Abstract
Acylcarnitine profiling in dried blood spots (DBS) is a useful method for high-throughput newborn screening of metabolic disorders, but differentiation of isobaric and isomeric compounds is not achievable. Chromatographic methods for separation have already been reported but are specific for short-chain acylcarnitines or time-consuming. The aim of this work was to develop a fast ultraperformance liquid chromatography (UPLC)–tandem mass spectrometry (MS/MS) method for separation and quantification of a large number of acylcarnitines, including dicarboxylic acylcarnitines and hydroxyacylcarnitines, in DBS and plasma samples. Acylcarnitines from DBS and plasma were converted to their butyl esters and analyzed by electrospray ionization MS/MS. Chromatographic separation was achieved using a UPLC system equipped with an ethylene-bridged hybrid C18 column. The correlation coefficients of the calibration curves (r 2) ranged from 0.990 to 0.999. The limit of detection ranged from 0.002 and 0.063 μM for all compounds, and the limit of quantification ranged from 0.004 and 0.357 μM. Precision ranged from 0.8 to 8.8 % and the mean recovery was 103 %. Profiles of acylcarnitine isomers were investigated in specimens obtained from patients diagnosed with different inborn errors of metabolism. Acylcarnitine concentrations were also measured in 58 term newborns and compared with flow injection analysis measurements. With this newly developed UPLC–MS/MS method, the simultaneous detection of 61 (13 of these labeled) acylcarnitines in DBS and plasma can be achieved in 15 min including postrun equilibration. The method has been validated and can be used as an important component of newborn screening methods as a second-tier test for discrimination and to confirm diagnosis.

From LC-MS/MS to UPLC-MS/MS: scheme of the method for simultaneous separation of acylcarnitine in dried blood spot and plasma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acylcarnitine profiling in dried blood spots (DBS) by flow injection analysis (FIA) electrospray ionization (ESI) tandem mass spectrometry (MS/MS) is a useful method for high-throughput newborn screening, which is important in the diagnosis of defects related to fatty acid β-oxidation and branched-chain amino acid metabolism [1]. However, this method cannot differentiate acylcarnitine as isomers, isobaric compounds, and contaminants. Although several high-performance liquid chromatography–MS/MS methods have been developed to separate isomers in serum and urine, they are time-consuming [2–5], need different derivatization steps [3, 5], or are focused on specific acylcarnitines [6, 7]. Moreover, isomeric separation has been performed especially for short-chain acylcarnitines [8, 9], and has not been applied to DBS samples.
Recently a new ultraperformance liquid chromatography (UPLC)–MS/MS method has been developed, but only for identification of C4 and C5 acylcarnitine isomers [10]. The method consists of two diagnostic tests targeted at either C4 or C5 isomers performed in around 10 min to obtain information about disease related to only these specific acylcarnitines.
The aim of our work was to develop a UPLC–MS/MS method for the separation and quantification of a large number of butylated acylcarnitines, including dicarboxylic acylcarnitines and hydroxyacylcarnitines found in DBS and plasma samples, for use as a second-tier test in newborn screening.
This method was successfully applied to analyze DBS and plasma samples of term infants. We also investigated acylcarnitines in patients with different metabolic diseases to assess the identification of characteristic isomers, which are indistinguishable by conventional screening methods.
The concentrations of butyrylcarnitine and isobutyrylcarnitine (C4 and isoC4) are increased in patients with short-chain acyl-CoA dehydrogenase (SCAD) deficiency (OMIM #201470) and with isobutyryl-CoA dehydrogenase deficiency (OMIM *604773), respectively. Five-carbon acylcarnitine has three isomers: isovalerylcarnitine, a diagnostic marker for isovaleryl-CoA dehydrogenase deficiency (OMIM #243500), 2-methylbutyrylcarnitine—concentration elevated in patients with short/branched-chain acyl-CoA dehydrogenase (SBCAD) deficiency, also known as 2-methylbutyryl-CoA dehydrogenase deficiency (OMIM #610006)—and 2,2-dimethylpropionylcarnitine (also known as pivaloylcarnitine), which is generated after administration of pivalate-conjugated antibiotics.
For C5:1 acylcarnitine there are two isomers—tiglylcarnitine and 3-methylcrotonylcarnitine—whose concentrations increase in β-ketothiolase deficiency (OMIM #203750) and in 3-methylcrotonyl-CoA carboxylase (MCC) deficiency (OMIM #210200).
Three C5OH isomers are involved in different forms of organic acidemias: 3-hydroxyisovalerylcarnitine, which appears in patients with MCC deficiency or 3-hydroxy-3-methylglutaryl-CoA lyase deficiency (OMIM #613898) in different types of 3-methylglutaconic aciduria (OMIM #250950, #302060, #258501, 250951), and two diastereoisomers of 2-methyl-3-hydroxybutyrylcarnitine, which appear in disorders of isoleucine metabolism such as 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency (OMIM #300438) and β-ketothiolase deficiency (OMIM #203750). C5OH is also an indicator for disorders of biotin metabolism such as multiple carboxylase deficiency, both early onset (holocarboxylase deficiency, OMIM #253270) and late onset (biotinidase deficiency, OMIM #253260).
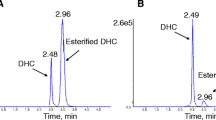
With respect to dicarboxylic acid modified carnitines, separation of methylmalonylcarnitine and succinylcarnitine is important to discriminate methylmalonic aciduria (MMA) from succinyl-CoA synthase deficiency. The discrimination between glutarylcarnitine (C5DC) and 3-hydroxydecanoylcarnitine (C10OH) is important in establishing the diagnosis of glutaric acidemia type 1 (OMIM #231670) as reported by Moore et al. [11].
The identification of all of these isomers is not feasible without chromatographic separation; thus, with our method, a rapid analysis is possible and can be applied to samples previously analyzed by FIA, making this method useful as a confirmatory test for current screening methods and follow-up of disease.
Experimental
Reagents and materials
Stable-isotope-labeled acylcarnitine internal standards—2 H9-dl-free carnitine (d 9-C0), 2 H3-dl-acetylcarnitine (d 3-C2), 2 H3-dl-propionylcarnitine (d 3-C3), 2 H7-dl-butyrylcarnitine (d 7-C4), 2 H9-l-isovalerylcarnitine (d 9-C5), 2 H6-dl-glutarylcarnitine (d 3-C5DC), 2 H3-dl-hexanoylcarnitine (d 3-C6), 2 H3-dl-octanoylcarnitine (d 3-C8), 2 H3- dl -decanoylcarnitine (d 3-C10), 2 H3-dl-dodecanoylcarnitine (d 3-C12), 2 H3-dl-myristoylcarnitine (d3-C14), 2 H3-dl-hexadecanoylcarnitine (d 3-C16), and 2 H3-dl-octadecanoylcarnitine (d 3-C18)—were purchased from ChromSystems Instruments & Chemicals (Munich, Germany).
Unlabeled acylcarnitines—l-carnitine·hydrochloride (C0), acetyl-l-carnitine·hydrochloride (C2), propionyl-l-carnitine·hydrochloride (C3), isobutyryl-l-carnitine·hydrochloride (iso-C4), butyryl-l-carnitine·hydrochloride (C4), isovaleryl-l-carnitine·hydrochloride (iso-C5), valerylcarnitine·hydrochloride (C5), hexanoyl-l-carnitine·hydrochloride (C6), octanoyl-l-carnitine·hydrochloride (C8), decanoylcarnitine hydrochloride (C10), dodecanoylcarnitine hydrochloride (C12), tetradecanoylcarnitine hydrochloride (C14), hexadecanoyl-l-carnitine·hydrochloride (C16), octadecanoyl-l-carnitine·hydrochloride (C18), and pivaloylcarnitine were generous gifts from Piero Rinaldo (Mayo Clinic, Rochester, MN, USA).
Acetonitrile, methanol, and n-butanol were from Carlo Erba (Milan, Italy). Trifluoroacetic acid (TFA) and acetyl chloride were from Sigma-Aldrich (Milan, Italy). All solvents were high-performance liquid chromatography grade, and water was Milli-Q water (Millipore, Billerica, MA, USA). Filter paper (S&S 903) was purchased from Schleicher & Schuell (Dassel, Germany). Eight-strip tubes and dome caps were from Explera (Ancona, Italy), and 96-well plates were purchased from Abgene (part of Thermo Scientific, Epsom, UK).
DBS control level 1 and DBS control level 2 were purchased from ChromSystems Instruments & Chemicals (Munich, Germany).
Sample collection
DBS and plasma were collected, on the third day after birth, from 58 healthy term newborns (mean gestational age 38.8 ± 0.7 weeks) admitted to the Neonatology Unit of the Pediatric Hospital of Padua. Patient compliance was expressed by written informed consent before the newborns were enrolled in the study.
DBS or plasma was also obtained from patients of different ages admitted to the Pediatric Hospital of Padua diagnosed with different mitochondrial enzyme deficiencies: SCAD deficiency (n = 2), medium-chain acyl-CoA dehydrogenase deficiency (n = 3), very long chain acyl-CoA dehydrogenase deficiency (n = 2), long-chain hydroxyl acyl-CoA dehydrogenase deficiency (n = 3), glutaric acidemia type 2 (n = 2), and carnitine palmitoyltransferase II deficiency (n = 2). Patients with organic acidemias such as MMA (n = 6), succinyl-CoA synthase deficiency (n = 2), MCC deficiency (n = 3), 3-hydroxy-3-methylglutaryl-CoA lyase deficiency (n = 1), propionic aciduria (n = 2), and isovaleric acidemia (n = 2) and with other diseases such as SBCAD deficiency (n = 2) were also enrolled. In all cases, diagnoses were confirmed by performing characteristic urine organic acid patterns, tissue enzyme assays, and/or mutation analysis.
Whole blood was drawn by heel prick and spotted on Guthrie filter paper, dried at room temperature, and stored at 4 °C until analysis.
Neonatal plasma was collected (about 10 μL) using heparinized capillary tubes to harvest blood from heel. Plasma aliquots were stored at -20 °C until analysis.
Preparation of standard solutions and calibrations curves
Internal standard solutions were prepared by reconstituting ChromSystems Instruments & Chemicals standards with 50 mL of methanol and adding 100 μL of this solution to each sample. To assess linearity, calibration curves were obtained by applying the stable isotope dilution method. Standard stock solutions at 5-10 mmol/L unlabeled acylcarnitines (C2, C3, isoC4, C4, isoC5, C5, C6, C8, C10, C12, C14, C16, C18) were prepared individually in water. A combined acylcarnitine spiking solution was prepared by diluting the respective stock solutions and this was diluted again to obtain five points in the range of concentration optimized for each acylcarnitine (Table S1). All dilutions were made with methanol, and 30 μL of each solution was added to DBS or plasma.
The endogenous concentrations of the analytes in all matrices were calculated from the value of the intercept with the y-axis of the calibration curves, obtained from peak area ratios of the analytes to the internal standard.
Sample preparation
A 3.2-mm DBS was punched into the strip tube and 100 μL of the reconstituted internal standard was added, the tube was sealed with a cap, and the mixture was sonicated for 20 min.
Plasma (6 μL) was added to 100 μL of the reconstituted internal standard, the mixture was frozen at –20 °C for 10 min, and was then centrifuged at 13,000 rpm for 10 min in a Biofuge Fresco centrifuge (Heraeus, Germany) to remove the precipitated proteins.
The supernatant obtained from DBS or plasma was transferred to a 96-well plate and evaporated under nitrogen flow at 60 °C. Acylcarnitines were derivatized to their butyl esters by adding 100 μL of freshly prepared butanol solution containing 5 % v/v acetyl chloride and heating the mixture at 60 °C for 20 min. Samples are evaporated to dryness under nitrogen flow, reconstituted in 50 μL acetonitrile/water (80:20, v/v), and injected into the UPLC analytical column for chromatographic separation.
The controls were handled in the same manner as patient specimens.
UPLC–MS/MS
MS/MS analysis was performed using a Micromass Quattro Ultima triple-quadrupole mass spectrometer (Waters, Milford, MA, USA) equipped with an ESI source operating in positive mode. Data were acquired with MassLynx 4.1 and processed for calibration and quantification of the analytes with QuanLynx supplied by Waters Technical Services (Etten-Leur, The Netherlands). Optimal fragmentation patterns were previously determined for each acylcarnitine at a concentration of approximately 10 μM.
Data were acquired in multiple reaction monitoring (MRM) mode, with source temperature 90 °C, desolvatation temperature 200 °C, capillary voltage 3.5 kV, and desolvation gas flow 400 L/h. The cone voltage and collision energy were determined for each acylcarnitine (Table 1). Each acylcarnitine concentration was calculated on the basis of the corresponding calibration curve. Compounds for which specific calibration curves were not available were quantified by applying the curve of a metabolite with similar structure or retention time. All acylcarnitines and corresponding labeled internal standards are reported in Table 1.
Sample introduction (7 μL) into a UPLC ethylene bridged hybrid C18 column (15 cm long × 2.1-mm inner diameter with 1.7-μm particles) from Waters was accomplished using an Acquity UPLC system (Waters, Manchester, UK). The chromatographic run was performed at 400 μL/min with a gradient elution of a mobile phase which consisted of solution A (H2O with 0.1 % TFA, v/v) and solution B (acetonitrile with 0.1 % TFA, v/v). The gradient began with 99 % solution A, then was programmed as follows: 0–0.1 min, gradient to 83 % solution A; 0.1–0.28 min, gradient to 76 % solution A; 0.28–0.35 min, gradient to 74 % solution A; 0.35-0.80 min, gradient to 71 % solution A; 0.80-1.71 min, gradient to 69 % solution A; 1.71-2.96 min, gradient to 66 % solution A; 2.96-4.50 min, gradient to 64 % solution A; 4.50-5.44 min, gradient to 44 % solution A; 5.44-6.37 min, gradient to 30 % solution A; 6.37-8.01 min, gradient to 18 % solution A; 8.01-11.30 min, gradient to 7 % solution A; 11.30-12.50 min, gradient to 5 % solution A; 12.50-13.50 min, gradient back to 99 % solution A; 13.50–15 min, hold at 99 % solution A to reequilibrate the column. The total run time was 15 min. The column temperature was 50 °C and the sample temperature was 5 °C. Flow to the mass spectrometer source was reduced by splitting 1:10 with a split tee positioned after the column in order to optimize ionization. Fig. 1 shows a representative total ion chromatogram of mixed standard solution. The chromatograms for different MRM functions, for a newborn DBS specimen, are shown in Fig. S1.
Statistical analysis
For statistical analysis, Prism 4.0 (GraphPad Software), STATISTICA 6.0 (StatSoft), and SPSS 15 (SPSS, Chicago, IL, USA) were used. Significance was defined as p < 0.05.
Results and discussion
Validation studies
Linearity
For the determination of linearity in DBS and plasma, blood from a healthy volunteer was spiked with unlabeled acylcarnitines to the achieve the final concentration ranges reported in Table S1. For regression analysis, the ratio of the analyte signal versus the internal standard signal was plotted against the analyte concentration. The mean slope, y-intercept, standard deviation (SD) of residuals, and coefficient of regression (r 2) are reported in Table S1. Correlation coefficients (r 2) of the calibration curves ranged from 0.990 to 0.999.
The limit of detection (LOD) was determined by a signal-to-noise ratio of 10:1 and the limit of quantification (LOQ) was estimated from the concentration of analyte that can be measured with an imprecision lower than 20 % and a deviation from the target below 20 %. Water and the sample (DBS or plasma) were spiked with decreasing concentrations of analytes to obtain the LOD and LOQ, respectively. The LOD ranged from 0.002 and 0.063 μM for all compounds, and the LOQ ranged from 0.004 and 0.357 μM. Detailed values are also given in Table S1.
Precision, accuracy, and recovery
Interassay and intra-assay precision were assessed over a period of 20 days by measuring DBS controls obtained from ChromSystems Instruments & Chemicals (quality control level 1 and quality control level 2) and two internal quality controls (quality control plasma 1 and quality control plasma 2) at two different concentrations of acylcarnitines. The within-run precision was determined by injection of a duplicate of the same sample in the same analytical session for two different concentrations for each metabolite. The within-day precision was obtained by measuring five replicates on the same day of a duplicate of the same specimen for two different concentrations of each metabolite. The between-day precision on 20 consecutive days was also determined for all metabolites at the same concentrations. The precision ranged from 2.7 to 8.8 % for DBS and from 2.9 to 15.2 % for plasma. All data are reported as the mean, SD, and percent coefficient of variation in Table S2.
Accuracy for DBS was estimated by comparison of the measured concentration (n = 80) in quality control material with expected values given by ChromSystems Instruments & Chemicals. Also, quality control material and proficiency testing specimens from the Newborn Screening Quality Assurance Program of the Centers for Disease Control and Prevention were analyzed and used as internal quality controls.
Since reference material for plasma is not available, accuracy for plasma samples was not estimated by referring to certified values. Accuracy for plasma was calculated by comparison of the measured levels of the spiked analyte with the expected concentration. Accuracy is reported as the percent bias of (measured value - expected value)/expected value (Table S3). The bias was lower than 20 % for all acylcarnitines in DBS controls. In plasma controls, the bias was lower than 30 %.
Recovery was determined from DBS (n = 12) and plasma (n = 20) samples spiked with unlabeled acylcarnitines, and was calculated as the percentage of the difference between the final and the initial concentration divided by the added concentration (Table S3). The mean recovery was 103 % and 102 % for DBS and plasma, respectively.
Concentrations of acylcarnitine in DBS and comparison with FIA
We investigated whether our method could be correlated with FIA. Acylcarnitine concentrations in DBS and plasma obtained from UPLC measurements were compared with those obtained using FIA. FIA measurements were performed on the same derivatized samples, which were introduced into the ESI source of a Quattro Ultima triple-quadrupole instrument by an Alliance HT 2795 separations module (Waters, Manchester, UK). Injections of 10 μL per sample were made into the mobile phase (acetonitrile/water/formic acid; 70:30:0.01, v/v/v) and a flow rate of 0.015 mL/min. The analysis time was 1.9 min per injection. The acylcarnitine profile was obtained by a parent scan of the ion at m/z 85.
The concentrations of metabolites measured in DBS and plasma from healthy newborns by FIA and UPLC, expressed as the mean ± SD, are reported in Table S4.
Similar values were obtained in DBS measurements, with agreement between the average values confirmed by a bias lower than 20 % and always within two SD of the mean. Bland–Altman and x–y plots were generated and the data showed a good fit (r 2 ranged from 0.86 to 0.95) for free carnitine, C2, C3, C12, C14, C16, and C18. The values for C4 and C5 obtained by FIA were higher, as expected, owing to the contribution of isomers, isobutyrylcarnitine and 2-methylbutyryl carnitine, respectively. The concentrations of C6, and C8 acylcarnitines were also higher in FIA measurements, probably owing to background signal. The FIA and UPLC values obtained for all acylcarnitines in DBS of healthy newborns are plotted in Fig. 2.
Comparison of the values obtained by FIA and UPLC analysis from plasma specimens showed that only C2 data were well correlated, whereas data for the other acylcarnitines were slightly or not correlated, and if correlation was present, the r 2 values obtained were low or very low (ranging from 0.8 to 0.1). FIA values were generally higher than UPLC values, denoting a background signal probably due to a matrix effect. The FIA and UPLC values obtained for all acylcarnitines in plasma of healthy newborns are reported in Fig. 3.
Separation of acylcarnitine isomers
DBS and plasma of patients with confirmed disease were examined to obtain isomer profiles to distinguish different types of disease. The identification of metabolites was confirmed against a standard. No interfering compound corresponding to metabolite masses was observed.
Figure 4 shows MRM chromatograms for separation of C4 isomers in DBS and plasma of a healthy newborn, in DBS of a patient with SCAD deficiency, and a corresponding labeled internal standard. The good separation provides rapid discrimination of SCAD deficiency from isobutyryl-CoA dehydrogenase deficiency.
The appearance of isomers was different in DBS and plasma of the healthy newborn: the isobutyrylcarnitine to butyrylcarnitine ratio was greater than 1 (mean 1.4) in DBS, whereas in plasma it was less than 1 (mean 0.9).
Isomeric forms of C4DC are significant for discrimination of MMA from succinyl-CoA synthase deficiency [12]. Figure 5 shows succinylcarnitine and methylmalonylcarnitine separation (retention times 6.23 and 6.35 min) in DBS and plasma of a healthy newborn, in DBS of a patient with MMA, and in DBS of a patient with succinyl-CoA synthase deficiency.
C5 isomers—pivaloylcarnitine (retention time 4.59 min), 2-methylbutyrylcarnitine (retention time 4.86 min), and isovalerylcarnitine (retention time 5.05 min)—are well separated, as shown in Fig. 6. The MRM chromatogram of m/z 311 shows the labeled internal standard of C5, whereas m/z 302.2 represents 2-methylbutyrylcarnitine and isovalerylcarnitine in DBS and plasma of a healthy newborn, in a patient with isovaleric acidemia, and in a patient with SBCAD deficiency. 2-Methylbutyrylcarnitine in the last patient is present as two unresolved peaks corresponding to R and S isomers [10]. The chromatogram at the bottom of Fig. 6 is for a standard solution containing C5 isomers spiked with pivaloylcarnitine. Isomeric separation of C5 isomers was useful in our laboratory for early identification in a case of suspected newborn DBS screening.
Acquired data are routinely compared with cutoff values by software. If an analyte crosses the cutoff value, a flag automatically appears. Following the standard procedure, the sample DBS sample is analyzed. A sample is classified as true-positive only if the first and second test results are positive. Then another DBS specimen is obtained from the infant. Here we report a case of a borderline result, identified early by UPLC analysis. The FIA spectrum showed a small increase (0.41 μM) of the peak at m/z 302 (cutoff 0.40 μM); the C5/C2 ratio was 0.04 (cutoff 0.03), but the C5/C3 ratio was below the cutoff limit. To exclude a false-positive result, with a concentration so close to the cutoff, UPLC analysis was performed and showed the presence of an abnormal quantity of 2-methylbutyrylcarnitine for a healthy newborn. Then a definitive diagnosis of SBCAD deficiency was made. The DBS spectrum and the chromatographic separation of the peak at m/z 302 are reported in Fig. S2.
Moreover, discrimination between C5DC and C10OH, which share the transition m/z 388 to 85, is also possible because of different retention times, 6.41 and 6.82 min, respectively (seen in Fig. S1, function 4, channel 388.2 > 85.2).
Chromatograms related to other isomeric peaks are reported in the electronic supplementary material. Figure S3 depicts ion chromatograms of m/z 318.2, corresponding to C5OH isomers. 3-Hydroxyisovalerylcarnitine present in specimens of plasma and DBS of a healthy newborn, and DBS of a patient with MCC deficiency, a patient with SBCAD deficiency, and a patient with 3-hydroxy-3-methylglutaryl-CoA lyase deficiency is shown. No specimens from a patient with 2-methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency were available; therefore, the peak of 2-methyl-3-hydroxybutyrylcarnitine was not verified. But for the patient with SBCAD deficiency, a shoulder before the peak at 2.60 min was identified at 2.55 min, presumably due to an isomeric form.
Hydroxyacylcarnitines were well identified and separated in DBS, whereas in plasma the peaks are near the LOD. In all samples, C4OH is present as two peaks, at 2.12 and 2.19 min (Fig. S4). Other hydroxyacylcarnitines were well identified in three patients with long-chain hydroxyl acyl-CoA dehydrogenase deficiency, and we found two isomeric forms, R and S, for each of C16OH, C18:2OH, C18:1OH, and C18OH. Minkler et al. [3] demonstrated the presence of isomeric forms of hydroxyacylcarnitine.
Moreover, in one of these patients (Fig. S5) we found an additional peak at m/z 470, presumed to be a C16:1OH isomer, and two additional peaks at m/z 496, related to C18:1OH. Because it was established that the patient had not been treated with cefotaxime at the time of blood sampling, we excluded this peak as being a metabolite of this antibiotic [13]. The identification of these extra peaks and correlation with diagnosis are in progress.
The chromatogram in Fig. S6 shows peak separation of C8 acylcarnitine from valproylcarnitine, which can be produced after treatments with the anticonvulsant drug valproic acid, at 7.05 and 6.86 min, respectively.
Conclusions
We have described the development of a rapid method for identification and quantification of a large number of acylcarnitines (48 metabolites and 13 labeled internal standards), including hydroxyacylcarnitines and dicarboxylic acylcarnitines in DBS and plasma. The use of UPLC–ESI-MS/MS enabled us to separate and quantify all these acylcarnitines in a 15-min chromatographic run. Analysis can be performed with a very small sample volume (one punch of DBS or 6 μL of plasma) and directly on derivatized samples used for newborn screening.
The method is capable of high precision and accuracy, and the separation of isomers makes it clinically suitable for accurate diagnoses as a second-tier test for newborn screening of metabolic diseases.
References
Chace DH, Kalas TA, Naylor EW (2003) Clin Chem 49(11):1797–1817
Ferrer I, Ruiz-Sala P, Vicente Y, Merinero B, Perez-Cerda C, Ugarte M (2007) J Chromatogr B Anal Technol Biomed Life Sci 860(1):121–126
Minkler PE, Stoll MS, Ingalls ST, Yang S, Kerner J, Hoppel CL (2008) Clin Chem 54(9):1451–1462
Maeda Y, Ito T, Suzuki A, Kurono Y, Ueta A, Yokoi K et al (2007) Rapid Commun Mass Spectrom 21(5):799–806
Minkler PE, Ingalls ST, Hoppel CL (2005) Anal Chem 77(5):1448–1457
Jauregui O, Sierra AY, Carrasco P, Gratacos E, Hegardt FG, Casals N (2007) Anal Chim Acta 599(1):1–6
Vernez L, Wenk M, Krahenbuhl S (2004) Rapid Commun Mass Spectrom 18(11):1233–1238
Maeda Y, Ito T, Ohmi H, Yokoi K, Nakajima Y, Ueta A et al (2008) J Chromatogr B Anal Technol Biomed Life Sci 870(2):154–159
Horvath TD, Stratton SL, Bogusiewicz A, Pack L, Moran J, Mock DM (2010) Anal Chem 82(10):4140–4444
Forni S, Fu X, Palmer SE, Sweetman L (2010) Mol Genet Metab 101(1):25–32
Moore T, Le A, Cowan TM (2012) J Inherit Metab Dis 35(3):431–435
Ogier De Baulny H, Dionisi-Vici C, Wendel U (2011) In: Saudubray JM, Van Den Berghe G, Walter JH (eds) Inborn metabolic diseases: diagnosis and treatment, 5th edn. Springer, Heidelberg, pp 277–296
Vianey-Saban C, Boyer S, Levrat V, Cheillan D, Piraud M, Guffon N, Maire I (2004) J Inherit Metab Dis 27(Suppl 1):94
Acknowledgment
We thank Piero Rinaldo of the Mayo Clinic (Rochester, MN, USA) for generous gifts of unlabeled acylcarnitine standards and for the collection of some patient blood samples used in this study.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1090 kb)
Rights and permissions
About this article
Cite this article
Gucciardi, A., Pirillo, P., Di Gangi, I.M. et al. A rapid UPLC–MS/MS method for simultaneous separation of 48 acylcarnitines in dried blood spots and plasma useful as a second-tier test for expanded newborn screening. Anal Bioanal Chem 404, 741–751 (2012). https://doi.org/10.1007/s00216-012-6194-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6194-1