Abstract
High-performance liquid chromatography linked to isotope ratio mass spectrometry (HPLC-co-IRMS) via a Liquiface© interface has been used to simultaneously determine 13C isotope ratios of glucose (G), fructose (F), glycerol (Gly) and ethanol (Eth) in sweet and semi-sweet wines. The data has been used the study of wine authenticity. For this purpose, 20 authentic wines from various French production areas and various vintages have been analyzed after dilution in pure water from 20 to 200 times according to sugar content. If the 13C isotope ratios vary according to the production area and the vintage, it appears that internal ratios of 13C isotope ratios \(\left( {R_{^{13} C} } \right)\) of the four compounds studied can be considered as a constant. Thus, ratios of isotope ratios are found to be 1.00 ± 0.04 and 1.02 ± 0.08 for \(R_{^{13} C_{G/F} }\) and \(R_{^{13} C_{Gly/Eth} }\), respectively. Moreover, \(R_{^{13} C_{Eth/Sugar} }\) is found to be 1.15 ± 0.10 and 1.16 ± 0.08 for \(R_{^{13} C_{Gly/Sugar} }\). Additions of glucose, fructose and glycerol to a reference wine show a variation of the \(R_{^{13} C}\) value for a single product addition as low as 2.5 g/L−1. Eighteen commercial wines and 17 concentrated musts have been analyzed. Three wine samples are suspicious as the \(R_{^{13} C}\) values are out of range indicating a sweetening treatment. Moreover, concentrated must analysis shows that 13C isotope ratio can be also used directly to determine the authenticity of the matrix.

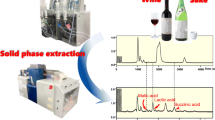
HPLC-co-IRMS chromatogram of a diluted sweet wine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The link between high-performance liquid chromatography (HPLC) and isotope ratio mass spectrometry (IRMS) has been commercially available since 2004 [1]. Recently, an instrument based on a similar process is commercialized by Isoprime/Elementar [2]. The process is based on a gas extraction from the liquid phase of the CO2 resulting from chemical oxidation of organic matter after compound separation by HPLC. Such a system allows isotopic analysis of polar and labile compounds such as amino acids [3], organic acids [4], standard small and macro-molecules [5], as well as kinetic studies [6]. The knowledge of δ13C ratios is often used in food authenticity. Until now, analyses were performed by EA-IRMS for solid and liquid bulk analysis [7, 8] and by GC-IRMS for volatiles compounds [9–11]. The achievement of the link between HPLC and IRMS allows the determination of δ13C ratios of new kinds of compounds, like sugars, that can be studied individually. Honey authenticity was the first application in food authenticity: δ13C data of honey sugars, separated by HPLC, allowed the detection of exogenous sugar adulteration [12].
Due to its economic impact, wine authentication is of importance. Since the 1990s, wine control is insured in the European Union by isotopic measurement together with an annual wine isotopic databank carried out in each wine-producing EU country. The method is based on analytical results provided by 2H-NMR and IRMS on ethanol (δ13C and 2H) and by IRMS on wine water (18O). The isotopic determinations are mainly used for sugar enrichment that is regulated and for watering a prohibited practice [13]. But several steps are required for data collection: wine databank constitution (grape harvest, must fermentation…), ethanol matrix extraction (distillation), and sample preparation. As these steps need high investment of people and time, some developments are made to find internal tracer allowing the authentication of a wine. With this objective, Cabanero et al. studied δ13C of glycerol and ethanol of dry wines by HPLC-co-IRMS [14, 15]. Their results showed that this technique is simple, fast, and the difference between δ13Cglycerol and δ13Cethanol could provide information on wine authenticity. But this difference is variable according to the origin and the type of wine [16]. Nevertheless, and to follow this idea, HPLC-co-IRMS was applied to sweet and semi-sweet French wines. The first reason was the challenge of simultaneous determination of δ13C isotope ratio of the four major components of wine (glucose, fructose, glycerol, and ethanol) with the objective of finding some links between the δ13C values of these four compounds. The second reason was the control of sugar authenticity in semi-sweet wines. Until now, the control is only possible when sugar concentration is higher than 40 g/L as technical problems appear for lower concentrations: the ethanol amount resulting from sugars fermentation would request too important volumes.
For this purpose, the HPLC-co-IRMS method validation was the first step of the work in order to demonstrate results accuracy and reproducibility. Then 20 authentic wines were analyzed by this method; δ13C results allowed the computation of various ratios of isotope ratios that appear to be constant in authentic wines. These results were applied to 18 commercial wines and 17 concentrated musts (CM) in order to determine the validity of the assumption and the applicability of these results for wine control area.
Material and methods
Reagents
All reagents were of analytical grade and used without any purification steps. Solutions of ammonium persulfate (Sigma-Aldrich), orthophosphoric acid (Fisher Scientific) were prepared in Milli-Q water (Elga). Sugars, d-(+)-glucose (99.5%) and d-(−)-fructose (99%), glycerol (analytical reagent) are Sigma and Fisher Scientific products, respectively. Helium (Linde 5.6) is the carrier gas and oxygen (Linde, 5.0) the combustion gas for the combustion reactor. Carbon dioxide (Linde, 4.5) is used as the reference gas calibrated with certified materials and regularly controlled using internally calibrated materials.
Samples
Each year, our laboratory develops part of an isotope data bank for French wines. This is our contribution to the EU requirement for all wine producer state members [17]. Grapes from various French wine production areas are received at the laboratory in order to make reference wines (experimental details in Ref. [18]). At the end of the fermentation, most of the wines are dry (no residual sugar) but in some cases, due to the initial grape sugar richness, some wines present residual sugars as yeasts are not able to transform all of them in ethanol; 20 sweet wines from various French regions and vintages were used to conduct this study (10 over the 20 samples are listed in Table 1, as a data illustration). Eighteen commercial wines as well as 17 CM were analyzed in order to check results. Wines were diluted with Milli-Q water from 20 to 200 times to reach concentrations of studied compounds lower than 1 g/L. Concentrated musts were dissolved in milli-Q water at 0.6 mol L−1. Twenty-five microliters of filtered (0.45 μm nylon filters) solutions were directly injected in the HPLC system.
EA-IRMS
Vario Micro Cube (Elementar), elemental analyzer (EA), is linked to the Isoprime isotope ratio mass spectrometer (IRMS, Isoprime). For ethanol analysis, EA is equipped with a liquid auto-sampler (injected volume: 1 μL); sugars and glycerol were analyzed on the Vario Micro cube settled in solid mode, using tin cups and 2 mg of compounds. EA works under 200 mL min−1 helium flux, a temperature of 950 °C in the oxidation tube and 550 °C in the reduction tube. The outlet is equipped with a column that physically retains the CO2 at 60 °C, carbon dioxide being released by temperature increases to 210 °C. Overall experiment duration is 600 s. Sample δ13C values are the average of duplicate measurements (δ < 0.30‰).
HPLC-co-IRMS
The link between high-performance liquid chromatography (Ultimate 3000, Dionex) and the IRMS mass spectrometer is ensured by an interface, called Liquiface© (Isoprime) allowing the chemical oxidation (co) of the organic compound in CO2. HPLC is equipped with a 120 position auto-sampler and a regulated temperature block, set at 80 °C, that contains the carbohydrate column 700 CH (Alltech, 300 mm × 6.5 mm). The volume injected is 25 μL and elution flux sets at 0.4 mL min−1, elution being ensured with Milli-Q water. “Peek” tubing ensures the link between the HPLC and the interface. On line, the eluted solution is mixed with ammonium persulfate (15% in mass), oxidative solution, and orthophosphoric acid solution (2.5% in volume) via a three-way Valco valve. In order to avoid any mixing problem, orthophosphoric acid and persulfate solution are prepared in the same flask, the two inlet tubings being dived into the same flask and brought with a peristaltic pump at a flow rate of 0.6 mL min−1 (Fig. 1). As the persulfate oxidative property is efficient at 90 °C, the mixture is warmed at 93 °C. At the oven outlet, the twisted tubing passes through a bath of water refrigerated by frequent ice addition in order to efficiently cool the solution and thus avoiding water vapor extraction simultaneously to CO2 gas; this system allows the obtaining of more stable analysis (Fig. 1). This cooled solution passes through a separating membrane, flushed by helium (15 mL min−1) on the outside, allowing the extraction of the CO2 elaborated in the reactor. This gas mixture (CO2 + H2O vapor) is sent in the IRMS (current trap, 300 μA) for isotope ratio analysis after crossing a Nafion membrane for gas drying (external helium flux, 50 mL min−1). Eluent and reagent solutions are previously de-aerated by bubbling helium for 1H and kept under helium flux during experiments to avoid any atmospheric CO2 contamination. In order to prevent any ammonium persulfate crystallization in the tubing, the system is rinsed with water as soon as unoccupied and left under water flux overnight. Under these conditions G, F, Gly, and Eth are fully separated (Fig. 2). Overall experiment duration, HPLC separation and gas analysis, is 1,200 s; for each sample, the analysis was repeated twice, and the results listed in Tables 1 and 2 correspond to the average value of these two measurements.
Calibration and isotopic computation
δ13C, the 13C/12C isotope abundance ratio, is expressed against international standard Vienna Pee Dee Belemnite and can be defined as:
where R corresponds to the sample (sa) and international standard (st) 13C/12C isotope ratio. In order to determine CO2 reference gas δ13C, international standards were analyzed: BCR-660 provides, after distillation, an ethanol with δ13C = −26.72 ± 0.09 ‰. Internal standards were also used: cane sugar and ethanol (−11.09 ± 0.30% and −13.27 ± 0.10‰, respectively) and beet ethanol (−28.37 ± 0.09‰). Two pulses of CO2 reference gas, calibrated against the international standard, were performed at the beginning of the elution run.
Results and discussion
HPLC using carbohydrate column allows the separation of sugars, glycerol and ethanol, then each eluted compound is chemically oxidized, in the Liquiface© device, in carbon dioxide which is analyzed in the IRMS. Elution is performed by water, the use of organic solvent is prohibited as the CO2 resulting of its oxidation by the persulfate will distort or even hide the expected information. Under the experimental conditions described in the “Material and methods” section, the fully separated and δ13C measurements of glucose, fructose, glycerol, and ethanol were achieved by HPLC-co-IRMS (Fig. 2).
Method validation
The critical point of this method corresponds to the chemical oxidation of the eluted compound and the CO2 gas extraction from the eluting solution. The overall performance of the system, i.e., any isotopic fractionation, needs to be checked. The linearity was controlled with a glucose solution at various concentration levels, from 0.02 to 1 g/L, each solution being analyzed three times; the peak area average values measured by the IRMS are presented in Fig. 3 as a function of glucose concentration. The excellent correlation (R ≈ 1) between concentration and peak area indicated that the experimental conditions are convenient for this concentration range, i.e., all the eluted molecules are oxidized in CO2, in the interface, and all the gas formed is recovered by the system. Linearity was found to be 0.02 σ‰/nA. Method accuracy has been controlled with the comparison of values of δ13C ratio determined by HPLC-co-IRMS and EA-IRMS for glucose. In Fig. 4 is plotted the variation of δ13C with glucose concentration: the values determined by the two methods are in the range ±0.5‰ indicating that any isotopic fractionation is observable in the concentration range 0.02 to 1 g/L. The accuracy of the method has been also investigated using δ13C of ethanol as it is systematically determined for authentic sample according to the official method [19]. As the distillate is composed of ethanol, water (<5% m/m) and other volatile compounds (<2% m/m) [20], data deviation between these two methods could be a result of HPLC product separation. This is not the case as for most of the samples the deviation is lower than 0.5‰ indicating satisfying determination of isotope ratio by HPLC-co-IRMS and the negligible influence of distillated volatile compounds on the determination of ethanol 13C/12C isotope ratio, in accordance with former results [14]. Reproducibility was also checked by injecting the same wine on a 3-day period. Standard deviation was determined for the studied species, glucose (0.17), fructose (0.06), glycerol (0.45), and ethanol (0.08) confirming the method robustness for the four studied compounds (Electronic Supplementary Material Table S1).
Wine products measurements
Authentic wines with residual sugar were studied to analyze isotope parameters of major wine compounds. Dilution is necessary to reach a concentration lower than 1 g/L to avoid any IRMS signal saturation. This dilution can be up to 200 times for sweet wines; sometimes, essentially for semi-sweet wines (4 to 12 g/L of sugars), two measurements need to be performed at two dilutions levels: one for ethanol and fructose measurements (dilution factor 200) and a second one for glucose and glycerol isotopic ratio determination (dilution factor 20). Under these experimental conditions, a full separation of G, F, Gly, and Eth is obtained allowing the simultaneous determination of δ13C of each compound (Fig. 1). Results listed in Table 1 corresponds to the average of two successive measurements: isotope ratio from one sample to another is different depending on vintage and geographical origin. This observation was expected as it is one of the reasons of the need to constitute an annual European wine databank. In Table 1 are listed the δ13C isotope ratio of sugars, glycerol, and ethanol. To our knowledge, it is the first time that fructose and glucose δ13C isotope ratio was determined. These ratios show systematically significant deviation between δ13C values of the precursors and compounds elaborated during the fermentation step. Sugars present a higher carbon-13 concentration regarding ethanol and glycerol. These results suggest a non-equivalent distribution of 13C on the sugars’ carbonated skeleton which is consistent with a previous work on glucose 13C NMR measurements [21, 22]. This conclusion is reinforced by studies on sparkling wine CO2 showing δ13C ratio higher than ethanol δ13C [23, 24] and our work on ciders that confirms a CO2 bubble δ13C ratio higher than the one of apple juice sugars (unpublished work). From Table 1, one can estimate that carbon-13 enrichment of sugars is not only due to some carbons on specific positions but to the overall enrichment of their carbonated skeleton that occurs during sugar biosynthesis. Indeed, sugars with higher carbon-13 concentrations systematically lead to ethanol with high 13C concentration.
By themselves, individual results do not provide any information on wine authenticity unless a data bank is elaborated with these parameters as it is done for ethanol stable isotopes. Nevertheless, information on wine authenticity can be reached using the ratio of the isotope ratio, \(R_{^{13} C}\), that appears to be independent of vintage and geographical origin. Indeed, internal ratio of sugars, ethanol or glycerol, will differ from an authentic wine as soon as a wine will be submitted to a post-fermentation addition of one of these compounds. It appears that, for authentic wines, glucose–fructose ratio, \(R_{^{13} C_{G/F} }\), and glycerol–ethanol ratio, \(R_{^{13} C_{Gly/Eth} }\), are found to be 1.00 ± 0.04 and 1.02 ± 0.08, respectively (Table 3). The ratio of isotopic ratios of fermentation products over sugars is found to be 1.15 ± 0.10. A ratio equal to unity indicates a comparable 13C concentration for the compounds while the ratio of 1.15 reveals a discrepancy of 13C concentration in the fermentation products. The low standard deviation value adds to the fact that they can be considered as internal constants in wines, i.e., any deviation from these values is characteristic of wine adulteration. Thus, some practices like sweetening and glycerol or ethanol addition can be checked by controlling the internal ratio of isotopic ratios.
Additions of glucose, fructose, and glycerol were performed on an authentic sweet wine to determine the addition impact on the \(R_{^{13} C}\). Three solutions of glucose, fructose, and glycerol (1 g/L) were prepared and various aliquots were added to the tested wine. Glucose and fructose are extracted from C4 plants (δ13C = −10.40‰, determined by EA-IRMS) while glycerol comes from C3 plant type (δ13C = −30.70‰, EA-IRMS data). Tested wine δ13C isotope ratios were −25.15, −25.37, and −27.81‰ for glucose, fructose, and glycerol respectively. \(R_{^{13} C_{G/F} }\) as a function of added sugar is presented in Fig. 5a. As expected, only one signal had its isotope value modified according to the type of added compound, single sugar or glycerol. C4 type sugar addition leads to an increase of δ13C ratio, i.e. an increase of \(R_{^{13} C_{G/F} }\) value is observed for fructose addition and a decrease for glucose addition. A similar observation occurs for a C3 type glycerol addition (Fig. 5b): as the added glycerol presented a lower δ13C ratio, \(R_{^{13} C_{Gly/F} }\) ratio increases with added glycerol. These experiments indicate that C4 type sugar and glycerol addition could be suspected at a level of 2.5 g/L as a deviation of the ratio of isotope is observed.
For sweet wines, an isotopic study of sugars is performed as follow: after wine distillation (600 mL), 1 g/L of yeast are added to the distillate residue in order to transform the sugars in ethanol. When the fermentation is completed, this re-fermented wine is distilled to recover the ethanol on which a new series of isotopic measurements are performed. For technical reasons, the volume of ethanol elaborated after re-fermentation and/or the volume of wine needed for distillation, wine sugars were not studied for sugar amounts under 40 g/L. Thus, this method based on HPLC-co-IRMS results was mainly developed to control wines with sugar content lower than 40 g/L. These results were applied to commercial wine: 18 samples of white wines, sweet and semi-sweet, as well as rosé wines have been studied, the δ13C data are listed on Table 1, \(R_{^{13} C}\) uncertainty evaluation, extracted from statistical distribution of data coming from measurements series and characterized with experimental standard deviation, can be computed considering twice the standard deviation value. An uncertainty of 0.1 has been applied to the set of ratios of isotope ratios. Under these conditions, 83% of the samples are in the range determined by the authentic samples.
Despite this extended uncertainty, three commercial wines, samples 26 to 28, are still out of range. Sample 26 present expected values for \(R_{^{13} C}\) of Glucose/Fructose (0.94), glycerol/ethanol (0.94) but higher values for \(R_{^{13} C}\) of fermentation products / sugars (from 1.30 to 1.39, Table 1). Despite a \(R_{^{13} C_{Gly/F} }\) value at the uncertainty limit (1.22), an adulteration can be suspected corresponding to a wine sweetening by C4 type sugars, glucose, and fructose or a concentrated must addition. Sample 27 is a different case as only \(R_{^{13} C_{Gly/Eth} }\) (0.94) is in the expected range while the other computed values are found to be 0.74 for \(R_{^{13} C_{G/F} }\), 1.80 and 1.92 for \(R_{^{13} C_{Eth/G} }\) and \(R_{^{13} C_{Gly/G} }\), 1.33 for \(R_{^{13} C_{Eth/F} }\) and \(R_{^{13} C_{Gly/F} }\). The information provided by these results seems to indicate that C4 type glucose and fructose were added to this wine after the fermentation. Moreover it can be estimated that the amount of added glucose and fructose must not be proportional: \(R_{^{13} C}\) deviation is stronger when glucose parameter is involved in the computation indicating a glucose addition more important. Finally, in sample 28, all the ratios related to glucose (G/F: 0.89; Eth/G: 1.28; Gly/G: 1.40) are out of range while the other ratios (Gly/Eth: 1.10; Eth/F:1.14, Gly/F: 1.25) are in the expected range. These data seems to correspond to a single C4 type sugar addition, a glucose addition.
The same experiments were performed on commercial concentrated musts and the results are listed in Table 2. The first 16 results presents a \(R_{^{13} C_{G/F} }\) of 1.02 that is in accordance with the ratio found in authentic wine showing that none of these concentrated musts have been adulterated by added sugars. Nevertheless, for one sample, sugar authenticity can be doubtful. Sample 17 has a \(R_{^{13} C_{G/F} }\) of 0.96 that is within the uncertainty but the individual values of glucose and fructose δ13C isotope ratio is rather high and does not seem compatible with expected values for C3 sugar type with a pure grape origin. Thus, this sample must have been adulterated by an addition of C4-type sugars.
Conclusion
HPLC-co-IRMS has been used for the study of major wine compounds: glucose, fructose, glycerol, and ethanol. Depending on initial concentrations and using adequate dilution, these four compounds are fully separated and the carbon-13 isotope ratio of each of them can be determined in a single run with good reproducibility and repeatability. The simultaneous determination of isotopic ratio prevents any isotopic fractionation that could occur when data are obtained by different techniques. Despite δ13C variability linked to the geographical origin and vintage, the intrinsic ratio of compound δ13C, \(R_{^{13} C}\), appears to be constant and characteristic of a non-adulterated wine. This intrinsic ratio is equal to 1.00 for sugars and fermentation products and 1.16 for the ratio of sugars and fermentation product δ13C ratio. These results were applied to commercial wines and concentrated must, considering an uncertainty of 0.1; with this latter condition, 83% of the commercial wines present the expected intrinsic ratio. Still, 3 out of 18 samples are out of range revealing sugar addition, glucose alone, glucose and fructose mixtures, or concentrated must at a post-fermentation level. In none of the samples, no adulteration by glycerol or ethanol addition was suspected. Moreover, as observed with concentrated must, the δ13C value also provides information on sugar origin as in one case, the isotope ratio allowed to characterize a C4 sugars addition.
As this technique is performed for the control of C4 sugar type addition, it needs to be checked for C3 sugar type addition and extended to other wine production areas. Computation of ratio of isotope ratio provides qualitative information on post-fermentation wine treatment, its quantitative application seems to be difficult but needs to be investigated. Moreover, carbon-13 isotope ratio determination of wines acids (tartaric, lactic, malic…) is the next challenge that should also provide precious information on wine authenticity.
Electronic Supplementary Material available: Table S1 provides δ13C of sugars, glycerol and ethanol, determined by HPLC-co-IRMS for the same wine over a period of 3 days.
References
Krummen M, Hilkert AW, Juchelka D, Duhr A, Schlüter HJ, Pesch R (2004) A new concept for isotope ratio monitoring liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 18:2260–2266
Morrison DJ, Taylor K, Preston T (2010) Strong anion-exchange liquid chromatography coupled with isotope ratio mass spectrometry using a liquiface interface. Rapid Commun Mass Spectrom 24:1755–1762
McCullagh JS, Juchelka D, Hedges RE (2006) Analysis of amino acid 13C abundance from human and faunal bone collagen using liquid chromatography/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom 20:2761–2768
Heur V, Tille S, Krummen M, Mollar PX, Hmelo LR, Hinrichs KV (2006) Online δ13C analysis of volatile fatty acids in sediment/porewater system by liquid chromatography isotope ratio mass spectrometer. Limmnol Oceanogr Methods 4:346–357
Godin JP, Hau J, Fay LB, Hopfgartner G (2005) Isotope ratio monitoring of small molecules and macromolecules by liquid chromatorgraphy coupled to isotope ratio mass spectrometry. Rapid Commun Mass Spectrom 19:2689–2698
Schierbeek H, te Braake F, Godin JP, Fay LB, van Goudoever B. Novel method for measurements of glutathione
Kelly S, Heaton K, Hoogewerff J (2005) The application of multi-element and multi-isotope analysis. Trends Food Sci Technol 16:555–567
Guyon F, Douet CH, Colas S, Salagoity MH, Medina B (2006) Effects of must concentration techniques on wine isotopic parameters. J Agric Food Chem 54:9918–9923
Meier-Augenstein W (1999) Applied gas chromatography coupled to isotope ratio mass spectrometry. J Chromatogr A 842:351–371
Jung J, Jaufmann T, Hener U, Munch A, Kreck M, Dietrich H, Mosandl A (2006) Progress in wine authentication: GC-C/P-IRMS measurments of glycerol and GC analysis of 2.3-butanediol stereoisomers. Eur Food Res Technol 223:811–820
Spitzke ME, Fauhl-Hassek C (2010) Determination of the 13C/12C ratios of ethanol and higher alcohols in wine by GC-C-IRMS analysis. Eur Food Res Technol 231:247–257
Cabanero AI, Recio JL, Rupérez M (2006) Liquid chromatography coupled to isotope ratio mass spectrometry: a new perspective on honey adulteration detection. J Agric Food Chem 54:9719–9727
Regulation CE 491–09
Cabanero AI, Recio JL, Rupérez M (2008) Isotope ratio mass spectrometry coupled to liquid and gas chromatography for wine ethanol characterization. Rapid Commun Mass Spectrom 22:3111–3118
Cabanero AI, Recio JL, Rupérez M (2010) Simultaneous stable carbon isotopic analysis of wine glycerol and ethanol by liquid chromatography coupled to isotope ratio mass spectrometry. J Agric Food Chem 58:722–728
Calderon G, Naulet N, Guillou C, Reneiro F (2004) Characterisation of European wine glycerol: stable carbon isotope approach. J Agric Food Chem 52:5902–5906
Etablissement d’une banque de données pour l’analyse par résonance magnétique nucléaire des produits du secteur viti-vinicole. R-CE 2729/00, 2000, 1–15
Chabreyrie D, Chauvet S, Guyon F, Salagoity MH, Antinelli JF, Medina B (2008) Characterization and quantification of grape variety by mean of shikimic acid concentration and protein fingerprint in still white wines. J Agric Food Chem 56:6785–6790
EU regulation 2676/90 and amendments a. 822/97/EC b
Bauer-Christoph C, Watcher H, Christoph N, Robmann A, Adam L (1997) Assignment of raw material and authentication of spirits by gas chromatography, hydrogen- and carbon-isotope measurements. Z Lebensm Unters Forsch A 204:445–452
Gilbert A, Silvestre V, Robins RJ, Remeaund G (2009) Accurate quantitative isotopic 13C NMR spectroscopy for the determination of the intramolecular distribution 13C in glucose at natural abundance. Anal Chem 81:8978–8985
Thomas F, Randet C, Gilbert A, Silvestre V, Jamin E, Akoka S, Remaud G, Segebarth N, Guillou C (2010) Improved characterization of the botanical origin of sugar by carbon-13 SNIF-NMR applied of ethanol. J Agric Food Chem 58:11580–11585
Calderone G, Guillou C, Reneiro F, Naulet N (2007) Helping to authenticate sparkling drinks with 13C/12C of CO2 by gas chromatography-isotope ratio mass spectrometry. Food Res Int 40:324–331
Cabanero AI, San-Hipolito T, Rupérez M (2007) GasBench/isotope ratio mass spectrometry: a carbon isotope approach to detect exogenous CO2 in sparkling drinks. Rapid Commun Mass Spectrom 21:3323–3328
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Wine Analysis with Guest Editor Isabelle Pianet.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 25.1 kb)
Rights and permissions
About this article
Cite this article
Guyon, F., Gaillard, L., Salagoïty, MH. et al. Intrinsic ratios of glucose, fructose, glycerol and ethanol 13C/12C isotopic ratio determined by HPLC-co-IRMS: toward determining constants for wine authentication. Anal Bioanal Chem 401, 1551–1558 (2011). https://doi.org/10.1007/s00216-011-5012-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5012-5