of the total gadolinium waste of 1,160 kg per year from German hospitals and clinics was given by Kümmerer et al. [20], and this value was confirmed by analytical results from the work of Knappe et al. [10].
Concerning gadolinium speciation, the number of investigations is rather low, and further quantitative speciation studies are urgently needed to understand the fate of the contrast agents in the environment, especially the uptake into biological systems. Hydrophilic interaction chromatography (HILIC) is a powerful analytical technique to separate polar and hydrophilic compounds, like Gd-based contrast agents, on a polar stationary phase and is a good alternative for all the compounds that are retained poorly on reversed-phase columns [21]. For the speciation of gadolinium chelate species in environmental waters, first Künnemeyer et al. [22, 23] and later Raju et al. [24] used HILIC, while Kahakachchi et al. [25] applied reversed-phase chromatography. In these works, several Gd-containing contrast agents were detected in environmental water samples, but so far, only little knowledge exists about the bioavailability and transport of Gd species into biological systems. However, a take up of free gadolinium by different plants is shown in the work of Yang et al., Sun et al. and Giussani et al. [26–28]. Also, the uptake of metal ions can be increased by the addition of complexing agents (e.g. EDTA, EDDS) [29–31]. However, nothing is known if and how contrast agents can be taken up by aquatic bio-systems such as fish, mussels or water plants. The latter would be of interest regarding the question if these substances can enter the food chain.
In this paper, the transport of gadolinium-based contrast agents discharged from a waste water plant into environmental waters will be discussed. Additionally, a speciation analysis using HILIC–ICP-MS of waste and river water was performed to identify the contrast agents of interest and to investigate the possible uptake of contrast agents by plants. For the latter purpose, the interaction of Gd-containing chelates with the model plant Lepidium sativum (cress) will be investigated, and it will be shown that the contrast agents are even transported to the leaves.
Experimental
Instrumentation
The chromatographic separation of contrast agents was performed using an Agilent 1100 series HPLC system comprising a G1379A micro vacuum degasser, a G1312A capillary pump, a G1313A autosampler, a G1316A column oven and a G1314A variable wavelength detector (Agilent Technologies, Waldbronn, Germany), which was coupled to an Agilent 7500cs ICP-MS (Agilent Technologies, Waldbronn, Germany), employing a G3285-80000 micro concentric PFA nebuliser and G1820-65337 Scott type spray chamber (Agilent, Waldbronn, Germany) cooled by a peltier element to 2 °C. The detection was performed with a quadrupole mass spectrometer equipped with an octopole collision cell system. The working parameters are listed in the Electronic Supplementary Material (Table S1). If not mentioned otherwise, the main isotope 158Gd+ was used for detection of Gd. For control of the HPLC and ICP-MS data analysis, the Masshunter Workstation (Agilent Technologies) software was used. Quantification was performed in peak area mode, and chromatograms were plotted with the Origin 8.6.0G software (OrginLab Corporation). For the analysis of the total gadolinium concentration, the plant samples were digested with the microwave digestor ultraClave III (MLS GmbH, Leutkirch, Germany).
HPLC conditions
The separation of the Gd contrast agents was carried out using a zwitterionic ZIC-HILIC column (250 mm × 2.1 mm i.d., 5 μm particle size, 200 Ǻ pore size, SeQuant GmbH, Germany). To prevent the contamination of the main column, a ZIC-HILIC precolumn (20 mm × 2.1 mm i.d., 5 μm particle size, 200 Ǻ pore size, SeQuant GmbH, Germany) was used. The contrast agents were separated in isocratic mode with a flow rate of 0.15 mL min−1. The mobile phase used in this study consists of 20 mmol L−1 ammonium acetate in 65/35 acetonitrile/water (pH 7.3), if not mentioned otherwise. The injection volume was 5 μL. The column temperature was set to 25 °C. Usually, the separation was finished after 25 min.
Hyphenation and ICP-MS conditions
The eluent from the column was transferred to the ICP-MS via a PEEK tubing with an inner diameter of 0.13 mm. All tubing and seals used were resistant to organic solvents. The considerable organic load of the mobile phase resulted in the deposition of elemental carbon on the quartz torch and at the walls of the sampler and the skimmer cone during preliminary measurements, and therefore, an additional gas flow (15 %) in the form of an Ar/O2 mixture (80:20) was added to the plasma gas. Direct measurement of total Gd was performed using an auto sampler G3160A (Agilent Technologies, Germany) and a Micro Mist (200 μL, 504–67, AHF-Analysetechnik AG, Germany) nebuliser coupled with the Agilent ICP-MS 7500cs.
Reagents and standards
The gadolinium-based MRI contrast solutions used in this investigation were Gadovist (Gd-BTDO3A, 1.0 mol L−1) and Magnevist (Gd-DTPA, 0.5 mol L−1) from Bayer Schering Pharma AG (Berlin, Germany), Omniscan (Gd-DTPA-BMA, 0.5 mol L−1) from GE Healthcare Buchler (Braunschweig, Germany), Dotarem (Gd-DOTA, 0.5 mol L−1) from Guerbet (Sulzbach, Germany) and Multihance (Gd-BOPTA, 0.5 mol L−1) from Nycomed GmbH (Konstanz, Germany). All chemicals were used in the highest purity available. Ammonium acetate (p.a., 98 %) was obtained from Merck KGaA (Darmstadt, Germany), and HPLC-grade acetonitrile was obtained from J. T. Baker (Deventer, Holland). Gadolinium standard stock solutions (1,000 mg L−1 Gd for ICP-MS measurement, traceable to NIST SRM Gd2O3 in 2–3 % HNO3, CertiPUR®) and internal standard solutions for ICP-MS measurement thulium (1,000 mg L−1 Tm, traceable to NIST SRM Tm(NO3)3 in H2O CertiPUR®) were procured from Merck KGaA (Darmstadt, Germany). All solutions were prepared in 18 MΩ de-ionized water (milli-Q water) that were filtered through a 0.22-mm Millipak 40 filter (Millipore, Molsheim, France). All dilutions of nitric acid were prepared from freshly prepared sub-boiling nitric acid (p.a., 65 %).
For the total Gd concentration measurements with ICP-MS, all standard solutions were prepared in 1 % HNO3 solution by diluting the Gd standard stock solution. Twenty micrograms per litre of Tm was added to all samples and calibration solutions as internal standard for ICP-MS detection of Gd. For the microwave digestion, freshly prepared sub-boiling nitric acid and hydrogen peroxide (30 %), Suprapur, Merck KGaA (Darmstadt, Germany) were used.
The HPLC mobile phase was prepared by dissolving the appropriate amount of ammonium acetate in water followed by addition of acetonitrile. The mobile phase was filtered prior to application.
Sampling of surface water samples
Water samples were taken from the Teltow channel, near Berlin, where the sewage from the WWTP (wastewater treatment plant) Stahnsdorf enters the channel. Samples of surface water were taken with a polypropylene container on a telescopic arm in a distance of about 2 m from the river bank. The sampling started 500 m upstream to the WWTP and ended 5,000 m downstream. Sampling was carried out in 2 h at ten locations.
The plants were taken near the river bank about 50 m downstream from the incoming wastewater. All samples were kept in polypropylene bottles. The solutions were filtered with a 0.20-μm membrane syringe filter and stored in the dark at 3 °C. A fraction of the solution was acidified with 2 vol% HNO3 and spiked with 20 μg/L thulium as internal standard, and the gadolinium concentration was determined using ICP-MS. As the concentrations of the contrast agents were below the limit of detection/quantification for HPLC–ICP-MS separations, a preconcentration step was necessary. For this purpose, the samples with a volume of 50 mL were evaporated with IR light by soft heating to a fraction of about 1:20 in open polytetrafluoroethylene vessels. The plants sampled in the river were washed in milli-Q water to prevent surface adsorption and dried under support of IR light by soft heating.
Plant experiment
L. sativum seeds were grown under controlled conditions in a glasshouse on cotton and watered by tap water. After 7 days, the shoots were transferred into a polytetrafluoroethylene vessel filled with a solution of 1 mg L−1 (Gd/tap water) of gadolinium-based contrast agent. The solutions were prepared from a main solution (10 mg L−1) and tap water. In comparison to the control group, no side effects such as phytotoxicity were observed. After 3 and 5 days, the topmost centimetre of the growing L. sativum plants were cut off, cleaned with water for removal of surface adsorption of gadolinium-based contrast agents and dried under support of IR light. For analyses of the distribution of contrast agents in leaves, stem and roots, the plants were grown for 2 days in a solution of 10 mg L−1 (Gd/tap water). The dry plant material (10 mg) was digested in 4 mL nitric acid (65 %) and 2 mL hydrogen peroxide (30 %) for 15 h in a 50-mL quartz tube and digested using a microwave-assisted extraction over 115 min with a power of 1,000 W and a pressure of 160 bar. The programme is compiled in the Electronic Supplementary Material (Table S2). The solution for the ICP-MS determination was evaporated in a heating block under heating (150 °C) to 1 mL, transferred into a 10-mL quartz flask, spiked with 20 μg/L thulium (169Tm) as internal standard and filtered with a 0.45-μm-membrane syringe filter.
Extraction of the Gd-based contrast agents from plants
The extraction of gadolinium-based contrast agents was performed according to the procedure developed by Kahakachchi et al. [25]. Dried, homogenised plant material (10 mg) was suspended with 2 mL water in a polyethylene tube, shaken for 1 min and stored afterwards. After 24 h, the contrast agents were extracted by ultrasound sonication, which was performed over 1 h with a power of 120 W with a Sonorex Digital 10p, type DK 102P (Bandelin electronic, Berlin, Germany). The yellow–green suspension was filtered with a 0.45-μm-membrane syringe filter. The extracted gadolinium species were analysed with ZIC-HILIC–ICP-MS (158Gd). For calculation of the extraction yield, the extract was spiked with 20 μg L−1 thulium as internal standard and analysed with ICP-MS (158Gd).
Results and discussion
Optimisation
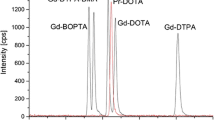
For the analysis of the five contrast agents Multihance (Gd-BOPTA), Magnevist (Gd-DTPA), Dotarem (Gd-DOTA), Omniscan (Gd-DTPA-BMA) and Gadovist (Gd-BTDO3A), the speciation method described by Raju et al. [24] was slightly modified to improve the separation of all five compounds. For this purpose, a longer ZIC-HILIC column (250 × 2.1 mm) was selected, and a higher pH was chosen. The pH of the surface water samples was in the range of 7.3 to 7.6. Therefore, a pH of 7.3 was chosen to prevent decomplexing of the gadolinium contrast agents in the samples [32]. The eluent was adjusted to 20 mmol L−1 ammonium acetate in 65/35 acetonitrile/water with a flow of 150 μL min−1. Under these conditions, the ionic contrast agents eluted first (Gd-BOPTA, Gd-DTPA, Gd-DOTA) followed by the neutral contrast agents (Gd-DTPA, Gd-BT-DO3A) as shown in Fig. 1.
Figures of merit
For each contrast agent, the figures of merit for the ZIC-HILIC–ICP-MS method were determined by a calibration procedure in the concentration range from 0 to 50 μg gadolinium per litre. A linear calibration curve with seven calibration points was obtained over the range of 0.5 to 50 μg L−1 for all substances of interest. The correlation coefficient was found to be better than 0.99 for all Gd contrast agents. All gadolinium species were detected by the 158Gd isotope as mentioned before. The limits of detection (LOD) and limits of quantification (LOQ) were determined by means of 3σ and 9σ in the integration range of each compound. With an injection volume of 5 μL, the LOD and LOQ for all contrast agents were found to be in the range of 51 ± 11 ng L−1 Gd and 153 ± 34 ng L−1 Gd, respectively (see Electronic Supplementary Material Table S3). This is roughly a factor of two worse compared to the work of Raju et al. [24] but is still sufficient for our work.
The LODs and LOQs presented in the Electronic Supplementary Material, Table S3 were obtained by the injection of the standards only. For environmental samples, these LODs are not sufficient, and thus, surface evaporation was used as a species-conserving enrichment procedure for the speciation studies in environmental waters. We have used a moderate enrichment factor of 1:20 only, and by doing this, the LOD and LOQ were improved to a concentration of 2 ± 1 ng L−1 Gd and 7 ± 2 ng L−1 Gd, respectively (See Electronic Supplementary Material, Table S3). More results are discussed in the next section.
Distribution of gadolinium and Gd-containing contrast agents in the Teltow channel
We have studied the dilution of Gd-based contrast agents coming from a wastewater treatment plant at hand of the Teltow channel. This river is very well suited as a model, because the water flow is controlled and regulated and the flow rate is rather independent on the amount of precipitation. In the small city of Stahnsdorf, a wastewater treatment plant is located where the cleaned wastewater is released into the Teltow channel. We have monitored the Gd concentration before and after the outlet of the wastewater treatment at several points. The concentrations were measured by ICP-MS, and the results are shown in Fig. 2. On the day of sampling, the highest value for a Gd concentration of 990 ng L−1 was directly measured in front of the outlet where the concentration is far above the geogenic background value of a few nanograms per litre, causing a strong gadolinium anomaly, which was already described in previous papers [10, 33]. The concentration of Gd decreased in the first kilometre downstream and remained constant already after 2 km at a value of about 99 ± 16 ng L−1. This decrease in concentration fits well to the calculated dilution factor of about 11 representing the ratio between the average upstream flow rate of the Teltow channel of about 561,000 m3/day [34] and the typical outlet water flow rate of the WWTP of about 51.000 m3/day [35]. It was assumed that the high Gd anomaly can be attributed to Gd-containing contrast agents. Therefore, the sampled water was investigated after preconcentration by a factor of about 20 with the speciation method described previously. A typical result for a sample taken directly at the outlet of the WWTP is shown in Fig. 3. Two different gadolinium-containing contrast agents can be separated and identified by comparison with the retention time of the standards: Dotarem (Gd-DOTA) and Gadovist (Gd-BT-DO3A). A small retention time shift was observed only for Gd-DOTA. It should be mentioned that the measured concentration varies strongly from day to day, and even the species composition can change also significantly during a day (data not shown here). Although on this day and location, two contrast agents have been detected, in a previous study, other contrast agents such as Gd-BOPTA and Gd-DTPA have been detected in other locations in the area of Berlin (e.g. Wannsee, Berlin) too [24]. This demonstrates that contrast agents are heavily applied in Berlin, but nothing is known about the total amount discharged per year or which of the contrast agents of interest are preferred in clinical applications.
Both contrast agents of Fig. 3 were also quantified by an external calibration using aqueous standards. The concentration of Gd-DOTA and Gd-BT-DO3A in the WWTP water outlet was found to be approximately 456 and 471 ng L−1, respectively. Further downstream, the concentration of both decreased to about 65 ng L−1 (Table 1). For higher concentrations of the determined compounds down to a few hundred nanograms per litre Gd, an external calibration with aqueous standards can be used. The concentrations of both species detected by HILIC–ICP-MS agree well with the total concentrations of Gd determined in the river water directly (Table 1). At lower concentrations, the good agreement could not be achieved, and losses of particulate matter during filtering or matrix effects from the river water caused by the preconcentration method have to be taken into account. In future works, we will improve our calibration strategy by investigation of a chromatographic internal standard and by use of standard addition methods for matrix containing samples.
However, so far, the data presented in Table 1 confirm already that there is no significant degradation or decomplexation of the gadolinium-based contrast agents over a distance of the first 5 km after the inlet of the wastewater into the Teltow channel, because no additional peaks or shifts in retention time are observed in the chromatograms.
The concentration of 34 ng L−1 measured before the wastewater treatment plant (−500 m) is also far above the geogenic background, which indicates another source or entrance pathway of these contrast agents into the environment further upstream.
Model plant experiments with L. sativum
The high Gd anomaly and the identification of the gadolinium-based contrast agents in the river water raise the question to which extent Gd is accumulated in water plants. A number of diverse plants were sampled from the Teltow channel (near Berlin) downstream of the wastewater plant. The extraction by ultrasound sonication was performed as described in the “Experimental” section. In these plant extracts (results are not shown here), we observed a clear signal of Gd in the chromatogram with a retention time similar to one of the investigated contrast agents (Gd-DOTA) which was also detected in the surface water, and thus, this finding had motivated us to study the uptake of contrast agents by plants in more detail, but under more controlled conditions.
As a model system, L. sativum (cress) plants were selected since they can quickly be grown in the laboratory, and our aim was to study the uptake of contrast agents by the root system of plants. Only by this experimental design can adsorption of the contrast agents on the outer plant surface be avoided, which is not possible in the case of water plant experiments. Additionally, this experiment would answer the question if Gd-containing contrast agents can enter the food chain.
For this purpose, the two different contrast agents Dotarem (Gd-DOTA) and Gadovist (Gd-BT-DO3A) which had been detected in the river water and additionally the open chain complex Multihance (Gd-BOPTA) were used for this model experiment. L. sativum seeds were grown under controlled conditions in a glasshouse on cotton and watered by tap water only. After 7 days, the shoots were transferred into a PTFE vessel containing a solution of the gadolinium-based contrast agents mentioned above with a concentration of 1 mg L−1 (Gd/tap water). No side effects such as phytotoxicity were observed for this concentration. For each contrast agent, after 3 and 5 days, the topmost centimetre of about 50 shoots was cut and dried. Ten grams of the dried plant material was digested with microwave extraction, and the total gadolinium concentration in solution was determined by ICP-MS. A bioconcentration factor (BCF) (Eq. 1) as described in Wild et al. [36] for gadolinium was calculated as the content of total gadolinium in the plant (wet weight) divided by the gadolinium concentration in the treatment solution. The results are compiled in Table 2.
Gd could be detected already in the leaves after 3 days for both contrast agents. This demonstrates that the agents are taken up in significant amounts in a relatively short time. Apparently, the filter system of the root, the casparian band, which normally prevents the uptake of large molecules, is inefficient for the Gd chelates. This might be due to the fact that the casparian band in the young tips of the roots is sometimes not fully established [37], and thus, the complexes can be easily taken up through the apoplastic pathway [30].
The BCF (see Table 2) is close to one for all contrast agents investigated and is independent of the treatment time. The slightly different values are probably due to an inhomogeneous distribution of the analytes in the plants and the very small sample sizes used. This finding suggests that there is no bioaccumulation over the time period of the experiment indicating a passive, only diffusion-driven transport/uptake mechanism in contrast to the active transport known for minerals. For further investigation, the BCF was determined for the roots, stem and leaves separately using Gd-DOTA (see Table 3). The highest factor is observed for the leaves, whereas the Gd concentration in the stem and the roots is lower compared to the treatment solution, and thus, a concentration gradient is established in the plant; therefore, the solution in the plant is not in equilibrium with the solution outside. Due to the fact that the water content is about 90 ± 4 % in all parts of the plant, a homogeneous distribution was expected. Uptake of organic compounds by plants is often described by use of the octanole/water coefficient which is based on the fact that hydrophobic substances show a higher, and hydrophilic substances a lower, BCF [38]; thus, our finding is in contrast to other experiments where, for hydrophilic substances such as our contrast agents, a much lower BCF was observed. To explain the transport and accumulation mechanism in more detail, further experiments, in particular on a long-term basis, are planned in our lab.
In summary, this experiment demonstrates that the uptake of Gd by plants and its transport to and enrichment in the leaves are possible, but it is not clear yet whether or not the contrast agent itself or a metabolite is transported. This will be investigated in more detail in the next section.
Detection of Gd-containing contrast agents in cress leaves
The detection of Gd in the previous experiments shows that the contrast agent is taken up directly by the roots of the cress plant. As the next step, it was necessary to find an effective extraction step to realize speciation analyses of the compounds in plant material. First, experiments just by grinding the plants with a mortar and filtration of the suspension showed a relatively low extraction recovery of about 25 % only. Alternatively to this procedure, Kahakachchi et al. [25] demonstrated that contrast agents from rat kidney tissues can be extracted by ultrasound sonication with water as a solvent. We have applied this procedure for extraction from plant leave cells. The same treatment of the plants has been performed as described in the previous section, but this time for all contrast agents of interest to see if they behave differently. At first, the extraction recovery was calculated as the concentration determined in the leaves of the plant after ultrasound sonication extraction divided by the total gadolinium determined after microwave digestion of the leaves. In each experiment, only a single contrast agent was used. The results obtained are compiled in the Electronic Supplementary Material (Table S4) for all five experiments. The extraction recovery ranged from 54 % for Gd-BOPTA up to 106 % for Gd-BT-DO3A, which demonstrates that ultrasound sonication with water is very efficient for the extraction of contrast agents from leaves. A necessary further optimised procedure with improved sample size, extraction time and solvent is under investigation.
For speciation analysis in the leave samples, a fraction (5 μL) of the extracts of the different plants treated with the contrast agents was injected into the HILIC–ICP-MS system. Typical chromatograms for the leaf extracts are shown in Fig. 4 for all five experiments. A single peak is observed for every contrast agent, and the retention time as well as the peak form fits very well with the chromatogram shown in Fig. 4 for an equimolar mixture of all five contrast agents. Only for Gd-DTPA could a slight shift in retention time be observed which might be related to the high matrix load of the extract injected into the column.
These first results confirm that the contrast agent itself is taken up by the plants and transported to the leaves with no or only minor modifications. Further experiments and application of complementing separation techniques are planned to investigate possible metal exchange processes in the plant system by which free Gd can be produced.
In summary, we have detected Gd-containing contrast agents in environmental surface water samples and could verify for the first time that an uptake of all investigated contrast agents by plants via the root system is possible.
Conclusions
A HILIC–ICP-MS speciation procedure was used to investigate surface water samples taken from the Teltow channel near Berlin. Two typical gadolinium-based contrast agents (Gd-DOTA, Gd-BT-DO3A) were found in these samples. The highest total gadolinium concentration of 990 ng L−1 was measured in samples taken directly in front of the outlet of a wastewater treatment plant. The concentration of the contrast agents decreased significantly after the first kilometre downstream due to dilution with the river water and then remained constant for the next 4 km at a value of about 99 ± 16 ng L−1.
In a model investigation, it was shown that under controlled conditions, the uptake of Gd contrast agents by roots of cress plants takes place. The BCF was found to be one in the leaves, so that the uptake of the contrast agents can be confirmed, but no significant accumulation is observed compared to the concentration of the spiked growing solution under short-term conditions. The BCF in the roots and stem is 0.2 and 0.1, respectively, which is a factor of five lower compared to the concentrations measured in the leaves. By application of an extraction by ultrasound sonication of cress plants with water as a solvent, the recovery for five contrast agents was improved. The recoveries are slightly different for all compounds investigated, and further improvements of the method will be carried out. In treated cress plants, all contrast agents investigated have been detected and identified in leaves by application of HILIC–ICP-MS.
It should be emphasised that many different and important questions remain still unanswered. So far, nothing is known about a long-term bio-enrichment, metal exchange processes, stability, degradation or metabolization of the contrast agents in the environment or in biological systems. Additionally, extensive environmental monitoring programmes are urgently needed to obtain more data about the present distribution and future increase of Gd-containing contrast agents in the environment and in particular in surface water samples.
References
Bousquet JC, Saini S, Stark DD, Hahn PF, Nigam M, Wittenberg J, Ferrucci JT (1988) Gd-DOTA characterization of a new paramagnetic complex. Radiology 166(3):693–698
Weinmann HJ, Brasch RC, Press WR, Wesbey GE (1984) Characteristics of gadolinium-Dtpa complex—a potential NMR contrast agent. Am J Roentgenol 142(3):619–624
Ersoy H, Rybicki FJ (2007) Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 26(5):1190–1197. doi:10.1002/Jmri.21135
Idée J-M, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C (2006) Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol 20(6):563–576. doi:10.1111/j.1472-8206.2006.00447.x
Muroff LR (2001) Contrast: current agents and issues. Appl Radiol 30:5–7
Möller P, Morteani G, Dulski P (2003) Anomalous gadolinium, cerium, and yttrium contents in the Adige and Isarco River Waters and in the water of their tributaries (Provinces Trento and Bolzano/Bozen, NE Italy). Acta Hydrochimica Et Hydrobiologica 31(3):225–239. doi:10.1002/aheh.200300492
Bau M, Dulski P (1996) Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci Lett 143(1–4):245–255
Elbaz-Poulichet F, Seidel JL, Othoniel C (2002) Occurrence of an anthropogenic gadolinium anomaly in river and coastal waters of Southern France. Water Res 36(4):1102–1105
Morteani G, Moller P, Fuganti A, Paces T (2006) Input and fate of anthropogenic estrogens and gadolinium in surface water and sewage plants in the hydrological basin of Prague (Czech Republic). Environ Geochem Heal 28(3):257–264. doi:10.1007/s10653-006-9040-6
Knappe A, Möller P, Dulski P, Pekdeger A (2005) Positive gadolinium anomaly in surface water and ground water of the urban area Berlin, Germany. Chemie der Erde 65:167–189
Rabiet M, Togola A, Brissaud F, Seidel JL, Budzinski H, Elbaz-Poulichet F (2006) Consequences of treated water recycling as regards pharmaceuticals and drugs in surface and ground waters of a medium-sized Mediterranean catchment. Environ Sci Technol 40(17):5282–5288. doi:10.1021/Es060528p
Nozaki Y, Lerche D, Alibo DS, Tsutsumi M (2000) Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and In. Geochimica Et Cosmochimica Acta 64(23):3975–3982
Zhu YB, Hattori R, Rahmi D, Okuda S, Itoh A, Fujimori E, Umemura T, Haraguchi H (2005) Fractional distributions of trace metals in surface water of Lake Biwa as studied by ultrafiltration and ICP-MS. Bull Chem Soc Jpn 78(11):1970–1976. doi:10.1246/Bcsj.78.1970
Zhu Y, Hoshino M, Yamada H, Itoh A, Haraguchi H (2004) Gadolinium anomaly in the distributions of rare earth elements observed for coastal seawater and river waters around Nagoya City. Bull Chem Soc Jpn 77(10):1835–1842. doi:10.1246/Bcsj.77.1835
Verplanck PL, Taylor HE, Nordstrom DK, Barber LB (2005) Aqueous stability of gadolinium in surface waters receiving sewage treatment plant effluent, Boulder Creek, Colorado. Environ Sci Technol 39(18):6923–6929. doi:10.1021/es048456u
Kulaksiz S, Bau M (2007) Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea. Earth Planet Sci Lett 260(1–2):361–371. doi:10.1016/j.epsl.2007.06.016
Lawrence MG, Jupiter SD, Kamber BS (2006) Aquatic geochemistry of the rare earth elements and yttrium in the Pioneer River catchment, Australia. Mar Freshw Res 57(7):725–736. doi:10.1071/Mf05229
Nozaki Y, Lerche D, Alibo DS, Snidvongs A (2000) The estuarine geochemistry of rare earth elements and indium in the Chao Phraya River, Thailand. Geochimica Et Cosmochimica Acta 64(23):3983–3994
Hennebrüder K, Wennrich R, Mattusch J, Stärk H-J, Engewald W (2004) Determination of gadolinium in river water by SPE preconcentration and ICP-MS. Talanta 63(2):309–316
Kümmerer K, Helmers E (2000) Hospital effluents as a source of gadolinium in the aquatic environment. Environ Sci Technol 34(4):573–577. doi:10.1021/es990633h
Hemstrom P, Irgum K (2006) Hydrophilic interaction chromatography. J Sep Sci 29(12):1784–1821. doi:10.1002/jssc.200600199
Künnemeyer J, Terborg L, Nowak S, Scheffer A, Telgmann L, Tokmak F, Günsel A, Wiesmüller G, Reichelt S, Karst U (2008) Speciation analysis of gadolinium-based MRI contrast agents in blood plasma by hydrophilic interaction chromatography/electrospray mass spectrometry. Anal Chem 80(21):8163–8170. doi:10.1021/ac801264j
Künnemeyer J, Terborg L, Meermann B, Brauckmann C, Möller I, Scheffer A, Karst U (2009) Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ Sci Technol 43(8):2884–2890
Raju CSK, Cossmer A, Scharf H, Panne U, Lück D (2010) Speciation of gadolinium based MRI contrast agents in environmental water samples using hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. J Anal At Spectrom 25(1):55–61
Kahakachchi CL, Moore DA (2010) Identification and characterization of gadolinium(III) complexes in biological tissue extracts. Metallomics 2(7):490–497
Yang LH, Wang XR, Sun H, Zhang HS (1999) The effect of EDTA on rare earth elements bioavailability in soil ecosystem. Chemosphere 38(12):2825–2833
Sun H, Wang XR, Wang Q, Wang HT, Wang LS, Chen YJ, Dai LM, Cao M (1997) The effects of chemical species on bioaccumulation of rare earth elements in wheat grown in nutrient solution. Chemosphere 35(8):1699–1707
Giussani A, Heinrichs U, Roth P, Werner E, Schramel P, Wendler A (1998) Biokinetic studies in humans with stable isotopes as tracers. Part 1: a methodology for incorporation of trace metals into vegetables. Isot Environ Heal Stud 34(3):291–296
Nowack B, Schwyzer I, Schulin R (2008) Uptake of Zn and Fe by wheat (Triticum aestivum var. Greina) and transfer to the grains in the presence of chelating agents (ethylenediaminedisuccinic acid and ethylenediaminetetraacetic acid). J Agric Food Chem 56(12):4643–4649. doi:10.1021/jf800041b
Nowack B, Schulin R, Robinson BH (2006) Critical assessment of chelant-enhanced metal phytoextraction. Environ Sci Technol 40(17):5225–5232. doi:10.1021/es0604919
Grcman H, Velikonja-Bolta S, Vodnik D, Kos B, Lestan D (2001) EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching and toxicity. Plant Soil 235(1):105–114
Kretschy D, Koellensperger G, Hann S (2011) Stability assessment of different chelating moieties used for elemental labeling of bio-molecules. Metallomics 3:1304–1309. doi:10.1039/C1MT00114K
Möller P, Dulski P, Bau M, Knappe A, Pekdeger A, Sommer-von Jarmersted C (2000) Anthropogenic gadolinium as a conservative tracer in hydrology. J Geochem Explor 69:409–414
Bittner R (2010) Wasserwirtschaftlicher Monatsbericht Oktober 2010. Senatsverwaltung für Gesundheit, Umwelt und Verbraucherschutz, Berlin
Möller K, Burgschweiger J (2008) Wasserversorgungskonzept für Berlin und für das von der BWB versorgte Umland (Entwicklung bis 2040). Berliner Wasserbetriebe
Wild SR, Jones KC (1992) Organic chemicals entering agricultural soils in sewage sludges: screening for their potential to transfer to crop plants and livestock. Sci Total Environ 119:85–119
Yeo AR, Yeo ME, Flowers TJ (1987) The contribution of an apoplastic pathway to sodium uptake by rice roots in saline conditions. J Exp Bot 38(192):1141–1153
Isnard P, Lambert S (1988) Estimating bioconcentration factors from octanol-water partition coefficient and aqueous solubility. Chemosphere 17(1):21–34
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Metallomics with guest editors Uwe Karst and Michael Sperling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 170 KB)
Rights and permissions
About this article
Cite this article
Lindner, U., Lingott, J., Richter, S. et al. Speciation of gadolinium in surface water samples and plants by hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. Anal Bioanal Chem 405, 1865–1873 (2013). https://doi.org/10.1007/s00216-012-6643-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6643-x