Abstract
Calcium-41 (t 1/2 = 105 years) can be used after a single dose to follow calcium metabolism over a subject's lifetime. The aims of this study were to expand a 41Ca kinetic model and estimate bone resorption in women with stable bone loss, compare the rates with those calculated with classical isotope studies, and to use the model to simulate dynamic changes in urinary 41Ca:Ca ratios and bone balance for the design and interpretation of 41Ca studies. Forty-two women >5 years post-menopause were given 41Ca intravenously. Bone mineral content and bone mineral density of total body were measured by dual-energy X-ray absorptiometry at the beginning of the study. Urine collections were made periodically for up to ∼5 years while subjects were free living. Urinary 41Ca:Ca ratios were measured using accelerator mass spectrometry. The isotope data were analyzed by compartmental modeling. Four compartments were necessary to fit the urinary tracer data and total bone calcium. The final model included pathways for absorption, distribution, urinary excretion, and endogenous excretion and was used to calculate rates of bone turnover. Estimates of bone resorption in a subset of the women (n = 13), studied previously in a 3-week balance and full kinetic study with 45Ca, agreed with those using 41Ca methodology. Thus, rates of bone resorption can be estimated from 41Ca urinary data in stable post-menopausal women. The model was used to simulate dynamic changes in urinary 41Ca:Ca ratios and bone balance, as a result of interventions that perturb calcium metabolism to aid in study design and interpretation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Qualitative changes in bone turnover that result in gain (or loss) of bone can be currently assessed using biomarkers [1, 2]. Dual-energy X-ray absorptiometry provides a quantitative measure of bone gain (loss) after the changes have occurred [3]. It would be useful to have an alternative method for determining more rapidly the rate of bone loss (or gain) following an intervention. A recent approach uses 41Ca, a radioisotope with a half-life (t 1/2 = ∼105 years), which is long compared to the lifespan of a biological entity, and thus behaves like a stable isotope. The excretion of 41Ca can be determined in single urine samples for years after a single dose because of the sensitivity of measurement by accelerator mass spectrometry (AMS) and the lack of abundance of 41Ca (natural abundance <10−15) in the environment [4]. Denk et al. administered 41Ca to post-menopausal women and analyzed urine data with a three-compartment model [5]. Changes in 41Ca urinary excretion after bisphosphonate intervention paralleled changes in biochemical markers and bone mineral density (BMD) [6], although they did not calculate rates of bone turnover.
While the potential for 41Ca for determining changes in bone turnover has been demonstrated, interpretation of the data still has some unanswered questions, namely, what changes in Ca metabolism alter the 41Ca:Ca ratio? Does a change in ratio always correspond to a change in bone balance? Do interventions at different times after dosing cause the same response in ratio? How large a change in metabolism is required for it to be detectable in the ratio? How long a recovery period (i.e., for bone balance to return to pre-intervention level) is required after stopping a treatment (e.g., as with bisphosphonate)? These questions can be addressed through dynamic modeling.
The purpose of this study was threefold: (1) to expand a kinetic model [5] for 41Ca in post-menopausal women who are beyond the rapid bone loss phase (i.e., have stable bone loss) [7] for calculating bone turnover rate, (2) to compare the values calculated for bone resorption with those calculated from previous isotope studies in a sub-group of the subjects, and (3) to use the model to simulate dynamic changes in urinary 41Ca:Ca ratios and bone balance for the design and interpretation of 41Ca studies.
Materials and methods
Subjects
Forty-two post-menopausal women aged 37 to 74 years were studied (Table 1). Subjects were excluded if they were less than 4 years post-menopausal to ensure that bone loss is in stable state. Also, subjects that used estrogen hormone replacement therapy, antiresorptive drugs, drugs for the treatment of bone disease, thiazide diuretics, thyroid medication, corticosteroids, non-prescriptive drugs, or consumed isoflavones were excluded since the following study will explore the effect of isoflavone-like treatment on bone metabolism. Potential subjects were also excluded if their medical history included mal-absorptive disorders, bone, liver, or kidney disease, hormonal abnormalities, cancer, or soy allergies. Serum follicle-stimulating hormone and luteinizing hormone were measured at screening to verify post-menopausal status.
The Human Institutional Review Boards at Purdue University, Indiana University-Purdue University Indianapolis, and Clarian IRB approved the protocol in this study as part of larger intervention studies.
Study design
Subjects participated in several studies including two previously reported [8, 9]. However, only the baseline or pretreatment data while the subjects were free-living were used here. Due to the long-term nature of the studies, the subjects for any one study were not studied simultaneously, but at intervals. Bone mineral content (BMC) and BMD of the lumbar spine, proximal femur, and total body were measured by dual-energy X-ray absorptiometry (Lunar DPX IQ or Prodigy, Madison, WI, USA) at the beginning of the study. Subjects were provided 500–648 mg calcium per day and 400–500 IU/day of vitamin D (Viactive, McNeil Nutritionals, LLC, Ft. Washington, PA, USA; Rexall Sundown, Inc., Boca Raton, FL, USA; Geritol, Glaxo Smith Kline, England) throughout the study period.
They were dosed with 50 to 1,000 nCi 41Ca (Argonne National Laboratories) in sterile saline by a single intravenous injection (six subjects received it in three divided doses). The larger doses (1 μCi) were administered initially, but smaller doses were administered in later studies as we learned that these were sufficient for detection. Both the size and administration schedule of the dose were modeled in the data analysis.
Sample collection
Urine samples (at least 500 ml) were collected beginning with the second void of the day every 10–20 days for 167–1,808 days after dosing. The wide time range in the baseline is indicative of multiple studies taking place over several years. Initially, urine collections were requested until the approximate total calcium excreted for each individual was established for determining the volume required for 41Ca analysis. Then, individual volume collections that provided the minimum calcium for analysis were then requested (e.g., 500 ml or 1 l) up to a maximum of a 24-h volume. The volume collected depended on the urinary calcium concentration of the particular subject. For approximately one out of every eight woman, it was determined that a 24-h urine collection was required to provide the 15–20 mg of CaF2 necessary to fill the AMS sample holder when mixed with silver. We lose some calcium during the processing, and though measurements can be made with less material, the focusing properties of our source are such that the highest output is obtained when our sample well is full (1.2 mm diameter, and 2 mm deep).
Sample analysis
Urine samples were collected in acid-washed containers, and the volume of each urine collection was measured. Approximately 10 ml of ammonium hydroxide was added to every liter of urine and mixed to ensure that the pH was at least 10. Then, 50 ml of saturated ammonium oxalate was added and mixed to separate the calcium by precipitation as calcium oxalate (CaC2O4) by letting the sample stand overnight. The supernatant was decanted, leaving the precipitate and some urine, which were then filtered. The precipitate was dissolved in 50 ml of approximately 0.5 M HNO3 solution and chromatographically purified by cation exchange using Bio-Rad AG 50W-X8 resin (Bio-Rad, Hercules, CA, USA). Calcium fluoride (CaF2) was precipitated with hydrofluoric acid, washed, dried in a vacuum oven, loaded into aluminum sample holders, and inserted into the ion source of the AMS to obtain the 41Ca:Ca ratio of the samples.
At the Purdue Rare Isotope Measurement Laboratory (PRIME Lab), the samples were measured during six runs that took place between 2000 and 2009. PRIME Lab is an NSF-supported AMS facility that also makes measurements for the life sciences. The AMS at PRIME Lab uses an upgraded FN (nominal 8 MV) tandem accelerator with a cesium sputter negative ion source, a 150-kV injector, and beam lines containing three large sector magnets. The detector is a gas-filled ionization detector that can discriminate between nuclear isobars. The AMS is approximately 60 m from ion source to detector. The mean value of each 41Ca:Ca isotope ratio was determined by normalizing the measured value against the measured values of two standards, one measured before and one measured after each unknown. The standards used had a 41Ca:Ca ratio of either 3.7 × 10−10 or 2 × 10−9. Each sample was measured multiple times, and the uncertainty was calculated from counting statistics and systematic errors [10]. In brief, the uncertainty from counting statistics is \( \sigma = 1/\sqrt {N} \), where N is the number of counts in the detector. Systematic errors most likely arise from small imperfections as the ion beam is jockeyed around the beam line. They were accounted for by calculating the standard deviation of the ratio each time a sample was measured. The total number of 41Ca measurements was about 630, and the average number of 41Ca measurements per subject was 15 with a range from 6 to 52. The typical precision of a measurement was 5% with a range from 2% to 7%. If a sample had precision over 10%, that indicates that something had gone awry with the AMS measurement, and the ratio was disregarded. The background due to chemical processing was regularly checked by running blanks over our chromatography columns, one a calcium solution and the other a urine specimen. The Purdue ion source generates CaF −3 ion currents of 50–500 nA with the corresponding 41Ca8+ ion detection rates of about 3 Hz for a sample 41Ca enrichment of about 5 × 10−10 of total calcium. A background of ≤5 × 10−13 is routinely achievable with samples collected prior to tracer administration. Background subtraction was performed on all unknowns as part of our analysis procedure but was unnecessary for most unknowns.
Total calcium in 24-h urine was measured in 17 subjects using atomic absorption spectrophotometry as previously described [11].
Kinetic modeling in steady state
Kinetic calculations during the baseline period assumed steady-state (i.e., that pool sizes were constant), so the rate of bone resorption equaled bone formation. A compartmental model was fitted to the urinary 41Ca:Ca data, measures of total bone calcium, and where available, daily urine Ca, for each subject using WinSAAM [12]. The dosing regimen (single or multiple i.v. doses of tracer) was simulated. The model was fitted using least squares procedures [12]. Modeling notation was L(i,j), for daily factional transfer from compartment j into compartment i, M(i) for compartmental mass in milligrams, and R(i,j) for daily mass transfer from compartment j into compartment i (i.e., R(i,j) = L(i,j) × M(j) (milligrams per day)). The mass of compartment 1 was calculated as the product of initial volume of distribution and plasma Ca concentration. Compartment turnover time (day) was calculated as the inverse of the sum of all the L(i,j) out of a compartment. Compartment mass, rates of calcium absorption, bone resorption (or formation), urine excretion, endogenous excretion, the diet necessary to maintain bone mass, the initial volume of distribution, and total bone calcium were calculated by the model. The initial volume of distribution was calculated based on i.v. tracer distribution (i.e., y-intersection of i.v. tracer). The dietary intake of calcium necessary to maintain bone mass was determined as an input to the model.
Dynamic simulations
To simulate calcium dynamics, when pool sizes change over time and bone formation and bone resorption rates differ (as would occur after a treatment or diet change), we modeled tracee (40Ca) and tracer simultaneously, using the population values for the parameters. In detail, we set up a duplicate model for tracee that had the same structure as the tracer model. The initial condition for each compartment in the tracee model was set to the steady-state mass of calcium as calculated by the tracer model. The 41Ca:Ca ratio was calculated as the tracer in compartment 1 divided by the amount of calcium in the analogous compartment in the tracee model, since we assume that urinary and serum ratios are equivalent [13]. We simulated both models for ∼250 days after administration of an i.v. dose (baseline period) and then simulated a treatment (or intervention) by introducing changes (in the range of 50–200 mg/day) in various pathways that would have an effect on bone balance (defined as the difference between bone deposition and bone resorption). We calculated the effect of the change on the 41Ca:Ca ratio and on bone balance since this determines bone mass.
Statistical analysis
Paired t test was used to compare the values of the bone resorption rate and urinary calcium excretion between this study and a previously reported 3-week balance study using 45Ca that shared a subset of subjects [14]. SAS statistical software package (version 9.1.3, SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Results
Kinetic modeling
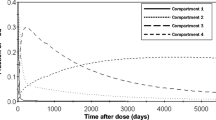
A three-compartment model was initially employed to fit the 41Ca data as in a previous study [5], but the model was expanded by including pathways for absorption, excretion into urine, and endogenous excretion. Modeling assumptions were based on a combined kinetic and balance study in post-menopausal women [14] that absorption was 20% of dietary intake, endogenous excretion was 150 mg/day and, if not measured, urinary Ca excretion was 150 mg/day. For subjects whose data were collected for <300 days, the value for L(2,3), which represents the fraction of Ca moving from compartment 3 to compartment 2, was set to the average value (2.5 × 10−3/day) calculated for subjects with data obtained for >300 days. While the three-compartment model fitted the urinary ratio data, it underestimated the mass of calcium in the body (estimated as 38% of BMC [15]) calculated as the sum of calcium mass in the three compartments. Thus, it was necessary to add a fourth compartment to the model (Fig. 1). We chose to define the pool based on knowledge of two forms of bone, trabecular and cortical, known to turnover at different rates. We refer to compartment 3, the faster turning over bone compartment, as “trabecular” and compartment 4 as “cortical” bone. The ratio of Ca in cortical vs. trabecular bone is considered to be 80:20 [16], and so the fractional transfer into compartment 4 was set so that its mass was four times that of compartment 3. Cortical bone turnover is reported to be 4.4%/year [17], which is a turnover time of over 20 years. As it was too slow to be determined from our data, release from compartment 4, L(2,4), was set using this value (10−4/day). Whole body calcium data were fitted by the sum of the mass of compartments 1, 2, 3, and 4. Fits of the four-compartment model to data are shown in Fig. 2. A plot of the distribution of tracer remaining in the body shows that from 250 until ∼1,800 days, most was in compartment 3, the “trabecular” bone compartment; thereafter, tracer was predominantly in compartment 4 (Fig. 3).
Compartmental model for calcium metabolism, with pools numbered arbitrarily with values for a fractional transfers (L(i,j), into compartment i from compartment j), mean ± SD (n = 42), b pool size and mass transfer, and c percent distribution of Ca from the compartments where the arrows start. L(6,1) was the average for subjects where urinary excretion was measured (n = 17). Absorption [L(1,8)/(L(1,8) + L(10,8)] was fixed at 20%, and endogenous excretion, L(10,1), was based on a rate of 150 mg/day [14]. Compartment 4 was necessary to fit both urinary tracer and bone calcium data, and L(2,4) was fixed based on cortical bone turnover of 4.4%/year [16, 17]
Calculated fits of the model (Fig. 1) (lines) to urine 41Ca:Ca ratio data (symbols) for three subjects. Triangle and square symbols indicate two subjects who received 1 μCi (right y-axis, 41Ca:Ca ratio × 10−9), while diamond symbol indicates a subject who received 50 nCi (left y-axis, 41Ca:Ca ratio × 10−10)
Simulation of distribution of the initial dose (fraction) between pools for the model in Fig. 1 over 5,000 days. Compartment 4 has the slowest rate of turnover, which is consistent with “cortical” bone, suggesting that interventions at 250 days post-dose mainly affect tracer in “trabecular” bone
The initial volume of distribution was calculated as 53 ± 23 L. The average transfer coefficients (Fig. 1a) were used to determine turnover times of 11 ± 5, 118 ± 92, 396 ± 187, and 10,000 days for compartments 1, 2, 3, and 4, respectively. The pool sizes in gram Ca and mass transfers in milligrams per day (calculated from averaging R(i,j) across all subjects, Fig. 1b) show the higher rate of resorption from “trabecular” vs. “cortical bone” (501 vs. 69 mg/day). Because we assume steady state, the rates of bone resorption (the sum of Ca transport from compartment 3 to compartment 2 and out of compartment 4) and bone formation (transport from compartment 2 to compartments 3 and 4) were the same (Fig. 1b). Calculated whole body Ca was 945 ± 171 g. The percent distribution of Ca from each compartment (Fig. 1c) shows, for example, that of calcium entering compartment 1, 50% will go to compartment 2, while 25% is excreted in urine and 25% in feces.
For a subset of the women (n = 13), bone resorption rates calculated by the model from only urine 41Ca data were compared to the rate calculated previously using 45Ca sampled in multiple tissues (serum, urine, and feces) following oral and i.v. tracer administration [14]. For these subjects, bone resorption rates calculated from the 41Ca and the 45Ca data were not statistically different (p = 0.21; Table 2).
Dynamic simulations of 41Ca:Ca ratio
To aid in experimental design and interpretation of 41Ca data, we simulated the effect of changes in calcium metabolism (representing an intervention or therapy) on predicted urinary 41Ca:Ca ratio and bone balance. We simulated a 1-μCi i.v. dose of 41Ca for a baseline period of 250 days and then changed some parameter values. Two assumptions were made: changes we introduced to pathways were the only changes that occurred in the system (i.e., there were no other homeostatic responses), and because plasma calcium is tightly controlled, the mass of tracee in the first pool (containing plasma) remained constant after any parameter change. So, if we perturbed a parameter that increased calcium entering the first compartment, we also increased a loss pathway to keep that pool constant. For example, if we increased calcium entry into plasma from bone resorption, we also increased urinary loss or, alternatively, decreased calcium absorption. In this way, pairs (or triplets) of parameters were altered in each simulation; 15 combinations were tested (Tables 3 and 4). The 41Ca:Ca ratio and bone balance were calculated at 500 days post-dose (i.e., after 250 days of intervention). The change in bone balance was dynamic or changing over time. If an intervention was continued (beyond 250 days), we found that it took more than 10 years for balance to return to pre-intervention values (Fig. 4).
Simulation of bone balance (the sum of “trabecular” and “cortical” bone balances) over time with a sustained intervention (increased calcium absorption, urine excretion, and “trabecular” bone deposition, simulation 8 in Table 3). For this case, the simulation predicts that total bone balance gain persists. It takes many years to return to the pre-intervention, or zero state
Results of each simulation are expressed in two ways, as percent change, so that the degree of change in the pathway can be compared to the resulting change in ratio (Table 3) and on an absolute basis (Table 4). The changes refer to the pathways and transport rates in Fig. 1b. Results for the specific questions posed are as follows:
-
1.
To answer what changes in Ca metabolism alter the urinary 41Ca:Ca ratio, all the simulations tested showed a change in the 41Ca:Ca ratio (Tables 3 and 4).
-
2.
Does a change in ratio always correspond to a change in bone balance? The change in ratio corresponded inversely with bone balance except for two cases, when a decrease (or increase) in absorbed calcium was matched by a decrease (or increase) in urine excretion (simulations 1 and 5, Table 4). In all other cases, increased bone balance was associated with decreased 41Ca:Ca ratio and vice versa.
-
3.
Do interventions at different times after dosing cause the same response in ratio? Simulation results were consistent with respect to changes in ratio and bone balance when the intervention was introduced at either 250 or 2,000 days post-dose (Table 5).
-
4.
To determine how small a change in metabolism can be detected in the ratio, we introduced interventions of varying size in absorption, excretion, and bone formation at 250 days post-dose and simulated for 50 days. As expected, the simulations showed that the change in the 41Ca:Ca ratio became larger as the magnitude of the intervention increased. Specifically, the model predicted that a change of less than 15 mg/day in bone balance changed the ratio about 5%, whereas a 30-mg/day change in bone balance caused a ∼10% change in the ratio (data not shown).
-
5.
To address how long a recovery period is required following an intervention for bone balance to return to the pre-intervention level, we simulated the model for baseline (250 days), intervention (50 days), and variable recovery periods, after model parameter values were reset to baseline values. The simulation showed that recovery of bone balance was dependent upon the magnitude of the intervention: when bone balance was perturbed by about 50 mg/day, a 50-day recovery time returns bone balance within 10 mg/day of its pre-intervention value, but more than 200 days of recovery is required to return bone balance to its original value.
Discussion
By extending a previous model [5] to fit urinary 41Ca data obtained over >6 months and calcium mass in bone, we estimated bone resorption rates for stable post-menopausal women. Because this tracer can be monitored for the lifetime of a subject after a single dose, potential uses for 41Ca include monitoring changes in a subject's bone metabolism due to disease or therapy. In these situations, metabolism would not be in steady state, and so we used the model to perform dynamic simulations that may help in the implementation and analysis of data in 41Ca studies with interventions.
We made several assumptions in expanding the model proposed by Denk et al. [5]. Two of these (absorption of calcium was 20% and rates of endogenous excretion of 150 mg/day) used the mean values from a study in a population of post-menopausal women [14]. Denk et al. [5] assumed 50% absorption as they administered their tracer orally. As we administered the dose i.v., we only assumed absorption of dietary calcium. We assumed steady state for the baseline period (but used a dynamic model for interventions). Bone loss 5 years after menopause is reported to be 1%/year [18, 19]. For whole body Ca of 945 g, this represents 26 mg/day. We found that a 10% change in 41Ca:Ca ratio is equivalent to about 30 mg/day in resorption (and with further simulation using the dynamic model, 26 mg/day represents a 7% difference in ratio). This is within the error of sample measurement of 2–7%. We conclude that the 1% loss/year is a relatively small amount daily and within experimental error, supporting our use of steady state for the baseline period.
The results for resorption determined from 41Ca data agreed with those from a study in one subject that included both oral and i.v. stable isotope tracer administration [20] and, as described in this paper, with 13 subjects that had blood sampling and complete collection of urine and feces for balance measurements [14]. This agreement suggests that bone resorption can be estimated using the less invasive 41Ca methodology, which means that after tracer administration, only single urine voids are required, not blood draws. Furthermore, Freeman et al. [21] reported there was no diurnal variation in the 41Ca signal.
We previously used a statistical approach to evaluate changes in 41Ca [9]. The current approach provides more quantitative information about calcium metabolism. Compared to the three-compartment model for 41Ca data published by Denk et al. [5], we found that most parameter values, including volume of distribution, transfer coefficients, and compartment turnover times, agreed. The turnover time of compartment 2 was slower, but quite variable, in our study (118 ± 92 days) compared to Denk et al. (28 days) and may be related to our low sampling density during the first 50 days, when compartment 2 peaks [5]. Our estimates of data fitting were similar; the relative residual from our model was 0.8 ± 10% similar to that reported by Denk et al. (0 ± 7%). The slightly larger residual may be due to our larger sample size and longer sampling time.
It should be noted that the compartments in the model based on the 3-week study [14] differ to those in the current study and that it is not possible to directly compare the parameter values. Specifically, the combined mass of all three compartments of the 3-week model (6 g) is comparable to only the first compartment (5 g, Fig. 1b) of the 41Ca model. The two models (from 3-week studies and over several years) could be combined into a six-compartment model, but this comprehensive model would only be useful when data collection spanned from hours to years.
We hope that the design of experimental studies will be improved by our dynamic simulations of the relationship of 41Ca:Ca to various calcium interventions and to bone balance. Following changes in calcium metabolic pathways, we predict that increased bone balance reduces the urinary 41Ca:Ca ratio, and conversely, decreased bone balance increases 41Ca:Ca ratios. However, the 41Ca:Ca ratio would change independent of bone balance if all the absorbed calcium is assumed to be excreted via urine. This would be unlikely because only 25% of calcium entering the first compartment (by absorption or exchange) was excreted in urine, although an intervention such as citrate which facilitates urine excretion might create this scenario. We note that the changes related to increased urinary excretion also apply to changes in endogenous fecal excretion. That is, because the same fraction of compartment 1 transfers to feces by endogenous excretion as to urine (25%), any changes in endogenous excretion can show the same effect on the ratio and bone balance as the changes in urine excretion. Therefore, changes in the urinary 41Ca:Ca ratio appear to reflect the changes in bone balance under normal physiological conditions.
The model simulation suggests that the time of intervention beyond 250 days post-dose does not significantly affect the calculated bone balance and ratio. Rather, the size and site of the intervention has a larger impact on the ratio. In a subset of 11 subjects from this study, in a blinded, randomized order, cross-over trial (50 days intervention and recovery periods with urine collections every 10 days), we observed a significant change in ratio of 5% [9]. That is, with a small sample size (n = 11), ratio changes ≥5% were required for significance. The model predicts that a 10% change in the ratio corresponds to a change in bone balance of about 30 mg/day. Either, perturbations in the 41Ca:Ca ratio larger than 5% (which corresponds to changes in bone balance of over 15 mg/day) or a larger sample size may be required to achieve statistically significant differences in intervention studies.
The duration of recovery needed between interventions is dependent on the magnitude of effect of an intervention, i.e., a longer recovery time is necessary following large changes in the urinary 41Ca:Ca ratio. The model predicts that for a 15-mg/day change in bone balance, which produces a significant change in 41Ca:Ca ratio, a 50-day recovery period is sufficient. However, for a 50-mg/day change in bone balance, a 50-day duration is sufficient for recovering bone balance to within 10 mg/day, which does not significantly impact the 41Ca:Ca ratio, but more than 200 days are required for complete recovery. This suggests that if multiple treatments are being assessed, it is important to compare a treatment period with the recovery periods that immediately bracket the treatment period in time as a large change in balance will require a longer recovery time.
Bone balance, or the rate of deposition minus the rate of resorption, is the main factor causing changes in 41Ca:Ca ratio, as most tracer is in the bone compartments by the time of an intervention. The ratio would not change when the amount of calcium absorbed increases, but only under the non-physiological condition of all this additional calcium being excreted. Therefore, the 41Ca:Ca ratio can be used to follow changes in bone metabolism as a proxy of bone balance. Future experiments are required to verify this predicted behavior, for example, measuring the urinary 41Ca:Ca ratio and comparing to short-term changes in calcium balance or long-term changes in bone calcium content in humans in response to various treatments that alter bone balance.
Conclusion
We developed a four-compartment model for 41Ca kinetics in post-menopausal women by fitting urinary 41Ca and total bone calcium data. The calculated results for bone resorption agreed with previous studies using 45Ca and sampling of multiple tissues. Therefore, we can conclude that the proposed model can be used when there is stable bone loss to estimate bone resorption using 41Ca measurements in urine. In addition, model simulations confirm that changes in urinary 41Ca:Ca ratio can capture changes in bone metabolism as a proxy for bone balance.
References
Looker AC, Bauer DC, Chesnut CH 3rd, Gundberg CM, Hochberg MC, Klee G, Kleerekoper M, Watts NB, Bell NH (2000) Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int 11(6):467–480
Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB (2006) An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol 62(10):781–792
Hui SL, Zhou L, Evans R, Slemenda CW, Peacock M, Weaver CM, McClintock C, Johnston CC Jr (1999) Rates of growth and loss of bone mineral in the spine and femoral neck in white females. Osteoporos Int 9(3):200–205
Jackson GS, Weaver C, Elmore D (2001) Use of accelerator mass spectrometry for studies in nutrition. Nutr Res Rev 14(2):317–334
Denk E, Hillegonds D, Vogel J, Synal A, Geppert C, Wendt K, Fattinger K, Hennessy C, Berglund M, Hurrell RF, Walczyk T (2006) Labeling the human skeleton with 41Ca to assess changes in bone calcium metabolism. Anal Bioanal Chem 386(6):1587–1602
Denk E, Hillegonds D, Hurrell RF, Vogel J, Fattinger K, Hauselmann HJ, Kraenzlin M, Walczyk T (2007) Evaluation of 41calcium as a new approach to assess changes in bone metabolism: effect of a bisphosphonate intervention in postmenopausal women with low bone mass. J Bone Miner Res 22(10):1518–1525
Recker R, Lappe J, Davies K, Heaney R (2000) Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15(10):1965–1973
Cheong JMK, Martin BR, Jackson GS, Elmore D, McCabe GP, Nolan JR, Barnes S, Peacock M, Weaver CM (2007) Soy isoflavones do not affect bone resorption in postmenopausal women: a dose-response study using a novel approach with 41Ca. J Clin Endocrinol Metab 92(2):577–582
Weaver CM, Martin BR, Jackson GS, McCabe GP, Nolan JR, McCabe LD, Barnes S, Reinwald S, Boris ME, Peacock M (2009) Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using 41Ca methodology. J Clin Endocrinol Metab 94(10):3798–3805
Elmore D, Conard N, Kubik PW, Fabryka-Martin J (1984) Computer controlled isotope ratio measurements and data analysis. Nucl Instrum Methods Phys Res B 5(2):233–237
Wastney ME, Martin BR, Peacock M, Smith D, Jiang XY, Jackman LA, Weaver CM (2000) Changes in calcium kinetics in adolescent girls induced by high calcium intake. J Clin Endocrinol Metab 85(12):4470–4475
Wastney ME, Patterson BH, Linares OA, Greif PC, Boston RC (1999) Investigating biological systems using modeling: strategies and software. Academic Press, San Diego
Neer R, Berman M, Fisher L, Rosenberg LE (1967) Multicompartmental analysis of calcium kinetics in normal adult males. J Clin Invest 46(8):1364–1379
Spence LA, Lipscomb ER, Cadogan J, Martin B, Wastney ME, Peacock M, Weaver CM (2005) The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr 81(4):916–922
Cooper AR, Forbes RM, Mitchell HH (1956) Further studies on the gross composition and mineral elements of the adult human body. J Biol Chem 223(2):969–975
O'Flaherty EJ (1998) A physiologically based kinetic model for lead in children and adults. Environ Health Perspect 106(Suppl 6):1495–1503
O'Flaherty EJ (2000) Modeling normal aging bone loss, with consideration of bone loss in osteoporosis. Toxicol Sci 55(1):171–188
Dawson-Hughes B (1996) Calcium and vitamin D nutritional needs of elderly women. J Nutr 126(4 Suppl):1165S–1167S
McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354(8):821–831
Freeman SPHT, King JC, Vieira NE, Woodhouse LR, Yergey AL (1997) Human calcium metabolism including bone resorption measured with 41Ca tracer. Nucl Instrum Methods Phys Res B 123:266–270
Freeman SPHT, Beck B, Bierman J, Caffee MW, Heaney RP, Holloway L, Marcus R, Southon JR, Vogel JS (2000) The study of skeletal calcium metabolism with 41Ca and 45Ca. Nucl Instrum Methods Phys Res B 172(1–4):930–933
Acknowledgements
This study is funded by Purdue University, University of Alabama Botanical Center for Age Related Diseases, and National Institutes of Health (NIH) grants P50 AT00477.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, WH., Wastney, M.E., Jackson, G.S. et al. Interpretation of 41Ca data using compartmental modeling in post-menopausal women. Anal Bioanal Chem 399, 1613–1622 (2011). https://doi.org/10.1007/s00216-010-4454-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4454-5