Abstract
Owing to the tight control of methamphetamine, it is presumed that phentermine, an amphetamine-type anorectic, has recently been considered a supplement for methamphetamine abusers in Korea. In addition, the abuse of other anorectics obtained by inappropriate means has become a social issue. Hair is a useful specimen to prove chronic drug use. Therefore, an analytical method for the simultaneous detection of phentermine, phendimetrazine, amfepramone, fenfluramine, mazindol, methamphetamine, and 3,4-methylenedioxymethamphetamine (MDMA), as well as their metabolites, which covers the major amphetamines and anorectic agents in Korea, in hair was established and validated using liquid chromatography–tandem mass spectrometry (LC-MS/MS). The drugs and their metabolites in hair were extracted using 1 % HCl in methanol and then filtered and analyzed by LC-MS/MS with electrospray ionization in positive mode. The validation results for selectivity, linearity, matrix effect, recovery, process efficiency, intra- and interassay precision and accuracy, and processed sample stability were satisfactory. The limits of detection ranged from 0.025 to 1 ng/10 mg hair and the limits of quantification were 0.25 ng/10 mg hair for every analyte except mazindol and phentermine, for which they were 10 ng/10 mg hair. The method was successfully applied for the segmental determination of selected anorectics, methamphetamine, MDMA, and their metabolites in hair from 39 drug suspects. Among the anorectics, phentermine and/or phendimetrazine were identified with or without methamphetamine in the hair samples. Closer supervision of the inappropriate use of anorectics is necessary. Also, hair analysis is useful for monitoring the abuse potential of unnoticed drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methamphetamine is traditionally the most abused drug in Korea. Even though new designer drugs such as 3,4-methylenedioxymethamphetamine (MDMA) and benzylpiperazine have recently been introduced, methamphetamine is still highly available as an illicit drug. On the other hand, our previous study [1] demonstrated that an amphetamine-like anorectic agent, phentermine, had been abused by drug users and is presumably considered an alternative or a synergist for methamphetamine abuse.
In addition to phentermine, other anorectics, such as phendimetrazine, amfepramone (diethylproprion), and mazindol, have also been approved by the Korea Food and Drug Administration for short-term use. Phentermine, phendimetrazine, and amfepramone are noradrenergic agents and stimulate norepinephrine release, which decreases food intake. These drugs are structurally similar to amphetamines but do not have much effect on the dopamine release by which amphetamines act [2]. Mazindol, a tricyclic imidazoindole, blocks norepinephrine reuptake to induce the anorectic effect [2]. The use of these agents is under the purview of the Narcotics Control Law in Korea. However, since these anorectic agents are currently used as therapeutic drugs, they are much easier to access than illegal amphetamines.
In addition to these commercially available anorectics, fenfluramine, which was once approved but never legally available in Korea, is of interest because it is occasionally detected in Chinese diet products that are available through the Internet or personal import by travelers [3]. Fenfluramine is a serotonergic agent that stimulates serotonin release and blocks its reuptake, which reduces food intake [2]. It is structurally similar to amphetamines and its use is also under the purview of the Narcotics Control Law.
Although phentermine, phendimetrazine and amfepramone are approved for short-term use, registration of such prescription records at the national level is not yet mandatory in Korea. Therefore, these drugs are often used chronically. Moreover, it has been reported that phentermine has become popular among known or suspected methamphetamine or MDMA users [1].
Hair analysis has been considered the best method to prove chronic use of anorectics. Results from hair provide conclusive evidence of previous drug use as well as information on the duration of drug use, depending on the growth rate of hair (about 1 cm/month), and the severity of drug abuse [4]. In previous studies, results from hair were used as evidence of addiction to some therapeutic drugs. For example, hair analysis demonstrated that opioid analgesics and benzodiazepines were illegally abused especially by medical professionals, who can easily obtain those drugs [5, 6]. Chronic use of some anorectic agents was also proved by hair analysis [1, 3, 7].
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) has recently become a popular tool for hair analysis owing to its sensitivity and the relatively modest sample treatment. Since amphetamines are volatile, their loss often occurs during the evaporation step of sample preparation [8, 9]. In particular, derivatization followed by additional evaporation, which is a step required in the gas chromatography–mass spectrometry method, could result in unsatisfactory reproducibility.
Therefore, in this study, an analytical method for the simultaneous detection of phentermine, phendimetrazine, amfepramone, fenfluramine, mazindol, methamphetamine, and MDMA, as well as their metabolites, phenmetrazine, diethylnorephedrine, norfenfluramine, amphetamine, and 3,4-methylenedioxyamphetamine (MDA) (Table 1, Fig. 1), in hair was established and validated using LC-MS/MS for the purposes of forensic and clinical applications. This covers the major amphetamines and anorectic agents in Korea. The method was applied to hair samples from selected known amphetamine users or suspected users.
Chemical structures of the analytes [10]
Materials and methods
Chemicals
All solvents were high-performance liquid chromatography grade. MDA-d 5, phentermine, fenfluramine, methamphetamine, MDMA, amphetamine, and MDA were purchased from Cerilliant (Round Rock, TX, USA). Phendimetrazine, mazindol, diethylnorephedrine, and norfenfluramine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Amfepramone and phenmetrazine were purchased from U.S. Pharmacopeia (Rockville, MD, USA).
Sample preparation
Anorectics, amphetamines, and their metabolites in hair were extracted as previously described [1, 11] with a minor modification. First, the hair was washed with 2 mL of methanol followed by 2 mL of distilled water and 2 mL of methanol twice, respectively. The hair was then cut, weighed accurately (approximately 10 mg), and incubated in 2 mL of 1 % HCl in methanol at 38 °C for 16 h. Then 50 μl of 1 μg/mL MDA-d 5 was added as an internal standard before incubation. The extract was evaporated to dryness under a stream of nitrogen. The residue was reconstituted in 100 μl of methanol and mobile phase A (1:9) and then filtered with a 0.45-μm polyvinylidenefluoride microporous membrane (Millipore, Bedford, MA, USA). Finally, 10 μl was injected into the LC-MS/MS system.
LC-MS/MS analysis
The LC-MS/MS analysis was performed with an Agilent 1200 liquid chromatograph and an MDS Sciex API 3200 Qtrap tandem mass spectrometer from Applied Biosystems (Foster City, CA, USA). The analytical column was a Zorbax Eclipse XDB-C18 (4.6 mm × 150 mm, 5 μm) column, which was maintained at 40 °C. The mobile phase consisted of 2 mM ammonium formate/0.2 % formic acid in water (A) and 2 mM ammonium formate/0.2 % formic acid in acetonitrile (B). The initial gradient conditions were 95 % solvent A at a flow rate of 1,000 μl/min, decreased to 60 % over a period of 1–14 min, then decreased to 5 % between 14 and 16 min, where it maintained until 22 min. The initial condition of 95 % solvent A was then restored from 22.1 to 26 min and the system was allowed to equilibrate. The total run time was 26 min.
The mass spectrometry system was operated using electrospray ionization (ESI) in positive mode. The optimum conditions were as follows: curtain gas, 20 psi; collisionally activated dissociation, medium; heated nebulizer temperature, 650 °C; nebulizing gas (GS1), 50 psi; and heater gas (GS2), 40 psi. Two multiple reaction monitoring (MRM) transitions were chosen for each analyte and one MRM transition was chosen for MDA-d 5. The MRM transitions, retention times, and conditions are shown in Table 2. Data were processed using Analyst 1.5.1.
Validation study
The analytical method was validated using spiked drug-free hair, which was pooled from five different volunteers with natural, dyed, and/or permed hair, as described in previous studies [12, 13]. The following parameters were evaluated: selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), matrix effect, recovery, process efficiency, intra- and interassay precision and accuracy, and processed sample stability. To demonstrate linearity, five sets of calibrators (10-100 ng/10 mg hair for mazindol and phentermine and 0.25-10 ng/10 mg hair for the others) were prepared and analyzed. The analyte concentration at which the signal-to-noise ratio was greater than 3 was chosen for the LOD and that with less than 20 % coefficient of variation (CV) for precision and less than ±20 % for bias was chosen for the LOQ [12]. The matrix effect, recovery, and process efficiency were determined by comparing the analysis of five neat standards, five extracts of blank hair spiked with analytes after extraction, and five extracts of blank hair spiked with analytes before extraction at low (10 ng/10 mg hair for mazindol and phentermine and 0.25 ng/10 mg hair for others) and high (100 ng/10 mg hair for mazindol and phentermine and 10 ng/10 mg hair for others) concentrations [13]. Method precision and accuracy were examined by analyzing drug-free hair samples spiked with low and high concentrations of each analyte, respectively. The five sets of each sample were analyzed on five different days. To examine processed sample stability, eight hair samples spiked at low and high concentrations, respectively, were prepared as described in “Sample preparation” and then pooled. Each aliquot was injected at 2.5-h intervals over 20 h.
Analysis of real samples
The hair samples from 39 drug suspects were divided into 120 segments of various lengths (minimum length 3 cm). They were washed with methanol and distilled water, cut, and weighed accurately (approximately 5-15 mg). Then, the drugs and their metabolites in hair were extracted and analyzed as described already.
Results
The results of the method validation are summarized in Table 3. No interferences were detected at the retention times of the analytes and the internal standards in ten different blank hair samples. Figure 2 shows the chromatograms of five anorectics, methamphetamine, MDMA, and their metabolites in fortified hair (10 ng/10 mg hair for mazindol and phentermine and 0.25 ng/10 mg hair for others). The intra- and interassay precision and accuracy were satisfactory, i.e., below 15 % and below 10 % for precision and between 80 and 120 % and between 100 and 115 % for accuracy at low and high concentrations, respectively. The regression coefficients of the calibration curves were higher than 0.995 on every occasion. The LODs varied significantly, ranging from 0.025 to 1 ng/10 mg hair. The LOQs were 0.25 ng/10 mg hair for every analyte except mazindol and phentermine, for which they were 10 ng/10 mg hair.
The mean and median values of the matrix effect, recovery, and process efficiency of each analyte and MDA-d 5 are shown in Table 4. The mean and median of the matrix effect of the analytes ranged from 82 % (MDMA) to 124 % (amfepramone) and from 81 % (MDMA) to 118 % (methamphetamine), respectively, at the low concentration, and from 49 % (mazindol) to 114 % (amfepramone) and from 50 % (mazindol) to 108 % (phendimetrazine), respectively, at the high concentration. Most analytes did not produce severe ion enhancement or suppression, except for mazindol at the high concentration. The coefficient of variation of all the analytes was below 10 % at both concentrations. The mean and median of the recovery were 82 % (mazindol) to 112 % (MDMA) and 82 % (amfepramone) to 111 % (MDMA) at the low concentration and 95 % (MDMA) to 131 % (mazindol) and 85 % (amfepromone) to 129 % (mazindol) at the high concentration, respectively. The mean and median of the process efficiency were 73 % (mazindol) to 124 % (amfepramone) and 73 % (mazindol) to 114 % (methamphetamine) at the low concentration and 66 % (mazindol) to 116 % (amfepramone) and 65 % (mazindol) to 110 % (phendimetrazine) at the high concentration, respectively. The linear regression analysis of the peak area plotted against the injection time confirmed that processed sample stability was also satisfactory, i.e., negative slopes were not indicated at the low and high concentrations of any of the analytes.
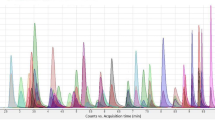
The results of the analysis of real samples are summarized in Fig. 3 and Table 5. Of the 39 subjects, 15 were men and 24 were women. All subjects were known or suspected illegal drug users. Their ages ranged from 23 to 54 years for the men and from 23 to 56 years for the women. Phentermine, phendimetrazine, and/or phenmetrazine were detected in the hair samples from the 39 subjects but other anorectics were not detected. Phentermine was identified alone in 15 subjects and along with methamphetamine and its metabolite, amphetamine, in ten subjects. Both phentermine and phendimetrazine (and/or its metabolite, phenmetrazine) were found in 13 subjects, among which methamphetamine and amphetamine were identified in three subjects. Both phendimetrazine and phenmetrazine without any other drug were detected in one subject (Fig. 3). As shown in Table 5, the concentration of phentermine ranged from 1.0 to 464 ng/mg (mean 29.7 ng/mg, median 6.8 ng/mg). The concentrations of phendimetrazine and phenmetrazine ranged from 0.03 to 94.7 ng/mg (mean 9.8 ng/mg, median 2.0 ng/mg) and from 0.07 to 231 ng/mg (mean 21.5 ng/mg, median 3.2 ng/mg), respectively. The concentrations of methamphetamine and amphetamine ranged from 1.1 to 110 ng/mg (mean 18.2 ng/mg, median 8.92 ng/mg) and from 0.04 to 13.8 ng/mg (mean 1.5 ng/mg, median 0.66 ng/mg), respectively. The chromatograms of five anorectics, methamphetamine, MDMA, and their metabolites in a real hair sample in which phenmetrazine, amphetamine, methamphetamine, phendimetrazine, and phentermine were detected are shown in Fig. 4. Their concentrations were below the LOQ and 0.79, 8.44, 0.03, and 1.24 ng/mg, respectively.
Discussion
Hair analysis has routinely been used in many forensic laboratories to prove drug abuse or for postmortem examination. Recently, its clinical application has expanded as a monitoring tool for drug therapy [14, 15] or for biomarker studies for disease [16] and alcohol addiction [17]. Since anorectics have high abuse potential, their abuse can be proved by segmental hair analysis along with individual prescription records in forensic and clinical areas.
Until now, studies on the simultaneous analysis of amphetamine-related drugs in hair have focused on illegal amphetamines, such as methamphetamine, amphetamine, MDMA, and MDA using gas chromatography–mass spectrometry [18, 19] or LC-MS/MS [20, 21]. In the present study, five anorectics (phentermine, phendimetrazine, amfepramone, fenfluramine, and mazindol), which have abuse probability because of local availability through legal or illegal means, were included among the analytes, along with methamphetamine, MDMA, and their selected metabolites.
The LODs of the current method show significant differences (40 times). In LC-MS/MS analysis, the matrix effect is considered an important factor in method sensitivity; however, no correlation was observed between the values of the LODs and the matrix effects in our study or in other studies on analytical methods for the simultaneous determination of drugs with similar structures [11, 22, 23], in which sensitivity variation was also observed. We presume that this difference could originate from the inconsistent degree of ionization of each analyte in the ESI source [24]. Another possible cause is that, since this method used a gradient condition for the mobile phase, the higher volatility of the mobile phase composition for an applied analyte may produce better ionization efficiency of the ESI source [25].
The validation results demonstrated that the current method was sensitive and reliable for use in routine analysis. However, the degree of ion suppression of mazindol at the high concentration was relatively significant compared with that of the other analytes. The extent of ion suppression differs between the types of samples and depends on how the samples are prepared. It also depends on the types and the concentrations of the analytes [26]. Mazindol is the only analyte that is not structurally similar to amphetamine. Considering the usefulness of a simultaneous analytical method, the degree of ion suppression of mazindol at the high concentration reported here was acceptable.
The results of the analysis of hair samples from drug suspects show that among the anorectics, phentermine and/or phendimetrazine were identified with or without methamphetamine, whereas amfepramone, fenfluramine, and mazindol were not. The abuse of phentermine has previously been reported using human hair [1, 7], but that of phendimetrazine was first proved by hair analysis in the current study. Most phendimetrazine abusers were multidrug abusers of phendimetrazine, phentermine, and/or methamphetamine (13 of 14 subjects).
In a previous study of self-administration by rhesus monkeys, variable but reliable self-administration of phentermine was found in part of the group, which suggested moderate abuse potential [27]. However, in a clinical group of long-term phentermine pharmacotherapy recipients, abrupt cessation did not induce amphetamine-like withdrawal and drug cravings [28]. Previous studies on phendimetrazine abuse are rare. One previous study of self-administration of phendimetrazine by rhesus monkeys showed no significant dependence potential [29]. Nevertheless, the previous [1] and current studies demonstrated that a growing number of suspected users of methamphetamine or MDMA have chronically used phentermine and/or phendimetrazine, probably because of the synergistic effects for methamphetamine or MDMA or because of economic or legal reasons for abuse of controlled drugs. Since both phentermine and phendimetrazine are prescription drugs, more controls are needed in the handling of these drugs by medical professionals.
Conclusion
In this study, a simultaneous analytical method for selected anorectics, methamphetamine, MDMA, and their metabolites in hair was validated and applied successfully to prove anorectics abuse. Among the anorectics, phentermine and/or phendimetrazine were identified with or without methamphetamine in hair samples from drug suspects. Closer supervision of the use of anorectics is therefore necessary. The current study also demonstrated that hair analysis is useful for monitoring the abuse potential of unnoticed drugs.
References
Lee S, Kim J, In S, Choi H, Chung H, Chung KH (2011) Forensic Sci Int 207:e5–e7
Bray GA (2000) Nutrition 16:953–960
Kaddoumi A, Wada M, Nakashima MN, Nakashima K (2004) Forensic Sci Int 146:39–46
Lee S, Han E, Park Y, Choi H, Chung H (2009) Forensic Sci Int 190:16–18
Kintz P, Villain M, Dumestre V, Cirimele V (2005) Forensic Sci Int 153:81–84
Lee S, Han E, In S, Choi H, Chung H, Chung KH (2011) J Anal Toxicol 35:312–315
Kim JY, Jung KS, Kim MK, Lee JI, In MK (2007) Rapid Commun Mass Spectrom 21:1705–1720
Holler JM, Vorce SP, Bosy TZ, Jacobs A (2005) J Anal Toxicol 29:652–657
Kudo K, Ishida T, Hara K, Kashimura S, Tsuji A, Ikeda N (2007) J Chromatogr B Anal Technol Biomed Life Sci 855:115–1120
http://www.chemspider.com/. Accessed 2 Jan 2011
Kim J, Lee S, In S, Choi H, Chung H (2011) J Chromatogr B Anal Technol Biomed Life Sci 879:878–886
Peters FT, Drummer OH, Musshoff F (2007) Forensic Sci Int 165:216–224
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030
Gottardo R, Fanigliulo A, Sorio D, Liotta E, Bortolotti F, Tagliaro F (2011) Forensic Sci Int (in press)
Gow R, Koren G, Rieder M, Van Uum S (2011) Clin Endocrinol (Oxf) 74:687–693
Jung HJ, Kim SJ, Lee WY, Chung BC, Choi MH (2011) Rapid Commun Mass Spectrom 25:1184–1192
Pirro V, Valente V, Oliveri P, De Bernardis A, Salomone A, Vincenti M (2011) Anal Bioanal Chem 401:2153–2164
Aleksa K, Walasek P, Fulga N, Kapur B, Gareri J, Koren G (2011) Forensic Sci Int (in press)
Merola G, Gentili S, Tagliaro F, Macchia T (2010) Anal Bioanal Chem 397:2987–2995
Tabernero MJ, Felli ML, Bermejo AM, Chiarotti M (2009) Anal Bioanal Chem 395:2547–2557
Chèze M, Deveaux M, Martin C, Lhermitte M, Pépin G (2007) Forensic Sci Int 170:100–104
Rust KY, Baumgartner MR, Meggiolaro N, Kraemer T (2011) Forensic Sci Int (in press)
Salomone A, Gerace E, Brizio P, Gennaro MC, Vincenti M (2011) J Pharm Biomed Anal 56:582–591
Covey TR, Schneider BB, Javaheri H, LeBlanc JCY, Ivosev G, Corr JJ, Kovarik P (2010) In: Cole RB (ed) Electrospray and MALDI mass spectrometry: Fundamentals, instrumentation, practicalities, and biological applications, 2nd edn. Wiley, New York
Hétu PO, Gingras ME, Vinet B (2010) Clin Biochem 43:1158–1162
Gosetti F, Mazzucco E, Zampieri D, Gennaro MC (2010) J Chromatogr A 1217:3929–3937
Stafford D, LeSage MG, Rice KC, Glowa JR (2001) Drug Alcohol Depend 62:41–47
Hendricks EJ, Greenway FL (2011) Am J Ther 18:292–299
Corwin RL, Woolverton WL, Schuster CR, Johanson CE (1987) Alcohol Drug Res 7:351–361
Acknowledgments
This research was partly supported by grants from the Korea Food and Drug Administration (11182-612) and the National Forensic Service (2011-05) in 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Kim, J., In, S. et al. Development of a simultaneous analytical method for selected anorectics, methamphetamine, MDMA, and their metabolites in hair using LC-MS/MS to prove anorectics abuse. Anal Bioanal Chem 403, 1385–1394 (2012). https://doi.org/10.1007/s00216-012-5950-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5950-6