Abstract
The use of a novel electrophoric derivatisation reagent, o-(pentafluorobenzyloxycarbonyl)-benzoyl chloride, for the quantitative determination of methylphenidate in plasma is described. The drug can be quantitatively measured down to 72 pg/mL plasma using only 250 μL of sample due to the extraordinary sensitivity of the derivatives under negative ion chemical ionisation mass spectrometry. Plasma samples were made alkaline with carbonate buffer and treated with extraction solvent n-hexane and reagent solution for 30 min, which, after concentration, was measured by GC-NICI-MS. The method is rapid as extraction and derivatisation occur in one single step. A stable isotope-labelled internal standard was used and its synthesis described. Full validation data are given to demonstrate the usefulness of the assay, including specificity, linearity, accuracy and precision, long-term stability, short-term stability, freeze–thaw stability, stock solution stability, autosampler stability, aliquot analysis, robustness, matrix effect, and prospective analytical batch size accuracy. The method has been successfully applied to pharmacokinetic profiling of the drug after oral application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit hyperactive disorder (ADHD) is a neurobehavioural problem mostly encountered with school-aged children at a high prevalence of 5–10% of the general population [1, 2]. The cyclic amphetamine analogue methylphenidate [dl-threo-methyl 2-phenyl-2-(piperidyl)actetate] (MPH) is widely used for the management of children having ADHD, with or without hyperactivity [3]. The psychostimulant drug displays a high intrinsic clearance due to the rapid hydrolysis of the methyl ester function [4] occurring in a stereoselective manner [5]. As a consequence, the plasma concentrations after oral therapeutic doses encountered are low, typically within c max of 10–20 ng/mL. There is an individual variability in the response to MPH concentrations that makes it necessary to adjust for optimal medication and elimination of toxicological side effects. This is of particular importance as children are the main target group of this medication [6]. Quantification in the lower picogram per millilitre range is hence desirable for reliable pharmacokinetic studies with MPH. Besides the treatment of ADHD, MPH improves attention, concentration, fine motor coordination and balance, and due to these sport-related benefits, the drug is considered as a doping agent [7, 8]. MPH is thus not permitted for use in competition by the International Olympic Committee.
Several analytical methods have been reported for the determination of MPH in plasma and urine. High-performance liquid chromatography has been used with fluorescence detection [9] as well as in combination with mass spectrometry (MS) [10–14] and tandem MS [15]. Heptafluorobutyryl-l-prolyl and trifluoroacety-l-prolyl derivatives were used for enantioselective measurement of the drug by GC-MS using either electron ionisation (EI) [16], positive ion chemical ionisation [17] or negative ion chemical ionisation (NICI) [17, 18]. Besides that, GC-MS of pentafluoropropyl derivatives have been used with EI [19, 20] and positive ion chemical ionisation [21] and, more recently, capillary electrophoresis ion trap mass spectrometry [22]. Besides the advantage of enantioselective detection, none of the methods achieved a limit of quantification (LOQ) below 0.4 ng/mL. A sensitive method has been described to detect 70 pg/mL plasma using GC-NICI-MS of the heptafluorobutyrate [23].
Recently, we have introduced a new derivatisation reagent, o-(pentafluorobenzyloxycarbonyl)-benzoyl chloride (PBBCl) that readily reacts with primary and secondary amines [24]. The obtained derivatives display the beneficial characteristics of a pentafluorobenzyl ester during NICI detection, resulting in striking sensitivity due to reduced fragmentation and high efficacy of the dissociative resonance electron capture process. The potential usefulness of this derivative for ultrasensitive determination of MPH has been preliminarily demonstrated [24].
The use of stable isotope-labelled analogues as internal standards comprises a major advantage of mass spectrometric detection. This standardisation method has previously been applied to the analysis of MPH [23].
For pharmacokinetic applications, robustness and short analysis time is a major concern since they involve the processing of a large a number of samples. Additionally, as the target group for MPH medication are children, reduction of sample size is of critical importance. It was therefore the aim of this study to elaborate a method for the determination of MPH in human plasma that meets the requirements of sensitivity, specificity, speed and ruggedness for pharmacokinetic applications and drug monitoring.
Experimental
Chemicals and reagents
Pentafluorobenzyl alcohol was purchased from ABCR (Karlsruhe, Germany). Phtalic anhydride was supplied by Sigma-Aldrich (Vienna, Austria). Methylphenidate was from Cerilliant (USA). All other substances, solvents and reagents of analytical grade were from Merck (Darmstadt, Germany). PBBCl reagent and isotope-labelled standard may be obtained from the corresponding author or via www.bpa-lab.com. Plasma samples were collected as part of a pharmacokinetic study and hence under approval of the corresponding authorities.
Synthesis of o-(pentafluorobenzyloxycarbonyl)-benzoyl chloride
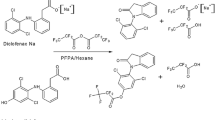
Synthesis of the PBBCl reagent was accomplished as previously described [24]. Briefly, phtalic anhydride (148 mg, 1 mmol) and pentafluorobenzyl alcohol (198 mg, 1 mmol) were dissolved in benzene and allowed to react at 100 °C for 2 h in the presence of pyridine. The intermediate acid was treated with thionyl chloride at room temperature for 1 h. Excess reagent was removed under nitrogen and the oily residue dissolved in 10 mL of dichloromethane, yielding a 10-mM solution of the PBBCl reagent.
Preparation of [18O2H3]-MPH
MPH HCl (10 mg) was dissolved in 800 μL H 182 O and 20 μL fuming HCl was added. The vial was closed under nitrogen and kept at 100 °C for 72 h. After cooling, the mixture was evaporated to dryness under nitrogen. The dry residue was dissolved in 300 μL of [2H3]-methanolic HCl (prepared by adding 20 μL of acetyl chloride to 0.5 mL of [2H3]-methanol) and left for 1 h at room temperature. The solvent and reagent were removed under nitrogen and the dry residue dissolved in acetonitrile. Isotope distribution was checked by GC-NICI-MS.
Plasma sample preparation and derivatisation
One nanogram of the internal standard [18O2H3]-MPH (50 μL of an acetonitrile working solution) was pipetted into a 5-mL glass tube and 0.25 mL of plasma was added. After vortexing, 0.25 mL carbonate buffer (pH 9.0) was added along with 1 mL of n-hexane and 100 μL of PBBCl reagent solution (1 mM in dichloromethane). It is crucial to add the internal standard immediately after thawing of the samples. The mixture was shaken on a rotary shaker for 30 min and phases were allowed to separate upon standing. The (upper) n-hexane phase was transferred to a fresh glass tube and solvent-evaporated under nitrogen. The dry residue was reconstituted in 80 μL of ethyl acetate and transferred to autosampler vials. Two microlitres were used for GC-NICI-MS analysis using m/z 380 and m/z 385 for the detection of MPH and internal standard (IS), respectively.
Gas chromatography–mass spectrometry
An ISQ quadrupole mass spectrometer coupled to a TRACE GC Ultra (Thermo Scientific, Vienna) was used. The GC was fitted with a BPX5 fused silica capillary column (15 m × 0.25 mm i.d., SGE). The injector was operated in the splitless mode at 280 °C. Helium was used as a carrier gas at a constant flow rate of 1.5 mL/min. Initial column temperature was 160 °C for 1 min, followed by an increase of 40 °C/min to 310 °C and an isothermal hold of 4 min. The mass spectrometer transfer line was kept at 315 °C. NICI was performed with methane as a moderating gas at an electron energy of 70 eV and an emission current of 0.250 A.
Analytical method validation
Calibration graphs were established in the range of 0.072–18.25 ng/mL plasma with nine calibration points in duplicate. For this purpose, blank plasma was spiked with the appropriate amounts of MPH and serial dilution of the highest calibrant with blank plasma. Standard solutions of MPH were prepared in acetonitrile and stored at −20 °C. Calibration curves were calculated by polynomial regression analysis (quadratic fit) weighting for 1/s² (s = standard deviation of duplicates). For linearity check, five individual calibration curves were measured and the coefficients of regression evaluated. Back-calculated values of all measured calibrants were correlated to their nominal values and the correlation coefficients calculated.
Inter-day precision was determined at 0.072- (LOQ), 0.210-, 1.5- and 15-ng/mL concentration levels by carrying five identical samples at each concentration level throughout the analytical sequence. Spiked samples were prepared from blank plasma. Intra-day precision was determined at 0.072- (LOQ), 0.210-, 1.5- and 15-ng/mL concentration levels by carrying five identical samples at each concentration level throughout the analytical sequence. Spiked samples were prepared from blank plasma. Accuracy of the methods was also tested at the aforementioned concentrations. Thus, the data from inter- and intra-day precision measurements were used to calculate the deviation of the values measured from the actual spiked values. Specificity was tested by analysing six different blank plasma samples. Stock solution stability was tested in acetonitrile and methanol after storage at −20 °C. To measure freeze–thaw stability, the samples were analysed immediately after spiking with the indicated amounts of MPH and after three freeze–thaw cycles. The long-term stability of stored samples was also investigated at the 0.210- and 15-ng/mL concentration levels after storage for 6 months at −20 °C. Short-term (benchtop) stability was assessed at the 1.5-ng/mL concentration level after 3 h at room temperature. Autosampler stability was determined by analysing a set of spiked samples at different concentrations together with the corresponding calibration curve at two different days. The samples were thereby left at ambient temperature until reanalysis. Aliquot analysis was validated by analysing 50% aliquots (250 μL sample, 1:1 dilution). Robustness towards GC temperature programming was determined by analysing one set of samples under different GC programme settings.
Matrix effect was investigated using six lots of matrix, including the haemolysed and hyperlipidaemic sample matrix. For each analyte and the internal standard, the matrix factor (MF) was calculated in each lot of matrix by calculating the ratio of the peak area in the presence of matrix (measured by analysing blank matrix spiked with analyte at a concentration of 0.210 ng/mL after extraction) to the peak area in the absence of matrix (pure solution of the analyte). The IS normalised MF was also calculated by dividing the MF of the analyte by the MF of the IS. The IS was added to the matrix or buffer together with the unlabelled target. Normalised MFs were calculated for each sample separately. For the assessment of accuracy and precision at prospective analytical batch size, five replicates of spiked samples at the 0.210-, 1.5- and 15-ng/mL concentration levels together with at least 90 blank plasma samples were extracted and chromatographed with a set of calibration standards in one single run. Quality control (QC) samples were analysed once before the blank plasma samples and again after analysis of 90 blank plasma samples. Accuracy was measured as bias (per cent deviation of the calculated versus the nominal values) and precision was expressed as coefficient of variation (%).
Results and discussion
Preparation of [18O2H3]-MPH
We have previously described the synthesis of [18O2]-MPH [23]. The labelling procedure described here offers a fast and inexpensive way to incorporate a specific label at the carboxylic acid group with an even higher isotopic label (M + 5). After hydrolysis in H 182 O and re-esterification with [2H3]-methanolic HCl, the 18O2H3-labelled product is obtained in quantitative yield. Unlabeled species were below 0.1%. No back exchange of label was observed under the conditions employed for the sample preparation and analysis. The increase in mass by 5 amu is also of advantage compared with the commercially available [2H3]-MPH labelled at the methyl ester carbon.
NICI mass spectrometry of MPH-PBB derivatives
As previously described, heptafluorobutyrate derivatives of MPH show a prominent fragment ion series at m/z 409/389/369/349, resulting from sequential loss of HF from the molecular ion [23]. Reduced fragmentation and production of more high-mass ions by NICI, however, occurs only to a certain extent when perfluoroacyl derivatives are measured. These derivatives produce molecular anions by resonance electron capture (REC), but fragmentation is quite intense, leading to the aforementioned ion series. Thus, the proportion of the total ion current is very low, no matter which fragment ion is chosen for quantification. Fragmentation under NICI conditions can, however, be drastically reduced to only a few fragment ions with striking intensity, as for pentafluorobenzyl derivatives of carboxylic acids undergoing dissociative REC that leaves the carboxylate anion with high stability and high abundance. PBB derivatives also show this fragmentation mechanism and yield similar results [24]. The NICI spectra of the PBB derivative of MPH and [18O2H3]-MPH are shown in Fig. 1a, b, respectively. The carboxylate anion is present with striking abundance and fragmentation is extremely reduced, resulting in more than 90% of the total ion current for the diagnostic fragment ions at m/z 380 (MPH) and m/z 385 (IS), respectively. This extremely efficient process accounts for the extraordinary sensitivity of these derivatives.
Gas chromatography of MPH-PBB derivatives
In comparison to heptafluorobuyrate derivatives, retention times are shifted dramatically due to the bulky PBB group, thus allowing detection at less interference from volatile matrix components. In Fig. 2, a typical chromatogram obtained after the analysis of plasma containing 0.072 ng/mL MPH is shown. There are no interfering peaks from matrix or reagent.
Plasma sample preparation and derivatisation
The method presented here provides a rapid, rugged and simple way for the analysis of MPH in plasma allowing processing of large sample batches. As children are the main target of this medication, a small sample size is desirable. The extraordinary sensitivity of the PBB derivative allows the use of only 250 μL of plasma by keeping a LOQ of 0.072 ng/mL. Extraction and derivatisation proceeds rapidly and is quantitative in this one-step procedure. The phase transfer reaction (extractive acylation) also eliminates excess reagent by hydrolysis in aqueous buffer and thus minimizes background contamination. As hydroxyls do not react under these conditions, interference from co-derivatised matrix components is low [24]. Even lower detection limits can be obtained, if desired, when larger plasma samples of 1 mL are extracted and the hexane phase is dried under nitrogen. Afterwards, derivatisation can be accomplished as described or with 100 μL of the neat reagent. Using this method, LOQs below 5 pg/mL can be obtained (results not shown).
Analytical method validation
The calibration graphs established were linear within the tested range of 0.072–18.25 ng/mL plasma with a mean regression coefficient (r) of 0.99959 (n = 5). The mean regression equation from five calibration curves was \( Y = 0.00{7}\left( {\pm 0.0{1}0{73}} \right) + 0.{24346}\left( {\pm 0.00{483}} \right) \times X + {3}.0{44}{{5}^{{ - {4}}}}\left( {\pm {2}.{67}{{7}^{{ - {4}}}}} \right) \times {X^{{2}}} \). Correlation (r²) of back-calculated values with their respective nominal values was 0.99959.
The coefficients of inter- and intra-day variation (precision) and accuracy of the spiked samples are presented in Table 1. It can be seen from these data that the method provides a highly precise and accurate assay for MPH in human plasma. This can be attributed at least in part to the use of a stable isotope-labelled internal standard. Mass spectrometry in combination with stable isotope dilution is a very powerful tool in external quality assessment schemes, and assays based on this technique can be regarded as reference procedures to validate other analytical methods.
Six different blank matrices were checked for interferences. In none of the samples was there background contribution above 25% LOQ.
Short-term stability was determined as the deviation between the concentrations obtained for samples subjected to the extraction immediately after thawing and those kept at room temperature for 3 h before extraction. In human plasma, deviation from immediately analysed samples was −7.6%. If, however, the internal standard was added immediately after thawing, the values obtained after 3 h at room temperature did not deviate substantially (2.9%). With porcine plasma as a matrix, deviation was significantly higher (−13.2%), but could also be compensated by the immediate addition of the internal standard (deviation −3.8%). It is well known that plasma esterases hydrolyse MPH to ritalinic acid; thus, the internal standard should be added immediately after thawing. This is another beneficial effect of the stable isotope-labelled standard as it is chemically identical to the target and thus compensates for loss due to hydrolysis of MPH. Nevertheless, long benchtop times should be avoided in any case during sample processing. It should be noted that we have also investigated the degradation of MPH in alkaline buffer and found no significant decrease during the time span of the sample workup.
For autosampler stability, the mean concentrations of samples chromatographed immediately after sample preparation and 5 days later were measured and differed between −5.3% at 0.210 ng/mL and −1.2% at 15 ng/mL Thus, MPH-PBB derivatives are stable at repeated analysis conditions.
Stock solutions of MPH in acetonitrile and methanol were stable for at least 31 months when stored below −20 °C. The measured difference was 1.08%.
For the assessment of long-term stability, plasma samples were stored below −20 °C in a freezer for 6 months and compared with the immediately analysed samples. Deviations ranged from −0.3% at LOQ to 0.7% at 15 ng/mL. Therefore, the samples can be considered as stable within 6 months of storage.
To determine the freeze–thaw stability of MPH, spiked samples were subjected to three freeze–thaw cycles. The results are shown in Table 2. Mean concentrations of samples undergoing three freeze–thaw cycles did not differ from that of the reference samples more than the critical 15%.
After analysing sample aliquots of 50% deviation was 0.76%, which shows that samples containing MPH may be measured with sufficient reliability in 125 μL sample aliquots (1:1 dilution).
Analysis of MPH with three different GC temperature programmes yielded deviations of 0.21% and 1.31% at 0.210 ng/mL and 0.17–0.27% at 15 ng/mL The method is thus robust against variations of the GC temperature programme.
Evaluation of the matrix effect resulted in an internal standard-normalised matrix factor of 1.02 with a coefficient of variation (CV) of 3.7%. This demonstrates that normal, lipidaemic and haemolytic plasma matrix yield equal results. This could be expected for a GC-MS method with appropriate sample preparation as the matrix effects are usually by far more pronounced in LC-MS applications, where ion suppression may lead to dramatic changes in MS response.
Accuracy at prospective analytical batch size has been estimated for a 90-sample batch. Deviation of samples analysed after sample batch from early analysed samples was 1.10% at 0.210 ng/mL, 2.55% at 1.5 ng/mL and 5.74% at 15 ng/mL. The method is thus suitable for analysing batch sizes up to 133 samples (18 calibrants + 90 batch samples + 15 QC samples).
We have applied the method described herein to the analysis of MPH during pharmacokinetic profiling of the drug. Figure 3 shows a typical time course from a human volunteer receiving 30 mg of the drug orally as a retard formulation. It should be noted here that a promising challenge is the determination of drugs in oral fluid for drug level monitoring. However, there are only few data on MPH levels in saliva, and ongoing studies will support the use of alternative matrices for noninvasive monitoring. Nevertheless, the presented method can be used for the analysis in minute amounts of plasma as well.
Conclusions
Due to the delicate targeting of the drug to children for the treatment of ADHD, an assay with the highest achievable sensitivity is desirable to minimize the sample size for therapeutic drug monitoring, which is crucial for adequate drug dosage because of the large intra-individual variability and tolerance. The method described fulfils these criteria exceptionally by reaching detection limits far below all assays published so far. The ease of extractive acylation keeps the sample preparation time to a minimum and allows large sample batches to be analysed in a short time. The use of a stable isotope-labelled internal standard adds an additional dimension of specificity and selectivity to the mass spectrometric detection, thereby also compensating ideally for losses during the sample workup procedure and derivatisation sequence. This is of particular interest for the analysis of MPH from the plasma matrix as esterase activities may lead to degradation which is time- and matrix-dependent. The extraordinary sensitivity of the assay must be attributed to the new derivatisation reagent, PBBCl. We have successfully applied this method to the bulk analysis of plasma samples for a preliminary pharmacokinetic study, demonstrating its ability for routine measurements.
References
Cantwell DP (1996) Attention deficit disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 35(8):978–987
Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJS, Jensen PS, Cantwell DP (1998) Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet 351(9100):429–433
Markowitz JS, Straughn AB, Patrick KS, DeVane CL, Pestreich L, Lee J, Wang Y, Muniz R (2003) Pharmacokinetics of methylphenidate after oral administration of two modified-release formulations in healthy adults. Clin Pharmacokinet 42(4):393–401
Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, Kraemer G, Breese GR (1983) Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther 226(2):382–386
Sun Z, Murry DJ, Sanghani SP, Davis WI, Kedishvili NY, Zou Q, Hurley TD, Bosron WF (2004) Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J Pharmacol Exp Ther 310(2):469–476
Scharman EJ, Erdman AR, Cobaugh DJ, Olson KR, Woolf AD, Caravati EM, Chyka PA, Booze LL, Manoguerra AS, Nelson LS, Christianson G, Troutman WG, Association A, American Association of Poison Control C (2007) Methylphenidate poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 45(7):737–752
Delbeke FT (1996) Doping in cyclism: results of unannounced controls in Flanders (1987–1994). Int J Sports Med 17(6):434–438
Hickey G, Fricker P (1999) Attention deficit hyperactivity disorder, CNS stimulants and sport. Sports Med 27(1):11–21
Zhu H-J, Wang J-S, Patrick KS, Donovan JL, DeVane CL, Markowitz JS (2007) A novel HPLC fluorescence method for the quantification of methylphenidate in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 858(1–2):91–95
Barbarin N, Mawhinney DB, Black R, Henion J (2003) High-throughput selected reaction monitoring liquid chromatography-mass spectrometry determination of methylphenidate and its major metabolite, ritalinic acid, in rat plasma employing monolithic columns. J Chromatogr B Anal Technol Biomed Life Sci 783(1):73–83
Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S (2000) Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 14(8):619–623
Marchei E, Farre M, Pellegrini M, Rossi S, Garcia Algar O, Vall O, Pichini S (2009) Liquid chromatography–electrospray ionization mass spectrometry determination of methylphenidate and ritalinic acid in conventional and non-conventional biological matrices. J Pharm Biomed Anal 49(2):434–439
Marchei E, Munoz JA, Garcia-Algar O, Pellegrini M, Vall O, Zuccaro P, Pichini S (2008) Development and validation of a liquid chromatography–mass spectrometry assay for hair analysis of methylphenidate. Forensic Sci Int 176(1):42–46
Yang Y, Kameoka J, Wachs T, Henion JD, Craighead HG (2004) Quantitative mass spectrometric determination of methylphenidate concentration in urine using an electrospray ionization source integrated with a polymer microchip. Anal Chem 76(9):2568–2574
Ramos L, Bakhtiar R, Tse FL (2000) Liquid–liquid extraction using 96-well plate format in conjunction with liquid chromatography/tandem mass spectrometry for quantitative determination of methylphenidate (Ritalin) in human plasma. Rapid Commun Mass Spectrom 14(9):740–745
LeVasseur NL, Zhu H-J, Markowitz JS, DeVane CL, Patrick KS (2008) Enantiospecific gas chromatographic–mass spectrometric analysis of urinary methylphenidate: implications for phenotyping. J Chromatogr B Anal Technol Biomed Life Sci 862(1–2):140–149
Aoyama T, Kotaki H, Honda Y, Nakagawa F (1990) Kinetic analysis of enantiomers of threo-methylphenidate and its metabolite in two healthy subjects after oral administration as determined by a gas chromatographic–mass spectrometric method. J Pharm Sci 79(6):465–469
Lin SN, Andrenyak DM, Moody DE, Foltz RL (1999) Enantioselective gas chromatography–negative ion chemical ionization mass spectrometry for methylphenidate in human plasma. J Anal Toxicol 23(6):524–530
Patrick KS, Ellington KR, Breese GR, Kilts CD (1985) Gas chromatographic–mass spectrometric analysis of methylphenidate and p-hydroxymethylphenidate using deuterated internal standards. J Chromatogr A 343(2):329–338
Patrick KS, Jarvi EJ (1990) Capillary gas chromatographic–mass spectrometric analysis of plasma methylphenidate. J Chromatogr A 528(1):214–221
Nakajima K, Kotaki H, Saitoh Y, Nakagawa F (1986) Determination of methylphenidate and its main metabolite in plasma by gas chromatography–chemical ionization mass spectrometry. Chem Pharm Bull 34(4):1701–1708
Bach GA, Henion J (1998) Quantitative capillary electrophoresis–ion-trap mass spectrometry determination of methylphenidate in human urine. J Chromatogr B Biomed Sci Appl 707(1–2):275–285
Leis HJ, Fauler G, Raspotnig G, Windischhofer W (2000) Negative ion chemical ionization for the determination of methylphenidate in human plasma by stable isotope dilution gas chromatography/mass spectrometry. J Mass Spectrom 35(9):1100–1104
Leis HJ, Windischhofer W (2010) o-(Pentafluorobenzyloxycarbonyl)benzoyl chloride: a novel electrophoric derivatisation reagent for amino compounds designed for negative ion chemical ionisation mass spectrometry. Rapid Commun Mass Spectrom 24(22):3320–3324. doi:10.1002/rcm.4775
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue on Analytical Sciences in Austria with Guest Editors G. Allmaier, W. Buchberger and K. Francesconi.
Rights and permissions
About this article
Cite this article
Leis, H.J., Schütz, H. & Windischhofer, W. Quantitative determination of methylphenidate in plasma by gas chromatography negative ion chemical ionisation mass spectrometry using o-(pentafluorobenzyloxycarbonyl)-benzoyl derivatives. Anal Bioanal Chem 400, 2663–2670 (2011). https://doi.org/10.1007/s00216-011-5048-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5048-6