Abstract
An analytical method utilizing chemical ionization gas chromatography-mass spectrometry was developed for the simultaneous determination of cyanide and thiocyanate in plasma. Sample preparation for this analysis required essentially one-step by combining the reaction of cyanide and thiocyanate with pentafluorobenzyl bromide and simultaneous extraction of the product into ethyl acetate facilitated by a phase-transfer catalyst, tetrabutylammonium sulfate. The limits of detection for cyanide and thiocyanate were 1 μM and 50 nM, respectively. The linear dynamic range was from 10 μM to 20 mM for cyanide and from 500 nM to 200 μM for thiocyanate with correlation coefficients higher than 0.999 for both cyanide and thiocyanate. The precision, as measured by %RSD, was below 9 %, and the accuracy was within 15 % of the nominal concentration for all quality control standards analyzed. The gross recoveries of cyanide and thiocyanate from plasma were over 90 %. Using this method, the toxicokinetic behavior of cyanide and thiocyanate in swine plasma was assessed following cyanide exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
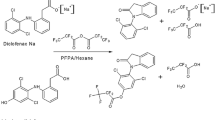
Cyanide, as HCN or CN−, is a deadly chemical that can be introduced into living organisms by a number of means, such as ingestion of edible plants (e.g., cassava, spinach), inhalation of smoke from cigarettes or fires, or accidental exposure during industrial operations (e.g., pesticide production) [1–3]. Once cyanide is introduced into cells, it inhibits cytochrome c oxidase, which subsequently causes cellular hypoxia, cytotoxic anoxia, and may eventually result in death [4]. Several literature sources have reported that the half-life of CN− is less than one hour in mammalian species (e.g., humans, rats, pigs), which makes confirmation of cyanide exposure via direct analysis difficult if a significant amount of time has elapsed between exposure and analysis [3, 5–8]. Therefore, other markers of cyanide exposure have been proposed. One such marker is thiocyanate (SCN−), the major metabolite of cyanide. In the presence of a sulfur donor (e.g., thiosulfate), about 80 % of cyanide is metabolized to thiocyanate through an enzyme catalyzed reaction (Fig. 1) [7–9].
Numerous procedures have been developed for the individual analysis of either cyanide or thiocyanate by gas-chromatography (GC) [2, 10–18]. While cyanide, as HCN, is volatile and may be analyzed by head-space GC [11–13], thiocyanate is not volatile. Therefore, SCN- must be chemically modified to a semi-volatile compound for analysis by GC. Methylation to methyl thiocyanate with dimethyl sulfate [15], conversion to cyanogen chloride by chloramine-T [18, 19], and alkylation with pentafluorobenzyl bromide (PFB-Br) [2, 10, 20] are among the methods that have been reported for analysis of SCN− by GC. After GC separation, CN− and SCN− have been detected using electron capture [21–23], nitrogen-phosphorus detection [24–26], and mass spectrometry (MS) [2, 10, 27]. Although each detector has advantages and disadvantages, MS detectors have several advantages, including extreme sensitivity and the ability to perform stable isotope dilution, which greatly increases the precision of most bioanalytical methods. Therefore, MS detectors are well-suited for detection of trace amounts of chemical substances from biological samples. For a recent review of methods for the analysis of cyanide and thiocyanate, refer to Logue et al. [3].
Within the last decade, there has been a single report of the simultaneous analysis of CN− and SCN− from biological fluids by GC-MS [10]. PFB-Br was used to yield volatile adducts of CN− and SCN− (Fig. 1) from saliva samples, and analysis was performed using electron ionization GC-MS. Although the extraction and analysis of CN− and SCN− were simple, the chemical modification of cyanide and thiocyanate was only 55–65 % efficient, the internal standard did not correct for variations in the derivatization reaction, and the method was only tested with saliva. The detection limits for the method were 1 μM for cyanide and 5 μM for thiocyanate. In addition, attempts to simultaneously analyze plasma CN– and SCN– using the Paul and Smith [10] method in our laboratory resulted in the inability to analyze low concentrations of CN– because of an unresolved interfering species. Therefore, although SCN– was easily analyzed from plasma by Paul and Smith [10] method, a novel method was necessary for simultaneous analysis of CN– and SCN– from plasma.
In the current report, a simple and sensitive chemical ionization-gas chromatography-mass spectrometry (CI-GC-MS) method for the simultaneous detection of cyanide and thiocyanate from plasma is presented. This method was used to determine cyanide and thiocyanate concentrations in swine plasma following cyanide exposure.
Experimental
Reagents and standards
Sodium cyanide (NaCN), sodium tetraborate decahydrate, sodium hydroxide (NaOH), and all solvents (HPLC-grade or higher) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Sodium thiocyanate (NaSCN) was purchased from Acros Organics (Morris Plains, USA). PFB-Br was obtained from Thermo Scientific (Hanover Park, IL, USA). The phase transfer catalyst, tetrabutylammonium sulfate (TBAS; 50 % w/w solution in water) was acquired from Sigma-Aldrich (St. Louis, MO, USA). Isotopically labeled internal standards, NaS13C15N (99 % 13C, 98 % 15N) and Na13C15N (99 % 13C, 98 % 15N), were acquired from Isotech (Miamisburg, OH, USA). HPLC-grade water was used to prepare all aqueous solutions. Single cyanide and thiocyanate stock solutions (1 mM each) were prepared and diluted to the desired working concentrations for all experiments. Note: Cyanide is toxic and is released as HCN in acid solutions. Therefore, all solutions were prepared in a well-ventilated hood, and aqueous standards were prepared in 10 mM NaOH.
Biological fluids
Swine (Sus scrofa) plasma was acquired from three sources: (1) plasma with EDTA anti-coagulant was purchased from Pelfreeze Biological (Rogers, AR, USA), (2) citrate anti-coagulated plasma was obtained through the Veterinary Science Department at South Dakota State University, and (3) cyanide-exposed plasma was acquired from Wilford Hall Medical Center (Lackland Air Force Base, TX). Upon receipt, the plasma was frozen and stored at −80 °C until utilized for optimizing analytical methodologies for sample analysis.
Four swine (about 50 kg each) were injected (intramuscularly) with different doses ranging from 7.5 to 15 mg/kg of potassium cyanide. Arterial blood samples were drawn, and plasma was taken from those blood samples at 13 different time points, including a baseline, 15 min, apnea (around 9 min), and ten additional time points post-apnea (2, 4, 6, 8, 10, 20, 30, 40, 50, and 60 min). The plasma samples were then shipped on ice to SDSU for analysis for CN− and SCN−. The swine study was conducted in accordance with the guidelines stated in “The Guide for the Care and Use of Laboratory Animals” in an AALAS (American Association for Laboratory Animal Science) accredited facility and were approved by the appropriate institutional review boards.
Sample preparation
Spiked and non-spiked biological samples (100 μL) were added to 2 mL micro-centrifuge vials. Aliquots (100 μL each) of Na13C15N (200 μM) and NaS13C15N (100 μM) were added to the sample vials as internal standards along with TBAS (800 μL of 10 mM TBAS in a saturated solution of sodium tetraborate decahydrate, pH 9.5) and PFB-Br (500 μL of a 20 mM solution in ethyl acetate). The solution was vortexed for 2 min, heated at 70 °C in a heating block for 1 h, and centrifuged for 4 min (room temperature) at 10,000 rpm (9,300×g) to separate the organic and aqueous layers. An aliquot (200 μL) of the supernatant organic layer was then transferred into a GC-MS autosampler vial fitted with a 200 μL glass insert for subsequent GC-MS analysis. The total sample preparation time was around 1.5 h and was essentially one step.
Gas chromatography-mass spectrometry
Prepared samples were analyzed for PFB-CN and PFB-SCN using an Agilent Technologies 6890 N gas chromatograph and a 5975B inert XL electron ionization/chemical ionization mass selective detector in CI mode with a 7683 B series autosampler. An 80 % dimethyl–20 % diphenyl polysiloxane capillary column (30 m × 0.25 mm ID, 0.5 μm film thickness; Restek, Bellefonte, PA) was used with helium as the carrier gas at a flow rate of 1 mL/min and a column head pressure of 8.10 psi. The injection (splitless, split delay 1 min) volume was 1 μL, and the injection port was held at 210 °C. The GC oven was initially heated to 60 °C. Upon injection, the temperature was increased to 165 °C at 7 °C/min and then elevated to 270 °C at a rate of 50 °C/min, where it was held for 1 min. The overall analysis time was 18.10 min with PFB-CN and PFB-SCN eluting at approximately 8.3 and 12.1 min, respectively. It is to be noted that the internal standards co-elute with the native species, thus, they all have same retention time as that of the native species. Attempts were made to shorten the overall run time, but the resolution and/or symmetry of the analyte peaks became unacceptable. The MS source and MS quad temperatures were 250 °C and 150 °C, respectively. Methane was used as a reagent gas for positive ion CI with electron energy of 150 eV. The abundant ions of PFB-CN [m/z, 208 (95 %) and 209 (5 %)], PFB-13C15N [m/z, 210 (91 %) and 211 (9 %)], PFB-SCN [m/z, 240 (93 %) and 241(7 %)], and PFB-S13C15N [m/z, 242 (91 %) and 243 (9 %)] were monitored with selected ion monitoring (SIM). It should be noted that the internal standards used only differed by two mass units. Therefore, mass carryover, (i.e., potential overlap between naturally occurring stable isotopes of the target analyte and the labeled internal standard) must be considered, especially at high analyte concentrations (e.g., a significant concentration of stable isotopes from the analyte at may contribute to the internal standard signal causing an overestimation of the internal standard). Therefore, a relatively large concentration of internal standard was used throughout the study.
Calibration, quantification, and limit of detection
Bioanalytical method validation was accomplished by generally following the Food and Drug Administration guidelines [28]. Aqueous cyanide and thiocyanate stock solutions (1 mM each) were used for preparing calibration and quality control (QC) standards. From the stock solutions, calibration standards for CN− (10, 20, 50, 100, 200, 500, and 1,000 μM) and SCN− (0.5, 1, 2, 5, 10, 20, 50, 100, and 200 μM) were prepared in swine plasma. To obtain a calibration equation, the average signal ratios from analyses (i.e., peak-area ratio of the analyte to the internal standard) were plotted as a function of CN− or SCN− concentration. Peak integration was performed manually from baseline to baseline in ChemStation software (Agilent Technologies, Santa Clara, CA). A non-weighted and a weighted (1/x 2) least-squares linear fit were used for cyanide and thiocyanate, respectively. The best model for each analyte was determined by a weighted sum-of-squares analysis.
For determining the upper limit of quantification (ULOQ) and lower limit of quantification (LLOQ), a percent relative standard deviation (%RSD) of <10 % (as a measure of precision) and a percent deviation within ±20 % back-calculated from the nominal concentration of each calibration standard (as a measure of accuracy) were used as inclusion criteria for the calibration standards. QC standards (N = 5) were prepared in swine plasma at three different concentrations—15 (low QC standard), 75 (medium QC standard), and 350 μM (high QC standard) for cyanide and 1.5 (low QC standard), 15 (medium QC standard), and 150 μM (high QC standard) for thiocyanate. The QC standards were analyzed in quintuplicate each day for 3 days and were run in parallel with the calibration standards. Intra-assay precision and accuracy were calculated from each day’s analysis, and inter-assay precision and accuracy were calculated from the comparison of the data gathered from three separate days. It should be noted that the inter-assay and intra-assay studies were conducted within 1 week.
The limit of detection (LOD) was found by analyzing multiple concentrations of CN− and SCN− below the LLOQ and determining the lowest concentration with a signal-to-noise ratio (peak-to-peak) of at least 3.
Selectivity, stability, and recovery
The assay selectivity was defined as the ability to differentiate and quantify the analytes (i.e., PFB-CN and PFB-SCN) in the presence of other components in the sample. Selectivity was determined by comparing three blank samples of swine plasma with spiked swine plasma (350 μM cyanide and 150 μM of thiocyanate) and determining if chemical components in the plasma interfered with the ability to quantify PFB-CN and PFB-SCN. The peak asymmetry (A s) was calculated by dividing the front half-width by the back half-width at 10 % peak height [29].
For evaluating the stability of cyanide and thiocyanate, swine plasma was spiked with high and low QC concentrations of each analyte. These samples were then stored under multiple conditions (−80 °C, −20 °C, 4 °C, and room temperature (RT)) and analyzed over multiple storage times. Cyanide and thiocyanate were considered to be stable under the conditions tested, if the calculated concentration of the stored sample was within 10 % of the initial concentration. For the long-term stability of cyanide and thiocyanate, three aliquots of spiked plasma were stored and analyzed (each in triplicate) on the day of preparation and after 1, 2, 5, 10, and 30 days of storage at the temperatures indicated. Freeze–thaw stability of CN− and SCN− and autosampler stability of PFB-modified CN− and SCN− were also evaluated. For freeze–thaw stability, three aliquots each of the high and low QC standards of both cyanide and thiocyanate were initially analyzed and then stored at −80 °C for 24 h. The samples were then thawed unassisted at room temperature. One set of samples was analyzed, and the non-analyzed samples were refrozen for 24 h at −80 °C. This process was repeated two more times. At the time of each analysis, internal standards were added to correct for variations due to sample preparation and instrumental errors. To determine the autosampler stability of PFB-modified CN− and SCN−, the cyanide and thiocyanate spiked plasma samples were reacted with PFB-Br, placed in the autosampler, and analyzed at approximately 1, 2, 6, 12, and 24 h.
For recovery experiments, three aliquots of high, medium, and low aqueous QC standards of cyanide and thiocyanate were analyzed and compared with plasma samples spiked with equivalent concentrations of cyanide and thiocyanate. The recoveries of cyanide and thiocyanate were calculated as a percentage by dividing the recovered analyte concentration by the calculated concentration of the appropriate aqueous QC standards.
Results and discussion
GC-MS analysis and selectivity
For simultaneous analysis of cyanide and thiocyanate by GC-MS, CN− and SCN− were reacted with PFB-Br to create semi-volatile species, PFB-CN and PFB-SCN (Fig. 1). Representative selected ion chromatograms (SIM) (i.e., m/z = 208 for PFB-CN and m/z = 210 for PFB-SCN) of non-spiked and spiked swine plasma and spiked water sample can be seen in Figs. 2 and 3. PFB-CN and PFB-SCN elute at 8.3 and 12.1 min, respectively. The peak shape for PFB-CN was sharp and symmetrical (A s = 1.14) while the peak for PFB-SCN showed some tailing (A s = 2.40). The method showed excellent selectivity for CN− and SCN− in the presence of other sample constituents. PFB-CN showed no interfering background signal (Fig. 2), and although a small PFB-SCN peak (8.3 μM) does elute from non-spiked swine plasma (Fig. 3, lower trace), this was attributed to endogenous thiocyanate in the plasma as confirmed by MS fragmentation [10, 18]. Multiple studies have shown the presence of SCN− in biological fluids (e.g., plasma, saliva, urine) from subjects not exposed to cyanide. This SCN− likely comes from multiple sources, such as foods (e.g., cheese, milk, cabbage family) [30–32]. The endogenous swine plasma SCN− concentrations for the swine plasma tested in our lab ranged from 8.2–46.6 μM. For comparison, the endogenous plasma SCN− concentrations from humans (non-smokers) range from 4.83–87.5 μM [33–35]. If the peak at 12.1 min is considered endogenous plasma SCN−, both analyte peaks were well-resolved from any interfering peaks (R s = 15 from the nearest peak at 7.7 min for CN− (not shown) and R s = 4 from the nearest visible peak at 11.8 min for SCN−).
Detection limit, calibration, and linearity
The LODs (signal-to-noise-ratios greater than 3:1) for cyanide and thiocyanate were found to be 1 μM and 50 nM, respectively. These limits of detection easily allow quantification of typical biological concentrations of both cyanide and thiocyanate and compare favorably with other similar methods [3]. Kage et al. [2] reported the detection limits for a similar GC-MS method to monitor cyanide and thiocyanate separately in whole blood to be 10 and 3 μM, respectively. Paul and Smith [10] reported limits of detection to be 1 μM for cyanide and 5 μM for thiocyanate from saliva samples.
Using the current method, both calibration curves for cyanide (unweighted) and thiocyanate (weighted, 1/x 2) were found to be linear with correlation coefficients of 0.9999. The calibration curves and the regression equation of both cyanide and thiocyanate in plasma samples are listed in Table 1. The LLOQ was found to be 10 μM for cyanide and 0.5 μM for thiocyanate. The ULOQ was 20 mM for cyanide and 200 μM for thiocyanate. The linear ranges for CN− and SCN− are also presented in Table 1. It is interesting to note that, while the typical linear ranges for GC-MS methods span two orders of magnitude [36–38], the linear range of CN− for this method, spanning over three orders of magnitude, is extraordinarily large. The linear range of SCN− is also excellent, although it does not cover a full three orders of magnitude. The stability of the calibration curve during the interday study was excellent as evident by the stability of the slope—0.0019–0.0022 for CN– and 0.016–0.017 for SCN−. LLOQs and ULOQs were not reported by Kage et al. [2] or Paul and Smith [10].
Accuracy and precision
The accuracy and precision of the method were determined by quintuplicate analysis of three different QC standards (15, 75 , and 350 μM for cyanide; 1.5, 15, and 150 μM for thiocyanate) on three different days (Table 2). The precision of the method was excellent, with both the intra-assay and inter-assay precisions below 9 % RSD. The accuracy for intra-assay and inter-assay analyses was also excellent (±9 % of nominal concentrations). Full accuracy and precision values were not reported for the Kage et al. [2] and Paul and Smith [10] methods, although for a single sample analyzed in quintuplicate, Paul and Smith [10] reported a %RSD of 11.6 % for CN−and 4.3 % for SCN−.
Assay recovery, stability, and robustness
Assay recoveries for cyanide and thiocyanate are reported in Table 3. The recoveries for both cyanide and thiocyanate were excellent at high, medium, and low analyte concentrations. The recovery of cyanide ranged from 91–99 % while the recovery for thiocyanate ranged from 92–93 %. These recoveries are greater than the 80 % recoveries reported by Kage et al. [2] and 55–65 % recoveries reported by Paul and Smith [10].
Cyanide and thiocyanate stabilities were evaluated in spiked plasma at −80, −20, 4 °C, or RT, and for three freeze–thaw (FT) cycles. Cyanide was stable for 2 days at −80, −20, and 4 °C and was quickly removed from plasma at RT (<1 h). For the freeze–thaw stability experiment, the concentrations of cyanide and thiocyanate after one FT cycle were within 10 % of the original concentration for both high and low QC standards (approximately 92 % for cyanide and 97 % for thiocyanate). For FT cycles 2 and 3, the concentrations of cyanide and thiocyanate were below 10 % of the initial concentration for both the high and low QC standards. The concentrations of cyanide and thiocyanate fell after each consecutive FT cycle but, for both cycles 2 and 3, the stability was near 80 % (±6 %) of the original concentration for both the high and low QC standards. Thus, FT experiments suggest that CN− and SCN− are stable for no more than 1 cycle. Low temperatures do increase the stability of CN− as compared with RT, presumably because microbial growth and the rate of enzymatic reactions for cyanide conversion are reduced [39]. Although this is the case, cyanide has been found to be generally unstable at low temperatures compared with other markers of cyanide exposure [40, 41].
The instability of CN− in biological samples was expected because HCN is volatile and is quickly lost from biological samples at pH values below 7–8 (HCN pKa = 9.2). CN− is also nucleophilic and may react with sulfur-containing compounds, aldehydes, or ketones to form cyano-adducts [42]. Previous studies have found that cyanide can convert to SCN− under common storage conditions [3]. Analysis of SCN− concentrations during the stability study shows that this was not the case. Alternatively, cyanide can also be produced from biological samples under certain storage conditions [42]. Many micro-organisms produce cyanide as a result of putrefaction or single-carbon metabolism [43–45], and non-specific oxidative reactions may produce cyanide from organic compounds. The loss of cyanide during the stability experiments indicate that cyanide generation does not occur or is only a minor process. Although it has been found that additives may help reduce cyanide loss or production (e.g., addition of silver ions can help stabilize CN− under storage and active oxygen-scavenging reagents, such as ascorbic acid, reduce cyanide production [40, 43]), the use of additives was not evaluated in this study.
Thiocyanate was stable for up to 5 days at −80, −20, or 4 °C and 1 day at RT. It has been found that SCN− can be converted to cyanide in the presence of erythrocytes [46], or oxidizing agents such as nitrite and hydrogen peroxide in samples under storage or during analysis [45, 47]. Our observations suggest that this mechanism is not a major loss mechanism in this study as no increase in cyanide occurred in the samples as thiocyanate levels decreased. Thiocyanate has also been found to bind to albumin or other proteins which may result in a decrease of free thiocyanate concentrations [48].
Derivatized cyanide and thiocyanate stabilities were evaluated in spiked plasma at approximately 1, 2, 6, 12, and 24 h after placement in an autosampler. The calculated concentrations of both cyanide and thiocyanate were within 10 % of the initial concentration at all times tested. Thus, both derivatized cyanide and thiocyanate were stable for at least 24 h when placed in an autosampler.
Application of the method
The method described in this paper was used to analyze plasma cyanide and thiocyanate concentrations in a toxicokinetics study of acute cyanide exposure in pigs. Figure 4 shows GC-MS total ion chromatographs (TIC) of plasma samples of potassium cyanide (7.5 mg/kg) exposed (upper trace) and non-exposed (lower trace) swine. The peaks of derivatized cyanide (i.e., PFB-CN) and derivatized thiocyanate (i.e., PFB-SCN) are observed at around 8.3 and 12.1 min, respectively. The method presented here performed very well in this study. The simple sample preparation allowed quick analysis of the large number of samples and standards generated from the study and the low LOQs allowed quantification of CN− and SCN− in all plasma samples. The full results of this toxicokinetic study will be published in the near future.
Conclusions
A simple analytical method for the simultaneous determination of cyanide and its major metabolite, thiocyanate, was developed using CI-GC-MS. The described analytical method includes one-step sample preparation and is sensitive, accurate, and precise with high recoveries. In addition, the method described yielded excellent detection limits for both CN− and SCN−, and large linear ranges for CN− and SCN− were observed. Sample preparation was minimal and only lasted approximately 1.5 h for single samples, and within a 24-h period, approximately 70 parallel samples were processed and analyzed. The ability to detect both cyanide and thiocyanate simultaneously provides efficiency and economy of samples and reagents, as well as a reduction in labor cost. The method presented was able to identify cyanide-exposed swine in a pig plasma samples in a toxicokinetics study through analysis of CN− and its major metabolite, SCN−.
References
Baskin SI, Petrikovics I, Kurche JS, Nicholson JD., Logue BA, Maliner BJ, Rockwood GA (2004) Insights on cyanide toxicity and methods of treatment. In: Flora SJS, Romano JA Jr, Baskin SI, Shekhar K (eds) Pharmacological perspectives of toxic chemicals and their antidotes. New Delhi, India: Narosa Publishing House
Kage S, Nagata T, Kudo K (1996) Determination of cyanide and thiocyanate in blood by gas chromatography and gas chromatography-mass spectrometry. J Chromatogr B Biomed Appl 675(1):27–32
Logue BA, Hinkens DM, Baskin SI, Rockwood GA (2010) The analysis of cyanide and its breakdown products in biological samples. Crit Rev Anal Chem 40(2):122–147. doi:10.1080/10408340903535315
Conn EE (1978) Cyanogenesis, the production of cyanide, by plants. In: Keeler RF, Van Kampen KR, James LF (eds) Effects of poisons in plants on livestock. Academic Press, San Diego, pp 301–310
Ballantyne B (1976) Changes in blood cyanide as a function of storage time and temperature. J Forensic Sci Soc 16(4):305–310
Isom GE, Baskin SI (1997) Enzymes involved in cyanide metabolism. In: Sipes IG, McQueen CA, Gandolfi AJ (eds) Comprehensive toxicology. Elsevier Science, New York, NY
Sousa AB, Manzano H, Soto-Blanco B, Gorniak SL (2003) Toxicokinetics of cyanide in rats, pigs and goats after oral dosing with potassium cyanide. Arch Toxicol 77(6):330–334. doi:10.1007/s00204-003-0446-y
Wood JL, Cooley SL (1956) Detoxication of cyanide by cystine. J Biol Chem 218(1):449–457
Ansell M, Lewis FA (1970) A review of cyanide concentrations found in human organs. a survey of literature concerning cyanide metabolism, 'normal', non-fatal, and fatal body cyanide levels. J Forensic Med 17(4):148–155
Paul BD, Smith ML (2006) Cyanide and thiocyanate in human saliva by gas chromatography-mass spectrometry. J Anal Toxicol 30(8):511–515
Calafat AM, Stanfil SB (2002) Rapid quantitation of cyanide in whole blood by automated headspace gas chromatography. J Chromatogr B 772:131–137
Dumas P, Gingras G, LeBlanc A (2005) Isotope dilution-mass spectrometry determination of blood cyanide by headspace gas chromatography. J Anal Toxicol 29(1):71–75
Felby S (2009) Determination of cyanide in blood by reaction head-space gas chromatography. Forensic Sci Med Pathol 5(1):39–43. doi:10.1007/s12024-008-9069-1
Odoul M, Fouillet B, Nouri B, Chambon R, Chambon P (1994) Specific determination of cyanide in blood by headspace gas chromatography. J Anal Toxicol 18(4):205–207
Funazo K, Tanaka M, Shono T (1981) Determination of cyanide or thiocyanate at trace levels by derivatization and gas chromatography with flame thermionic detection. Anal Chem 53:1377–1380
Funazo K, Kusano K, Wu HL, Tanaka M, Shono T (1982) Trace determination of cyanide by derivatization and flame thermionic gas chromatography. J Chromatogr 245:93–100
Liu G, Liu J, Hara K, Wang Y, Yu Y, Gao L, Li L (2009) Rapid determination of cyanide in human plasma and urine by gas chromatography-mass spectrometry with two-step derivatization. J Chromatogr B Anal Technol Biomed Life Sci 877(27):3054–3058. doi:10.1016/j.jchromb.2009.07.029
Thomson I, Anderson RA (1980) Determination of cyanide and thiocyanate in biological fluids by gas chromatography-mass spectrometry. J Chromatogr 188(2):357–362
Segal HS (1962) The microdiffusion separation and determination of microgram quantities of thiocyanate in corn. J Agric Food Chem 10:10–12. doi:10.1021/jf60119a004
Youso SL, Rockwood GA, Lee JP, Logue BA (2010) Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta 677(1):24–28. doi:10.1016/j.aca.2010.01.028
Maseda C, Matsubara K, Shiono H (1989) Improved gas chromatography with electron-capture detection using a reaction pre-column for the determination of blood cyanide: a higher content in the left ventricle of fire victims. J Chromatogr 490(2):319–327
Chen SH, Wu SM, Kou HS, Wu HL (1994) Electron-capture gas chromatographic determination of cyanide, iodide, nitrite, sulfide, and thiocyanate anions by phase-transfer-catalyzed derivatization with pentafluorobenzyl bromide. J Anal Toxicol 18(2):81–85
de Brabander HF, Verbeke R (1977) Determination of thiocyanate in tissues and body fluids of animals by gas chromatography with electron-capture detection. J Chromatogr 138(1):131–142
Takekawa K, Oya M, Kido A, Suzuki O (1998) Analysis of cyanide in blood by headspace solid-phase microextraction (SPME) and capillary gas chromatography. Chromatographia 47(209–214)
Zamecnik J, Tam J (1987) Cyanide in blood by gas chromatography with NP detector and acetonitrile as internal standard. Application on air accident fire victims. J Anal Toxicol 11(1):47–48
Boadas-Vaello P, Jover E, Llorens J, Bayona JM (2008) Determination of cyanide and volatile alkylnitriles in whole blood by headspace solid-phase microextraction and gas chromatography with nitrogen phosphorus detection. J Chromatogr B Anal Technol Biomed Life Sci 870(1):17–21. doi:10.1016/j.jchromb.2008.05.031
Frison G, Zancanaro F, Favretto D, Ferrara SD (2006) An improved method for cyanide determination in blood using solid-phase microextraction and gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 20(19):2932–2938. doi:10.1002/rcm.2689
Food and Drug Administration (2001) Guidance for industry bioanalytical method validation. US Department of Health and Human Services, FDA, Rockville, MD
Foley JP, Dorsey JG (1984) A review of the exponentially modified Gaussian (EMG) function: evaluation and subsequent calculation of universal data. J Chromatogr Sci 22:40–46
Levine MS, Radford EP (1978) Occupational exposures to cyanide in Baltimore fire fighters. J Occup Med 20(1):53–56
Dalferes ER Jr, Webber LS, Radhakrishnamurthy B, Berenson GS (1980) Continuous-flow (autoanalyzer I) analysis for plasma thiocyanate as an index to tobacco smoking. Clin Chem 26(3):493–495
Connolly D, Barron L, Paull B (2002) Determination of urinary thiocyanate and nitrate using fast ion-interaction chromatography. J Chromatogr B Anal Technol Biomed Life Sci 767(1):175–180
Pettigrew AR, Fell GS (1973) Microdiffusion method for estimation of cyanide in whole blood and its application to the study of conversion of cyanide to thiocyanate. Clin Chem 19(5):466–471
Hasuike Y, Nakanishi T, Moriguchi R, Otaki Y, Nanami M, Hama Y, Naka M, Miyagawa K, Izumi M, Takamitsu Y (2004) Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association 19(6):1474–1479. doi:10.1093/ndt/gfh076
Glatz Z, Novakova S, Sterbova H (2001) Analysis of thiocyanate in biological fluids by capillary zone electrophoresis. J chromatogr A 916(1–2):273–277
Logue BA, Kirschten NP, Petrikovics I, Moser MA, Rockwood GA, Baskin SI (2005) Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 819(2):237–244. doi:10.1016/j.jchromb.2005.01.045
Panchal JG, Patel RV, Menon SK (2011) Development and validation of GC/MS method for determination of pramipexole in rat plasma. Biomed Chromatogr 25:524–530
Gerace E, Salomone A, Fasano F, Costa R, Boschi D, Di Stilo A, Vincenti M (2011) Validation of a GC/MS method for the detection of two quinolinone-derived selective androgen receptor modulators in doping control analysis. Anal Bioanal Chem 400(1):137–144. doi:10.1007/s00216-010-4569-8
Cipollone R, Ascenzi P, Tomao P, Imperi F, Visca P (2008) Enzymatic detoxification of cyanide: clues from Pseudomonas aeruginosa Rhodanese. J Mol Microbiol Biotechnol 15(2–3):199–211. doi:10.1159/000121331
Lundquist P, Rosling H, Sorbo B (1985) Determination of cyanide in whole blood, erythrocytes, and plasma. Clin Chem 31(4):591–595
Seto Y (2002) False cyanide detection. Anal Chem 74(5):134A–141A
Askeland RA, Morrison SM (1983) Cyanide production by Pseudomonas fluorescens and Pseudomonas aeruginosa. Appl Environ Microbiol 45(6):1802–1807
Knowles CJ (1976) Microorganisms and cyanide. Bacteriol Rev 40(3):652–680
Seto Y (1995) Oxidative conversion of thiocyanate to cyanide by oxyhemoglobin during acid denaturation. Arch Biochem Biophys 321(1):245–254. doi:10.1006/abbi.1995.1392
Seto Y (1996) Determination of physiological levels of blood cyanide without interference by thiocyanate. Jpn J Toxicol Environ Health 42:319–325
Seto Y, Tsunoda N, Ohta H, Shinohara T (1993) Determination of blood cyanide by headspace gas chromatography with nitrogen-phosphorus detection and using a megabore capillary column. Anal Chim Acta 276:247–259
Vessey CJ, Wilson J (1978) Red cell cyanide. J Pharm Pharmacol 30:20–26
Pollay M, Stevens A, Davis C Jr (1966) Determination of plasma-thiocyanate binding and the Donnan ratio under simulated physiological conditions. Anal Biochem 17(2):192–200
Acknowledgments
The research was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Allergy and Infectious Diseases, Inter Agency Agreement Number Y1-OD-0690-01/A-120-B.P2010-01, Y1-OD-1561-01/A120-B.P2011-01, and the USAMRICD under the auspices of the US Army Research Office of Scientific Services Program Contract No. W911NF-11-D-0001 administered by Battelle (delivery order 0079, contract no TCN 11077). We gratefully acknowledge the funding from the Oak Ridge Institute for Science and Education (ORISE). The authors would also like to acknowledge Dr. George Perry, Associate Professor, Animal and Range Science of South Dakota State University, for making arrangements to provide swine plasma. The authors are thankful to Susan M. Boudreau, RN, BSN, Maria G. Castaneda, MS, Toni E. Vargas, PA-C, MHS, and Patricia Dixon, MHS, from the Clinical Research Division, Wilford Medical Center, Lackland A F B, TX, for providing cyanide exposed swine plasma samples for these studies. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, the National Institutes of Health, or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhandari, R.K., Oda, R.P., Youso, S.L. et al. Simultaneous determination of cyanide and thiocyanate in plasma by chemical ionization gas chromatography mass-spectrometry (CI-GC-MS). Anal Bioanal Chem 404, 2287–2294 (2012). https://doi.org/10.1007/s00216-012-6360-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6360-5