Abstract
The analysis of persistent organic pollutants is a real challenge due to the large number of compounds with varying chemical and physical properties. Gas chromatography with electron capture detection or mass spectrometry has been the method of choice for the past 50 years. Comprehensive two-dimensional gas chromatography (GCxGC) coupled with micro-electron capture detector (μECD) is a new method that can analyze polychlorinated biphenyls (PCBs), organochlorine pesticides (OCs) and chlorobenzenes (CBz) in a single analytical run with enhanced selectivity and sensitivity over single column methods and can also be used to screen for other halogenated organics in environmental samples. An accredited routine method using commercially available LECO GCxGC-μECD and a column combination DB-1 × Rtx-PCB has been developed to analyse PCBs/OCs/CBz in soils, sediments and sludges. The method provides quantification of Aroclors and Aroclor mixtures to within 15% of target values and sub-nanogrammes per gramme detection limits.

Enhanced separation by GCxGC
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial chemicals have been used for almost 200 years in a wide variety of applications as solvents, precursors, reagents, surfactants, flame retardants and pesticides [1, 2]. Over 100,000 chemicals are currently in use with over 30,000 considered to be in wide commercial use (>907 kg/year) [3]. Many of these compounds are persistent, toxic and bioaccumulative, and many of them are halogenated. Unfortunately, only a small number of these compounds are regulated. The Stockholm Convention on persistent organic pollutants (POPs) [4, 5] came into force on May 2004 in order to reduce and eliminate 12 compounds including nine legacy organochlorine pesticides (OC), the industrial chemicals, polychlorinated biphenyls (PCBs) [6] and the unintentionally produced polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) [7]. An additional nine pesticides, flame retardants and surfactants were added to the list in 2009, and three additional groups of compounds are currently under review. All of the Stockholm compounds are halogenated compounds, and most of them, especially the recently added ones, are routinely detected in environmental samples.
Very large amounts of many Stockholm POPs [8] (~1 million tonnes of PCBs and 2 million tonnes of DDT alone) were manufactured during the last century and used in numerous industrial applications and as pesticides and flame retardants. The dioxin-like polychlorinated naphthalenes (PCNs) were produced in amounts of about 15% to 20% of the PCBs and were used as flame retardants in airplane cloth and uniforms in World War I, electrical insulators for cabling prior to the development of polymeric materials and as antifungal agents in gas masks [9]. PCNs were replaced in many applications by PCBs because they were considered less toxic. Although it was known that many of these early halogenated compounds were toxic, it was not until the discovery of gas chromatography (1951) and its combination with sensitive detectors like the electron capture detector (ECD, 1958) and mass spectrometer that these compounds could be analysed at levels low enough to be used for the protection of human health and the environment. The ECD is very sensitive to halogenated organics, but not selective. Early methods split extracts into a number of fractions. Fractions were run on multiple columns to separate compounds for accurate quantification. Mass spectrometry provides greater selectivity, but many of the compound groups (e.g. PCBs, PCDD/Fs and polybrominated diphenyl ethers (PBDEs)) contain isomers and congeners of varying toxicity and must be chromatographically separated because they may not be separated mass spectrometrically. There currently is no single GC column phase that can separate all 209 PCBs or 210 PCDD/Fs, and therefore, at least two columns must be used for accurate quantification [2].
Comprehensive two-dimensional gas chromatography (GCxGC) [10–26] is a method that can connect two GC columns of different phases using a modulator to significantly increase peak capacities and correspondingly selectivity. The modulator is a thermal or valve-controlled device that traps compounds from the first column and then re-injects them in a very tight band onto the second column. The enhanced separation is achieved because compounds are subjected to two independent separations (GC column phases). Orthogonal separation occurs when the two columns use different separation mechanisms, operating independently in the two dimensions (synentropy across dimensions is zero) [10]. Structured chromatograms are distinctly visible in orthogonal GCxGC chromatograms for structurally related compounds such as PCBs, PCDD/Fs, PCNs and polychlorinated alkanes (PCAs) allowing easier group-type identification [14, 16, 18, 19]. Isomers line up as bands in the chromatogram, and congeners groups or homologues appear as separate bands. Reversing the phases provides an alternate approach with different patterns and separations and can be effective where coelutions of critical target analytes are a challenge.
The modulation process produces very narrow GCxGC peaks that elute in the second column (dimension), requiring fast scanning detectors to record data. Detectors such as the time-of-flight (TOF) mass spectrometer (MS) or ECD are the detectors of choice due to their high scanning rate (>20 Hz) and ability to accurately characterize the narrow GCxGC peaks. TOF mass spectrometers can perform mass spectral deconvolution of overlapping peaks when fragmentation patterns of coeluting peaks are different [16, 21, 22] but are significantly less sensitive to higher molecular weight and polyhalogenated compounds than the ECD. The enhanced selectivity of GCxGC, especially for analysis of compounds groups that contain congeners or homologues like PCBs, PCDD/Fs or PCN, can enable a less selective detector such as the ECD to be used. The micro-electron capture detector (μECD) has a much smaller inner volume than classical ECDs and is an excellent detector for quantitative analysis and screening of polyhalogenated organics by GCxGC [19, 23]. This paper describes the development of a routine accredited method (to ISO 17025 [27]) for the analysis of PCBs, OCs, chlorobenzenes (CBz) and screening of polyhalogenated organics in soil, sediment and sludge by GCxGC-μECD.
Experimental
Standards and reference materials
PCBs standards (BP-MS, BP-MS2, BP-MS3 and BPMS-PARH; see Table 1 for composition) were obtained from Wellington Laboratories (Guelph, ON, Canada). The BP-MS, BP-MS2 and BP-MS3 solutions were combined and used for multilevel calibration, while the PCB-PARH (82 PCB congeners) standard solution was used for spiking and alternate standard verification. A CBz standard mixture of 15 CBz and an OC standard mixture of 23 compounds were acquired from Absolute Standards Inc. (Hamden, CT, USA) for calibration and UltraScientific (North Kingstown, RI, USA) for spiking and as an alternate standard. Decachlorobiphenyl and 1,3,5-tribromobenzene (North Kingstown, RI, USA) were used as surrogates.
The six-level calibration standard solutions containing PCBs/OCs/CBz were prepared by combining BP-MS, BP-MS2 and BP-MS3 which contain 82 PCB congeners and the OC and CBz standard solutions and diluting in iso-octane to produce a calibration series with concentrations ranging from 1 to 500 ng/ml. All calibration solutions contained 4,4′-dibromo-octafluoro-biphenyl at a concentration of 10 ng/mL, which was used as an internal standard for PCB congeners' quantification.
Sediment reference material Standard Reference Material 1944 (SRM1944, New York/New Jersey Waterway Sediment) from the National Institute of Standards and Technology (NIST; Gaithersburg, MD, USA) and sludge reference material CNS-312 from the Resource Technology Corporation (RTC; Laramie, WY, USA) were used to determine precision and accuracy of the method.
Sample extraction and internal standard spiking
The sediment samples, collected from various locations in Ontario, were air-dried, crushed and sieved, if necessary, using a 2-mm sieve or other suitable means to ensure homogeneity and to remove stones and organic fragments. A portion of the soil/sediment/sludge sample was then weighed (5 g), and surrogate standards, decachlorobiphenyl and 1,3,5-tribromobenzene (in acetone), were added to the sample in each ASE cell prior to extraction to reach a final concentration of 50 ng/mL. Along with the samples, a fortified sample was prepared by spiking blank material with BPMS-PARH standard solution and OCs/CBz spiking solutions in acetone (UltraScientific) at a concentration of 50 ng/mL. The blank material consisted of samples previously extracted by Soxhlet extraction and analyzed to show no detectable residues of the analytes to be tested.
Extraction and sample preparation
Pressurized liquid extraction (accelerated solvent extraction, ASE 200, Dionex Corporation, Sunnyvale, CA, USA) was used to extract the samples with 25% dichloromethane/75% hexane (v/v) as the extraction solvent. The ASE conditions were as follows: one cycle extraction at 100 °C, heat time 5 min, purge time 90 s and flush volume 60%. The resulting extract is then subjected to a single-stage silica cartridge cleanup procedure prior to instrumental analysis (1 g, Varian, Mississauga, ON, Canada). The cartridge was conditioned with 5 mL 25% dichloromethane/75% hexane (v/v); the sample was then applied and gravity eluted with 15 mL 25% dichloromethane/75% hexane (v/v). The extracts were evaporated to 5 mL final volume in iso-octane using a Turbovap LV evaporating system (Caliper Life Sciences, Hopkinton, MA, USA). Copper treatment (15 min shaking each sample with copper granules or powder followed by 4 to 24 h time to settle out) was applied to all samples to remove the sulphur interferences prior to instrumental analysis. No fractionation of extracts was carried out prior to analysis. A similar sample preparation method was developed for analysis of polycyclic aromatic hydrocarbons and adopted for this method because it recovers most non-polar and slightly polar organic compounds [28–30, 32].
Instrumental setup
A LECO Corp GCxGC-μECD system (Benton Harbour, MI, USA) was used for all the analyses. This system was equipped with a stationary quadruple jet dual-stage modulator, a modified Agilent 6890 gas chromatograph with split/splitless injector and μECD detector.
The following chromatographic column combination was used: a 30-m, 0.25-mm i.d., 0.25 μm film thickness DB-1 (100% dimethylpolysiloxane) from J&W Scientific (Folsom, CA, USA) as the first dimension column; a 1.6-m, 0.18-mm i.d., 0.18-μm film thickness Rtx-PCB from Restek Corp. (Bellefonte, PA, USA) as the second dimension column; and a 0.3-m, 0.1-mm i.d. IP-Deact. column (Restek Corp.) as the transfer line to ECD. The connections between the first dimension, second dimension and IP-Deact columns were made using SGE Siltite mini unions 0.25 col. (Canadian Life Sciences, Peterborough, ON, Canada). One-microlitre samples were injected into the split/splitless injector operated in splitless mode at a temperature of 250 °C with a 4-mm i.d. gooseneck liner (Restek Corp.). Helium gas with a purity of 99.999% was used as carrier gas with a flow rate of 1.2 mL/min. The μ-ECD system was operated at 300 °C, with 5% methane in argon as make-up gas at a flow-rate of 150 ml/min and data acquisition rate of 50 Hz. ChromaTof software (LECO Corp.) was used for data acquisition and analysis.
Results and discussion
Optimization of GCxGC separation
Many studies have shown the numerous advantages of GCxGC which include powerful separation capability through increases of peak capacity of >20 times over conventional single dimensional methods, orthogonal separation for structurally related compounds (e.g. PCBs and PCAs), improved sensitivity due to the modulation process producing significantly narrower and taller GC peaks and improved separation of analytes from the matrix interferences [14, 16, 18, 20–22]. After reviewing potential column combinations, DB-1 × Rtx-PCB was selected as the column combination of choice because it showed distinct orthogonal separations for the PCBs. Similar to other published reports [14, 16], PCB congeners are separated as bands in the two-dimensional space according to their degree of chlorination as well as to the degree of ortho substitution. The mono-ortho PCBs and non-ortho PCBs 37, 77, 81, 126 and 169 elute later in the second dimension due to the selectivity of Rtx-PCB for the compounds that are planar or may adopt a planar configuration [14].
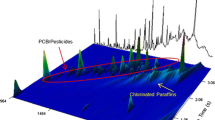
Figure 1 shows the analysis of PCBs, OCs and CBz in a single injection. The greatest challenge in developing the method was to optimize the separation of the 118 target compounds from three different classes of halogenated organic contaminants in a reasonable run time. This was accomplished by analyzing the separate components: PCBs, OCs and CBz to determine preliminary values and then a combined standard mixture containing PCBs, OCs and CBz to evaluate the effects of the different parameters (e.g. modulation time, modulator temperature offset, oven temperature programming and carrier gas flow rate). The optimal parameters produced an analytical run of 45 min with the following temperature program: starting oven temperature of 80 °C (hold for 2 min) to 160 °C at 10 °C/min, then to 280 °C at 4 °C/min (hold for 5 min); the secondary oven had a 35 °C temperature offset to the first dimension oven program. The modulation period was 4 s with a modulator temperature offset of 35 °C. These temperature offsets and the 4-s modulation period assured that the target analytes from the same class are not wrapped around within the group and to eliminate a split across the 2D chromatogram, and the best possible separation was achieved. In addition to the above parameters, 0.3 m of the 1.6-m second dimension column was left in the first oven, preceding the modulator, and an IP-Deact. (intermediate-polarity deactivated fused silica) transfer line from the second dimension column to the ECD played an important role in avoiding wrap-around and in the optimization of target analyte separations.
After optimization, a total of seven coelutions remained unresolved: three within-class (PCB4/PCB10, PCB90/PCB101 and 1,2,3,5-tetrachlorobenzene (1,2,3,5-TCB)/1,2,4,5-tetrachlorobenzene (1,2,4,5-TCB)) and four between-class (heptachlor-epoxide/PCB74, gamma-chlordane (γ-CHLA)/PCB60, cis-nonachlor/PCB114 and methoxychlor (DMDT)/PCB171). The within-class coelutions are challenging separations also present in the classical GC-ECD analysis when using a DB-1 column [21] and were not further resolved by GCxGC. Since the calibration standard analysed does not contain all the PCBs present in the environment, there is the risk of reporting a positive bias for some PCBs (e.g. PCB138/163/164 not separated with this method) [31].
GCxGC quantification
The GCxGC calibration was performed using a PCB/OC/CB standard mixture at six different concentrations ranging from 1 to 500 ng/mL to develop the calibration curves used for quantification. The regression coefficients were satisfactory (0.995 to 0.999) using a linear calibration; however, quantitative results for the lowest and the highest calibration standards exhibited greater error than the other calibration standards. Thus, second order curve for quantification was used, and this resulted in regression coefficients ranging between 0.9996 and 0.9999, showing significantly lower error for the lowest and highest calibration standards. An external standard calibration method was used for the OCs and CBz, and an internal standard calibration procedure was used for PCB quantification. Prior to injection, 10 μL of 4,4′-dibromo-octafluoro-biphenyl internal standard solution at 1 μg/mL was added to each sample. Determination of an analyte's retention time when using a less selective detector like an ECD is critical for accurate quantification; therefore, the PCB internal standard was also used to check retention time stability between runs [18].
Retention time reference peaks were used to take into account any retention time shifts due to matrix effects in real samples and to simplify data processing for the analyst. Retention times were adjusted using 1,3,5-tribromobenzene as retention reference for all CBz, p,p′-DDE for the OCs and three PCBs based on their first dimension elution order: PCB52 for the first group of PCBs, PCB151 for the second group and PCB199 for the third. If any of the references were shifted, their retention times were updated, and the samples were then reprocessed.
A number of closely eluting analyte pairs were not baseline separated and typically required manual baseline manipulation in order to obtain proper integration and accurate quantification: PCB44/aldrin, PCB70/oxy-chlordane, PCB85/p,p′-DDE and PCB99/α-chlordane. Some other analyte pairs not baseline resolved by GCxGC did not require manual manipulation by the analyst: PCB28/PCB31 and PCB123/PCB118/PCB149.
Instrument performance
Instrument performance was assessed initially by analysing ten replicates of the PCBs/OCs/CBz calibration standard at 1 and 20 ng/mL levels. Quantification was carried out as a continuing calibration procedure with the 1- and 20-ng/mL standards used as daily instrument calibration confirmations. Experimental results of the 20-ng/mL standard solution showed a relative standard deviation (%RSD) ranging from 0.2% to 10% for PCBs, 2% to 7% for CBz and 2% to 14% for OCs (endrin was the poorest performer due to degradation in the injector port). The instrument precision at the low level (1 ng/mL) was calculated for nine replicates, and the %RSD ranged from 2% to 13% for PCBs, 2% to 14% for CBz and 1% to 11% for OC (beta-BHC had the highest RSD, 27% at this concentration).
To further assess the instrument performance, between-run results of eight replicates at 20 ng/mL were evaluated, and the data showed %RSD between 2% and 14% for PCBs (where PCB31 and PCB44 had the highest values), 5% and 15% for OCs with the exception of endrin at 21%, and 3% and 14% for CBz (Table 1, selected PCBs, OCs, CBz and Electronic Supplementary Material Table S1 for all analytes).
Method performance
Replicates of clean sediment samples spiked with the PCBs/OCs/CBz spiking solution were extracted, cleaned-up and analysed by GCxGC, and their recoveries were used to assess the method performance. The within-run precision (repeatability) was calculated from ten replicates processed in the same analytical run, while the between-run precision (reproducibility and accuracy) was determined from multiple replicates analysed from different analytical runs on different days (N = 21 for PCBs, N = 13 for OCs and N = 15 for CBz). Results presented in Table 2 for selected PCBs, OCs and CBz (for all the complete set of target analytes, see Electronic Supplementary Material Table S2), expressed as relative standard deviation (percent), show that the recoveries are within method acceptance criteria (±50%) and within ±10% for the majority of the analytes in the between-run results.
The statistical method detection limits (MDL), calculated by analyzing eight replicates of a clean sediment matrix spiked with the lowest level of the analytes in the calibration curve (1 ng/mL), are reported in Table 2 (Table S2 for all analytes) and exhibit the excellent sensitivity of the method. MDLs varied from 0.1 to 1.6 ng/g for PCBs, 0.14 to 0.83 ng/g for CBz and 0.12 to 0.50 ng/g for OCs. Since a quadratic fit was used for quantification, the minimum quantification limits (MQLs) were set as the lowest point of the calibration curves. The MQLs were 5 ng/g for di-PCBs, 2 ng/g for tri-PCBs and 1 ng/g for the remainder of compounds (Electronic Supplementary Material Table S2).
Only one potential interference, triclosan (a disinfectant in cleaning products), which elutes very close to the PCB60/γ-chlordane pair, was detected by GCxGC. No other significant interferences of the target analytes were observed.
Uncertainties
The estimation of overall uncertainty [33], which includes combined and expanded uncertainties, was based on data collected from different spiked samples that cover all the target parameters analysed over a period of time (Table 2 and Electronic Supplementary Material Table S2). The combined uncertainty (μ c) was calculated as the square root of the sum of the squares of individual uncertainty components (μ x, relative standard deviation times the design concentration of the individual component) and the uncertainty of “near zero” data (μ 0, method MDLs). Since the number of data points was less than 30 (n = 21 for PCBs, n = 13 for OCs and n = 15 for CBz), μ x was multiplied by the appropriate Student's t for 95% confidence. The expanded uncertainties were expressed as two times the combined uncertainty at the 95% confidence level. The uncertainties of the weighing procedure and sub-sampling were found to make negligible contributions, and therefore were not included in the final uncertainty budget [32–34]. The uncertainties were about on average 15% to 25% for most PCBs and 20% to 30% for most OC pesticides and CBz.
Accuracy—reference materials analysis
Two different reference materials were used to determine the efficiency of the extraction, clean-up and instrumental procedures and to assess the accuracy of the GCxGC-ECD method: a sediment (SRM1944) and a sludge (CNS312). For SRM1944, the data obtained from ten within-run replicates and eight between-run replicates were compared to the certified values. SRM1944 is a sediment CRM that contains many different compound groups, and as shown in Fig. 2a, the main components (PCBs, OCs and CBz are present in the chromatogram) and other compounds like PCDD/Fs, PBDEs and PCNs were also detected. The analytical results from NIST1944 are depicted in Fig. 2b and are in excellent agreement with the certified values for all analytes for both the between- and within-run data, indicating the method is accurate and rugged. Similarly, when CNS312, a biosolid reference material (N = 8 replicates), was analysed with the current GCxGC method (Fig. 3), the data was also in excellent agreement with the certified values. The error bars represent the standard deviations for both the CRM certified and GCxGC values. Some of the compounds such as heptachlor epoxide and γ-chlordane are reported as coelutions with different PCBs. The result for γ-chlordane is very close to its certified value, and the result for heptachlor epoxide appears slightly biased high. The detection of additional compounds enables the analyst to provide screening information or possible further sample preparation and/or concentration and analysis for some of these additional compounds.
PCB total (Aroclor)
PCB commercial mixtures were produced in a number of combinations with the Aroclor mixtures being the most well known and most produced. PCBs have been quantified using Aroclor technical mixtures as calibration standards for many years. This has resulted in difficulties in comparing data because not all laboratories used the same Aroclors or combination (mixtures) of Aroclors for quantification. Environmental and biological samples undergo weathering or degradation, changing their patterns and therefore are no longer neat Aroclors. All of these practices and processes introduce variability and bias into the analysis. This method quantifies 82 PCB congeners, and the final selection of congeners was determined so that most Aroclors could be determined as accurately as possible and that results can be compared with classical “Aroclor matching” procedures. A number of different Aroclors and Aroclor mixtures were analyzed and compared to the sum of the 82 target PCB congeners. As shown in Table 3, all of the results are within 15% agreement of the concentration of the Aroclor analyzed.
Samples
To further emphasize that the GCxGC method provides a very good estimate of the PCB total, two samples previously analysed in our laboratory using the classical GC method are presented in Table 3. Both the low level and high level PCBs results are in excellent agreement (<10%).
In addition to accurately quantifying target compounds, this two-dimensional technique detects and screens for other classes of contaminants present in sludge and sediment samples. Figure 4a shows the chromatogram of a sludge sample that contains numerous additional peaks. After a preliminary assessment of the “unknown” compounds, the presence of polychlorinated terphenyls was identified in the sludge using GCxGC-time of flight mass spectrometry. Figure 4b shows the chromatogram of a sediment sample that contains target analytes and numerous additional compounds including PCA (shown as typical bands), dioxins, furans, PBDEs and other unknown contaminants.
A few challenges were encountered when analysing “real” sediment extracts: the presence of PCA bands interfered with some of the higher chlorinated PCBs (i.e. PCB170 and PCB180), making their quantification a challenge, and the presence of triclosan that elutes closely to γ-chlordane/PCB60 has to be monitored. The analyst should pay careful attention to the γ-chlordane retention time and compare it with a control sample, such as matrix spike when using ECD to ensure it is not misidentified as triclosan.
Conclusions
Comprehensive GCxGC-μECD can be used as a sensitive and selective routine method for the analysis of PCBs, OCs and CBz in sediments, soils and sludges in a single analytical run. The method can also be used to perform analytical triage to screen for additional compounds, for additional extract processing and testing or for identification and monitoring of new and emerging halogenated compounds present in sample extracts. Fractionation of sample extracts prior to instrument analysis, a prerequisite in classical GC-ECD methods, is not required resulting in significant savings of time and analytical costs. GCxGC requires more time to review the chromatograms than a single one-dimensional GC run, but because using GCxGC, all analytes can be determined in a single injection, versus up to six separate injections (dual column injections for PCBs, OCs and CBz); data review is often faster than for separate classical GC analyses. The DB-1 × Rtx-PCB column combination used for GCxGC analysis yields excellent separation of analytes and minimal coelution of analytes. This is important when analysing compound groups that contain isomers, congeners or homologues that may not be distinguished by more expensive mass spectrometric detectors. This method is accurate and precise for the standards and reference materials tested, and the sum of the 82 target PCB congeners results in quantification of Aroclors or Aroclor mixtures to within 15% enabling comparison with historical Aroclor matching results. The instrument is commercially available, and comprehensive two-dimensional gas chromatography requires minimal additional training to be used as a routine analytical method for the analysis of halogenated organics.
References
Howard PH, Muir DCG (2010) Environ Sci Technol 44:227–2285
Reiner EJ, Boden AR, Chen T, MacPherson K, Muscalu AM (2010) LC-GC Europe 23(3):60–70
Muir DCG, Howard PH (2006) Environ Sci Technol 40:7157–7166
Stockholm Convention Secretariat (2001) UNEP. Available at http://chm.pops.int. Accessed 3 March 2011
Kannan N (2000) In: Paasivirta J (ed) The handbook of environmental chemistry, vol 3. Springer, Heidelberg, pp 127–157
Cochran JW, Frame GM (1999) J Chromatogr A 843:323–368
Reiner EJ (2010) Mass Spectrom Rev 29(4):526–559
Weber R, Watson A, Forter M, Oliaei F (2011) Waste Manage Res 29:107–121
Heyward D (1998) Environ Res 76:1–18
Venkatramani CJ, Xu J, Phillips JB (1996) Anal Chem 68:1486–1492
Beens J, Boelens H, Tijssen R, Blomberg J (1998) J High Res Chromatogr 21:47–54
Beens J, Tijssen R, Blomberg J (1998) J High Res Chromatogr 21:63–64
Bertsch W (2000) J High Res Chromatogr 23(3):167
Korytar P, Leonards PEG, de Boer J, Brinkman UAT (2002) J Chromatogr A 958:203–218
Dalluge J, Beens J, Brinkman UAT (2003) J Chromatogr A 1000:69–108
Focant JF, Sjodin A, Turner WE, Patterson DG Jr (2004) Anal Chem 76:6313–6320
Ryan D, Morrison P, Marriott P (2005) J Chromatogr A 1071:47–53
Korytar P, Leonards PEG, de Boer J, Brinkman UAT (2005) J Chromatogr A 1086:29–44
Danielsson C, Wiberg K, Korytar P, Bergek S, Brinkman UAT, Haglund P (2005) J Chromatogr 1086:61–70
Korytar P, Parera J, Leonards PEG, Santos FJ, de Boer J, Brinkman UAT (2005) J Chromatogr A 1086:71–82
Adahchour M, Beens J, Vreuls RJJ, Brinkman UAT (2006) Trends Anal Chem 25:438–454
Adahchour M, Beens J, Vreuls RJJ, Brinkman UAT (2006) Trends Anal Chem 25:540–553
von Muhlen C, Khummueng W, Alcaraz Zini C, Bastos Caramao E, Marriott PG (2006) J Sep Sci 29:1909–1921
Korytar P, Haglund P, de Boer J, Brinkman UAT (2006) Trends Anal Chem 25:373–396
Ramos L (2009) In: Barcelo D (ed) Comprehensive two dimensional gas chromatography, vol 55, Comprehensive analytical chemistry. Elsevier, Amsterdam
Muscalu AM, Reiner EJ, Liss SN, Chen T (2010) Int J Environ Anal Chem 90:1–13
International Organization for Standardization (ISO) (2005) Standard 17025 General requirements for the competence of testing and calibration laboratories
Ontario Ministry of the Environment, Method 3412 (2008) The analysis of PCB congeners in soil and sediment by gas chromatography-electron capture detection
Ontario Ministry of the Environment, Method 3270 (2008) The analysis of PCB, organochlorine pesticides and chlorobenzenes in soil and sediment by gas chromatography-electron capture detection
Ontario Ministry of the Environment, Method 3425 (2008) The analysis of poly aromatic hydrocarbons by gas chromatography isotope dilution mass spectrometry
Frame GM, Wagner RE, Carnahan JC, Brown JF Jr, May RJ, Smullen LA, Bedard DL (1996) Chemosphere 33:603–623
Ontario Ministry of the Environment, Method 3487 (2010) The analysis of PCB, organochlorine pesticides and chlorobenzenes in soil and sediment by two dimensional gas chromatography-electron capture detection
Ontario Ministry of the Environment, LSBSOP 0030 (2010) Laboratory services branch guidelines for the determination and documentation of uncertainty of chemical measurements
EURACHEM/CITAC Guide (2000) Quantifying uncertainty in analytical measurement
Acknowledgements
The authors would like to thank Jack Cochran (Restek) and Frank Dorman (Penn State) for providing valuable information on chromatographic separations. We would like to thank Olivier Niquette (LECO Corporation), Adrienne Boden, Paul Helm, John Bodnar, Vedrana Pantelic and Sathi Selliah of the Ontario Ministry of the Environment for providing advice and support during the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Copies of analytical methods are available from LaboraotryServicesBranch@Ontario.ca.
Published in the special issue Comprehensive Multidimensional Separations with Guest Editors James Harynuk and Philip Marriott.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muscalu, A.M., Reiner, E.J., Liss, S.N. et al. A routine accredited method for the analysis of polychlorinated biphenyls, organochlorine pesticides, chlorobenzenes and screening of other halogenated organics in soil, sediment and sludge by GCxGC-μECD. Anal Bioanal Chem 401, 2403–2413 (2011). https://doi.org/10.1007/s00216-011-5114-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5114-0