Abstract
Micron-sized particles have primarily been used in microfabricated flow cytometers for calibration purposes and proof-of-concept experiments. With increasing frequency, microparticles are serving as a platform for assays measured in these small analytical devices. Light scattering has been used to measure the agglomeration of antibody-coated particles in the presence of an antigen. Impedance detection is another technology being integrated into microflow cytometers for microparticle-based assays. Fluorescence is the most popular detection method in flow cytometry, enabling highly sensitive multiplexed assays. Finally, magnetic particles have also been used to measure antigen levels using a magnetophoretic micro-device. We review the progress of microparticle-based assays in microflow cytometry in terms of the advantages and limitations of each approach.

Multiplexed assays using coded microspheres provide sensitive assays for on-site microflow cytometers
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As conventional flow cytometers with advanced capabilities mature as large, powerful laboratory systems for obtaining highly complex information, a new generation of “personal flow cytometers” has evolved. These benchtop, user-friendly cytometers focus on specific functions [1, 2] and include less flexible laser selection [3–5], less sensitive detectors [4], and/or elimination of sheath fluid [3]. These smaller systems can often accomplish most popular tasks, for example cell counting, measurement of cell viability, antibody quantitation, or detection of cell death. The Luminex family of cytometers, in particular, has advanced the use of microparticle-based assays to provide multiplexed analytical capability while keeping the optics relatively simple. With developments in microfabrication and microfluidics, for example chip design optimization and reduction of component size, developers are miniaturizing current cytometers even further to create systems for point-of-use applications, and some of these systems will continue to employ particle-based assays [6].
Currently, three companies specialize in flow cytometers that are designed specifically for particle-based assays: Luminex Corporation, Becton–Dickinson, and DiaSorin [2]. Commercially-available particles with various functionalized surfaces are also available from many sources (for example Bangs Laboratories, BD Biosciences, Invitrogen, Luminex Corporation, Sigma–Aldrich, or Spherotech) for use in calibration or assays. Microparticles are synthesized from a variety of materials, for example polystyrene (PS) or poly(methyl methacrylate) (PMMA), and are supplied with surface groups, for example carboxyls and amines, that can be readily modified for attachment of recognition molecules [7]. Particles can be purchased with diameters from tens of nanometers to hundreds of microns. For fluorescence measurements, microspheres with dyes doped within or attached to the surface are available in a wide range of colors.
Microparticle-based assays often rely upon antibodies as capture molecules on the surface of the microparticle. Although other types of capture molecule can be used, the high specificity and affinity of antibodies in the presence of complex sample matrices has made them a reagent of choice. In 1977, Horan and Wheeless published a manuscript in Science detailing the first microsphere-based immunoassays [8]. Since that time, others have followed with technology of increasing sophistication for microparticle-based assays interrogated using flow cytometry [2]. Capture antibodies are generally immobilized on to the microparticle surface using available reactive groups, for example amines, hydroxyls or thiols, but carboxyls are the most frequently used [7]. Carboxyl functionalization followed by exposure to ethyldimethylaminopropylcarbodiimide and N-hydroxysuccinimide provides a mild procedure for antibody attachment to the microparticle surface. The attached antibody captures antigen from a sample on to the surface of the microsphere. The signal can then be generated as an aggregation event measured using light scattering or electrical or magnetic properties. More frequently, a fluorescent tracer is included in the particle-capture antibody–antigen complex, and fluorescence is measured.
Light scattering in microflow cytometers
Light scattering is a staple phenomenon for detecting and characterizing particles in modern flow cytometry. A light beam directed at a particle can interact through reflective, refractive, and diffractive effects. Then, information about a particle or aggregate of particles can be derived from the change of direction and intensity of a scattered light beam. Collecting scattered light at various angles from the incident beam has been reported to provide different types of information about the particle, including both size and density [9]. The diameter of the particle should fall into the range of 1–50 wavelengths of the incident light beam.
Typically, forward-scattered light (0.5–5° from the incident beam) can provide approximate information about the size of a particle [10]. It should, however, be noted that an increase in the intensity of forward-scattered light does not always correlate with increasing particle size. Side-scattered light (15–150° from the incident beam) is often collected at 90° and provides information about smaller particles and structures within particles. Proportionally more light is scattered by small particles or internal structures at a wide angle than at a small angle, and thus side-scattered light can provide information about the relative roughness (or shape) of a larger particle surface and the granularity of its internal structures. Measuring side-scattered light and forward-scattered light has become a standard in flow cytometry for biomedical research, because this behavior enables cells to be distinguished by size and granularity, providing insight into mixed populations, viability, or changes in internal structures.
Using the information derived from scattered light at different angles, particles can be classified and studied. Shvalov et al. used light-scattering data from a scanning flow cytometer to distinguish lymphocytes, erythrocytes, polystyrene particles, and milk-fat particles of various size and refractive index [9]. These cells and particles generated different scattered-light profiles dependent on scattering angle. Steen custom-built a flow cytometer to characterize viruses of different size by light scattering [11]. This device could easily distinguish particles with diameters in the range 70–300 nm [12].
In the 1980s, Masson and coworkers presented a strong body of work describing particle-counting agglutination immunoassays (PACIA) [13–16]. Initially, PACIA was publicized as a replacement for expensive assays utilizing radioactive labels for characterization of antigen and antibody interactions. In these assays, polystyrene particles coated with antigens were incubated with antibodies to cause agglutination or aggregation of the particles. Key aspects of this assay were:
-
1.
use of an antibody with multivalent binding sites to enable particle–particle interaction;
-
2.
determination of particle concentrations that would allow aggregation; and
-
3.
prevention of non specific interactions between particles.
The samples before and after agglutination were measured in an optical particle counter based on light scattering. Aggregated particles were larger in size than unaggregated particles and resulted in more side-scattered light.
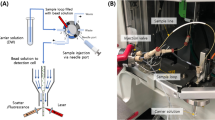
In 2003, Pamme, Koyama, and Manz described a microfluidic device that used light scattering to analyze agglutination immunoassays (Fig. 1) [17]. The device used was fabricated in poly(methyl methacrylate) (PMMA) and used a design that focused particles in two dimensions into an optical interrogation region. The scattered laser light was collected at 15° and 45° to the incident beam. Particles ranging from 2 to 9 μm in diameter were distinguished in this system, which is significantly larger than the 70–300 nm range reported previously [12]. Using this system, an agglutination immunoassay for C-reactive protein was performed. Scattered-light intensity was plotted against C-reactive protein concentration to show that higher protein concentration resulted in scattered light of greater intensity. The authors allude to other antigen–antibody pairs that can be characterized by this particle-based assay in a microfabricated flow cytometer.
(a) Schematic diagram of a microflow cytometer used for agglutination particle-based immunoassays using light-scattering detection. (b) Photograph of the experimental device, showing the microfabricated cytometer chip, and the stage used to align the optical fibers at 15° and 45° to the incident He–Ne laser beam. (c) Dot plot of 15° scattering intensity and 45° scattering intensity obtained from a mixture of microparticles of different sizes. Reproduced with permission from the Royal Society of Chemistry [17]
Light scattering is a well-studied detection method with many advantages to developers of microflow cytometry assays. Using just a beam of light of suitable wavelength (relative to the particle) and detectors at various angles, information regarding size, shape, and granularity of a particle are easily derived. Additionally, scattered light signals tend to be strong and do not need the most advanced or expensive detectors. Yet, distinguishing particles differing in diameters of a few microns can be challenging, and thus agglutination assays usually have limited sensitivity. Light scattering also lacks the specificity, sensitivity, and multiplex capability of the fluorescence detection that is enabled by an ever increasing number of fluorophore/antibody combinations.
Electrical detection in microflow cytometers
Electrical detection was the first particle-detection method used in flow cytometry [10]. Based on the work of Wallace Coulter [18], the Coulter principle demonstrates that electrical charge can be used to detect, size, and count particles in solution. The Coulter counter was subsequently developed, and detects changes in conductance, when particles or cells (generally non-conductive), pass through a conductive microchannel between two electrodes. This breakthrough led to the development of automated hematology and laid the foundation for the Coulter blood count or the complete blood count (CBC) medical diagnostic test [19, 20].
Advances in microfabrication methods have drawn many research groups to develop Coulter counters on chip-based platforms. The first examples used direct current (DC) or low-frequency alternating current (AC) [21, 22]. Later, microdevices using more sophisticated AC designs were developed to measure electrochemical impedance spectra of particles [23]. Microfabricated cytometers could also measure impedance at high (10 MHz) and low (0.5 MHz) frequencies to distinguish mixed particle populations [24, 25]. In this work and studies that followed [24–27], single-shell [28] and double-shell [29] particle models were used to distinguish particles, cells without nuclei, and cells with nuclei.
Holmes et al. has demonstrated a microfabricated flow cytometer for rapid analysis of microspheres using impedance for particle detection (Fig. 2) [30]. The device uses an elegant combination of electrical impedance and fluorescence detection to analyze immunoassays on the surface of microspheres. Dielectrophoresis was used to focus particles into an interrogation region on the chip. Impedance was then used to size particles and trigger the acquisition of fluorescence data within the optical interrogation region immediately downstream of the impedance interrogation region. Fluorescence was ultimately used as the reporter for the immunoassays because of its sensitivity and the ability to use selective fluorescent tracers of different wavelengths to distinguish multiple target populations.
(a) Schematic diagram of the impedance interrogation region of a microflow cytometer using electrical detection. Two pairs of parallel electrodes are at the top and bottom of the microchannel. As a particle passes through the interrogation region, the electrode pairs perform a differential measurement of the particle, taking the difference between the detection and reference volumes. (b) Schematic diagram showing the impedance-based microflow cytometer of Sun et al., which includes dielectrophoretic particle focusing, electrical detection, and dielectrophoretic sorting. Reproduced with permission from the Royal Society of Chemistry [30]
Although optical detection methods (light scattering and fluorescence) have emerged as the standard for flow cytometry, electrical methods have advantages that maintain relevance in cytometer technology. Electrical detection necessitates smaller, less expensive equipment compared with fluorescence-based flow cytometers. Additionally, impedance measurements do not require labels, for example fluorescent antibodies. Fluorescent markers increase the time and cost of sample preparation. Thus, in the analysis of microparticles by microfabricated devices, electrical detection has a place because of potential benefits in portability, cost, and time. The limitations of electrical methods include limited capacity for multiplex analyses and possible changes in signal because of sample matrix components.
Fluorescence detection in microflow cytometers
With the popularity of fluorescence in conventional flow cytometry, microfabricated flow cytometers with fluorescence detection capabilities have also become a common target for most research groups developing microflow cytometers [6]. Small lasers and filter sets are available for use with a range of fluorescence dyes and can thus be used to interrogate coded microspheres, facilitating multiplexed assays. Many fluorescent tracers, in the form of small molecules, proteins, and nanoparticles, enable sensitive quantification of antigen bound to the coded microspheres. Since 1985, particles have been attached to small molecules in single-target assays to make them detectable using flow cytometry [10]. Fulton et al. demonstrated the multiplexing capabilities of FlowMetrix microspheres, which incorporate different amounts of two fluorescent dyes (emitting at 585 nm and >650 nm) into 5.5-μm polystyrene microspheres, to distinguish as many as 64 distinct analytes (Fig. 3) [31]. Luminex Corporation has since advanced this technology to fabricate 200-microsphere sets distinguishable on its proprietary benchtop flow cytometer. Carson and Vignali used the coded microspheres to analyze 15 different cytokines in a single assay, including members of the interleukin family, tumor necrosis factor-alpha, tumor growth factor-beta 1, and interferon-gamma [32]; a plethora of similar multiplexed analyses, using a variety of recognition molecules, were reported in the following decade.
Multiplexed cytokine analysis using FlowMetrix microparticles in a flow cytometer. (a) A schematic representation of the reagents used in the FlowMetrix sandwich immunoassay. A key point is the color-coded fluorescence of the particles, because the 488 nm excitation beam causes the emission of 580 nm and 660 nm light as well as the 519 nm light resulting from the Alexa488 tracer. (b) FL2 (580 nm) and FL3 (660 nm) emission are plotted against each other to show microsphere set identification, which was coupled to cytokine assays for multiplexing. (c) Comparison of single FlowMetrix assays, multiplexed FlowMetrix assays, and ELISA data for cytokine analysis. Reproduced with permission from Elsevier [31]
In 1999, Fu et al. reported an early microflow cytometer that detected particles (specifically green fluorescent protein-expressing bacteria) using fluorescence [33]. But this system did not make use of the fluid focusing usually essential in flow cytometry. Beyond reducing clogs and debris accumulation on the channel surface, fluid focusing is important for reproducible interrogation of single particles. Microflow cytometers with dielectrophoretic focusing have been shown to detect microparticles by laser-induced fluorescence using optical fibers [34] and microscope objectives [35]. Hydrodynamic focusing was used by Simonnet and Groisman to detect particles with an accuracy for fluorescence detection comparable with that obtained using a commercial BD FACScalibur system [36].
Our group at the Naval Research Laboratory has developed a microparticle-assay platform to measure bacteria and toxins in a microfabricated flow cytometer using hydrodynamic focusing to align the particles one-by-one in the interrogation region [37, 38]. Capture antibodies were immobilized on the surface of fluorescently coded microparticles of uniform size. These particle sets were mixed together to perform multiplexed assays. A mixture of biotinylated tracer antibodies was added to the microparticles after exposure to the sample containing antigen. Streptavidin-phycoerythrin was added to the particles before measurement within the microflow cytometer.
The previously reported device design can be seen in Fig. 4. Chevron-shaped grooves altered the path of the sheath fluid to completely surround and focus the sample stream in front of two laser beams. Excitation from green and red lasers was delivered using optical fibers; additional fibers collected the scattered light signals and three colors of fluorescence. The collected data were processed to identify microsphere sets, calculate the associated phycoerythryin fluorescence, and create dose–response curves for each antigen tested. The sensitivity of detection of six bacteria and toxins in a multiplexed assay was comparable with that of the benchtop Luminex system using the same reagents.
A system layout scheme for a microflow cytometer based on use of optical (light scattering and fluorescence) detection to detect bacteria and toxins. Optical fibers were placed in the channel’s interrogation region to pipe in excitation light at 532 nm and 635 nm. Four PMTs detected emitted light at 635 ± 5 nm (for light scattering at 45°), 670 ± 10 and ≥700 (for fluorescence particle identification at 135°), and 565 ± 10 nm for phycoerythrin (at 90°). The graphic on the left is reproduced with permission from the American Chemical Society [37, 38]. On the right, a micrograph shows the microfluidic channel with chevron grooves in the top and bottom to focus the sheath fluid completely around the sample stream
Fluorescence measurements are particularly good for multiplexed analyses using coded beads and for distinguishing signal from background. Measurements can be quantitative and quite sensitive. Advances in chemistry and biotechnology have led to the emergence of bright and easily conjugated organic molecules, proteins, and inorganic nanoparticles. These luminescent tags are easily attached by covalent bonds or biotin–avidin interactions to a vast and quickly evolving choice of affinity molecules, primarily antibodies. Yet, the primary limitation of the use of fluorescence is also the requirement for a fluorescent dye or dye-labeled affinity tag, which increases the number of sample preparation steps and time.
Magnetic beads for target localization and detection
The use of magnetic force to induce migration, or magnetophoresis, has been explored as a method to separate and analyze particles and cells [39, 40]. Manipulations by magnetophoresis of latex particles [41] or red blood cells [42] in paramagnetic, metal-ion media are examples of proof-of-concept demonstrations toward biological assays. While promising, the fledgling technology usually requires low flow rates to demonstrate adequate migration for analysis because the magnetic force exerted on the bioparticles is relatively weak. The method also requires the use of paramagnetic ionic solutions, for example MnCl2, that are not often used in biological assays and thus can result in unexpected interference or interactions.
Because of their ease of synthesis and surface manipulation [43, 44], magnetic nanoparticles have become popular, and magnetophoresis has started to gain further relevance as an analytical technique. Particles and cells can be labeled with superparamagnetic nanoparticles to further increase the response to a magnetic field [45]. The increase in magnetism of the labeled particle renders the need for the metal-ion media obsolete. Wilhelm et al. have published a wide breadth of work on cellular internalization of magnetic nanoparticles and the resulting magnetophoresis [46–51].
Pamme and Manz explored magnetophoresis of particles and particle aggregates in flow on a microfluidic chip for separation purposes [52]. Superparamagnetic particles of different size and magnetic susceptibility in laminar flow were separated by a perpendicular magnetic field. The magnetophoretic device controlled particle movement and separation using the strength and gradient of the magnetic field, and the flow rate. Particles of larger size and magnetic susceptibility were deflected to a greater extent than smaller particles with less magnetic susceptibility, and nonmagnetic latex particles were not deflected. Aggregates of superparamagnetic particles were deflected more than the single superparamagnetic particle, because of their greater size. Recently, Peyman et al. have used this device design as a method of sample preparation for sandwich immunoassays (Fig. 5) [53]. Because magnetic particles are attracted toward one side of the microchannel by the magnet, they cross laminar flow streams of reagents and wash buffers in continuous flow.
A continuous-flow reactor using magnetophoresis to deflect magnetic particles through multiple reagent streams in laminar flow, performing consecutive binding and washing steps of a sandwich immunoassay. Reproduced with permission from the Royal Society of Chemistry [53]
Kang and Park used magnetophoresis to perform multiplexed immunoassays using superparamagnetic nanoparticles and nonmagnetic polystyrene microparticles [54]. A typical sandwich immunoassay format was used in these experiments. Red or yellow-green fluorescent microspheres were coated with goat anti-rabbit IgG or goat anti-mouse IgG. Superparamagnetic nanoparticles were also conjugated to the same antibodies to act as a magnetic tracer. After microparticles were incubated with mouse or rabbit IgG, the magnetic nanoparticle tracer was added. After sample preparation, the particle complexes were placed within the flow of the microfluidics device. The fluidic channel consisted of an “H” type design in which a permanent magnet was placed on one side to deflect microparticles complexed with magnetic nanoparticles.
Hahn et al. continued the development of this device in subsequent years. In 2007, slight modifications to the chip design were implemented (Fig. 6) [55]. Channel dimensions were optimized and a nickel-based magnetic microstructure was introduced. These changes resulted in the detection of two allergens for household dust mites, Dermatophagogoides farinae and Dermatophagoides pteronyssinus, in the sera of 44 patients. Further, in a very recent paper, the group detected three analytes using a reagent mixture and a charge-coupled device (CCD) for detection of microparticle position (Fig. 7) [56]. Besides providing a higher level of multiplexing from the addition of another color fluorescent microsphere, the CCD camera and data-acquisition program provided greater automation, throughput, and quantitation.
Schematic diagram of a magnetophoretic immunoassay procedure used in the device prepared by Hahn et al. The microchannel contained a magnetic Ni microstructure that changed the microparticle position depending on the amount of bound mite antigen. Reproduced with permission from the American Chemical Society [55]
Overlaid images of fluorescent microparticles flowing (right to left) in a microchannel: (a) without an external permanent magnet, (b) in the presence of a permanent magnet 4 mm from the channel, and (c) in the presence of a permanent magnet 2 mm from the channel. Reproduced with permission from the Royal Society of Chemistry [56]
Currently, magnetism is most often used with microparticles for pre-concentration and sample preparation before detection [39]. Yang et al. used magnetic microspheres to perform immunoassays with fluorescence-labeled antibodies in a microfabricated flow cytometer in a completely lab-on-a-chip setting [57, 58]. In this work, a permanent magnet was attached to a PDMS chip containing microfabricated mixers, pumps, and valves for sample preparation of a sandwich immunoassay. After on-board sample preparation using magnetic immobilization, the microspheres were sent through the optical detection region of the system. In this system, the use of magnetic force to preconcentrate target antigen and prepare samples enabled the detection of a virus at 103 PFU mL−1.
Magnetic particles have a role in microflow cytometry, but their principle benefits are in target capture and sample processing rather than detection in flow. Processing devices based on utilization of magnetic particles are amenable to integration with microflow cytometer systems. Furthermore, the availability of fluorescent magnetic beads provides opportunities to use the same reagents for both sample processing and analysis. Magnetophoresis is still an emergent technology in microflow cytometry, and the approach is ripe for innovative applications.
Conclusions
Microparticle-based assays have become important to flow cytometry because of the ease of antibody conjugation, the availability of coded beads for multiplexing, and the realization that particles can be used to detect targets too small to generate a significant scattered light signal. Because of their increasing popularity and wide utility, researchers have been implementing particle-based assays in microfabricated flow cytometers. This combination offers the prospect of rapid, multiplexed assays within a compact, portable system. Three main detection techniques have been used in microflow cytometers. The classic detection platform, derived from Coulter counters, is based on electrical impedance as a particle passes between electrodes in a microchannel. Optical detection methods, which use information from scattered light and emitted fluorescent light to characterize arrays of particle, are also popular and well studied. More experimental techniques, for example magnetophoresis, are being developed; these rely on positional analysis within a channel to determine analyte levels. The microflow cytometers that use microparticle assays implement a variety of detection methods, demonstrating the need to understand and fully utilize all available technology for point-of-care device development.
References
Ateya DA, Erickson JS, Howell PB Jr, Hilliard LR, Golden JP, Ligler FS (2008) The good, the bad, and the tiny: a review of microflow cytometry. Anal Bioanal Chem 391:1485–1498
Vignali DA (2000) Multiplexed particle-based flow cytometric assays. J Immunol Meth 243:243–255
www.accuricytometers.com/files/Accuri_Revolutionizes_Flow_Cytometry.pdf
Shapiro HM (2005) Practical flow cytometry, 5th edn. Wiley and Sons, Hoboken NJ
Kim JS, Ligler FS (eds) (2010) The microflow cytometer. PanStanford Publishing, Singapore, 400pp
Taitt CR, Shriver-Lake LC, Anderson GP, Ligler FS (2010) Surface modification and biomolecule immobilization on polymer spheres for biosensing applications. In: Hurst SJ (ed) Biomedical nanotechnology. Springer, New York, In Press
Horan PK, Wheeless LL Jr (1977) Quantitative single cell analysis and sorting. Science 198:149–157
Shvalov AN, Surovtsev IV, Chernyshev AV, Soini JT, Maltsev VP (1999) Particle classification from light scattering with the scanning flow cytometer. Cytometry 37:215–220
Shapiro HM (2003) Practical flow cytometry, 4th edn. Wiley–Liss, New York
Steen HB (1990) Light scattering measurement in an arc lamp-based flow cytometer. Cytometry 11:223–230
Steen HB (2004) Flow cytometer for measurement of the light scattering of viral and other submicroscopic particles. Cytom A 57:94–99
Colletcassart D, Magnusson CGM, Cambiaso CL, Lesne M, Masson PL (1981) Automated particle-counting immunoassay for digoxin. Clin Chem 27:1205–1209
Colletcassart D, Mareschal JC, Sindic CJM, Tomasi JP, Masson PL (1983) Automated particle-counting immunoassay of C-reactive protein and its application to serum, cord serum, and cerebrospinal-fluid samples. Clin Chem 29:1127–1131
Fagnart OC, Cambiaso CL, Sindic CJM, Masson PL (1985) Particle-counting immunoassay of a fetuin-like antigen in serum and cerebrospinal-fluid. Clin Chem 31:1820–1823
Castracane CE, Cambiaso CL, Retegui LA, Gilbert I, Ketelslegers JM, Masson PL (1984) Particle-counting immunoassay of human somatotropin. Clin Chem 30:672–676
Pamme N, Koyama R, Manz A (2003) Counting and sizing of particles and particle agglomerates in a microfluidic device using laser light scattering: application to a particle-enhanced immunoassay. Lab Chip 3:187–192
Coulter WH (1956) High speed automatic blood cell counter and cell size analyzer. Proc Natl Electron Conf 12:1034–1040
Maeurer HC (1964) Time-saving method for blood cell counting with the coulter counter. Bibl Haematol 18:19–20
Blades AN, Flavell HC (1963) Observations on the use of the Coulter model D electronic cell counter in clinical haematology. J Clin Pathol 16:158–163
Larson UD, Blankenstein G, Ostergaard S (1997) Microchip coulter particle counter. Proceedings in Transducers 1319
Koch M, Evans AGR, Brunnschweiler A (1998) Design and fabrication of a micromachined Coulter counter. Proceedings in Micromechanics Europe155–158
Ayliffe HE, Frazier AB, Rabbitt RD (1999) Electric impedance spectroscopy using microchannels with integrated metal electrodes. J Microelectromech Syst 8:50–57
Cheung K, Gawad S, Renaud P (2005) Impedance spectroscopy flow cytometry: on-chip label-free cell differentiation. Cytom A 65:124–132
Gawad S, Cheung K, Seger U, Bertsch A, Renaud P (2004) Dielectric spectroscopy in a micromachined flow cytometer: theoretical and practical considerations. Lab Chip 4:241–251
Gawad S, Schild L, Renaud PH (2001) Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 1:76–82
Sun T, Holmes D, Gawad S, Green NG, Morgan H (2007) High speed multi-frequency impedance analysis of single particles in a microfluidic cytometer using maximum length sequences. Lab Chip 7:1034–1040
Pauly H, Schwan HP (1959) Impedance of a suspension of ball-shaped particles with a shell; a model for the dielectric behavior of cell suspensions and protein solutions. Z Naturforsch B 14B:125–131
Asami K, Takahashi Y, Takashima S (1989) Dielectric properties of mouse lymphocytes and erythrocytes. Biochim Biophys Acta 1010:49–55
Holmes D, She JK, Roach PL, Morgan H (2007) Bead-based immunoassays using a micro-chip flow cytometer. Lab Chip 7:1048–1056
Fulton RJ, McDade RL, Smith PL, Kienker LJ, Kettman JR Jr (1997) Advanced multiplexed analysis with the FlowMetrix system. Clin Chem 43:1749–1756
Carson RT, Vignali DA (1999) Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Meth 227:41–52
Fu AY, Spence C, Scherer A, Arnold FH, Quake SR (1999) A microfabricated fluorescence-activated cell sorter. Nat Biotechnol 17:1109–1111
Lin CH, Lee GB, Fu LM, Hwey BH (2004) Vertical focusing device utilizing dielectrophoretic force and its application on microflow cytometer. J Microelectromechanical Syst 13:923–932
Holmes D, Morgan H, Green NG (2006) High throughput particle analysis: combining dielectrophoretic particle focusing with confocal optical detection. Biosens Bioelectron 21:1621–1630
Simonnet C, Groisman A (2006) High-throughput and high-resolution flow cytometry in molded microfluidic devices. Anal Chem 78:5653–5663
Kim JS, Anderson GP, Erickson JS, Golden JP, Nasir M, Ligler FS (2009) Multiplexed detection of bacteria and toxins using a microflow cytometer. Anal Chem 81:5426–5432
Golden JP, Kim JS, Erickson JS, Hilliard LR, Howell PB, Anderson GP et al (2009) Multi-wavelength microflow cytometer using groove-generated sheath flow. Lab Chip 9:1942–1950
Gijs MA, Lacharme F, Lehmann U (2009) Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem Rev. doi:10.1021/cr9001929
Watarai H, Suwa M, Iiguni Y (2004) Magnetophoresis and electromagnetophoresis of microparticles in liquids. Anal Bioanal Chem 378:1693–1699
Watarai H, Namba M (2001) Magnetophoretic behavior of single polystyrene particles in aqueous manganese(II) chloride. Anal Sci 17:1233–1236
Watarai H, Namba M (2002) Capillary magnetophoresis of human blood cells and their magnetophoretic trapping in a flow system. J Chromatogr A 961:3–8
Osaka T, Matsunaga T, Nakanishi T, Arakaki A, Niwa D, Iida H (2006) Synthesis of magnetic nanoparticles and their application to bioassays. Anal Bioanal Chem 384:593–600
Iida H, Takayanagi K, Nakanishi T, Osaka T (2007) Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J Colloid Interface Sci 314:274–280
Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L (2005) Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol 23:1418–1423
Wilhelm C, Gazeau F, Roger J, Pons JN, Salis MF, Perzynski R et al (2002) Binding of biological effectors on magnetic nanoparticles measured by a magnetically induced transient birefringence experiment. Phys Rev E Stat Nonlin Soft Matter Phys 65:031404
Wilhelm C, Gazeau F, Bacri JC (2002) Magnetophoresis and ferromagnetic resonance of magnetically labeled cells. Eur Biophys J 31:118–125
Wilhelm C, Cebers A, Bacri JC, Gazeau F (2003) Deformation of intracellular endosomes under a magnetic field. Eur Biophys J 32:655–660
Wilhelm C, Billotey C, Roger J, Pons JN, Bacri JC, Gazeau F (2003) Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials 24:1001–1011
Fortin JP, Wilhelm C, Servais J, Menager C, Bacri JC, Gazeau F (2007) Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc 129:2628–2635
Billotey C, Wilhelm C, Devaud M, Bacri JC, Bittoun J, Gazeau F (2003) Cell internalization of anionic maghemite nanoparticles: quantitative effect on magnetic resonance imaging. Magn Reson Med 49:646–654
Pamme N, Manz A (2004) On-chip free-flow magnetophoresis: continuous flow separation of magnetic particles and agglomerates. Anal Chem 76:7250–7256
Peyman SA, Iles A, Pamme N (2009) Mobile magnetic particles as solid-supports for rapid surface-based bioanalysis in continuous flow. Lab Chip 9:3110–3117
Kim KS, Park JK (2005) Magnetic force-based multiplexed immunoassay using superparamagnetic nanoparticles in microfluidic channel. Lab Chip 5:657–664
Hahn YK, Jin Z, Kang JH, Oh E, Han MK, Kim HS, et al (2007) Magnetophoretic immunoassay of allergen-specific IgE in an enhanced magnetic field gradient. Anal Chem 2214–2220
Hahn YK, Chang JB, Jin Z, Kim HS, Park JK (2009) Magnetophoretic position detection for multiplexed immunoassay using colored microspheres in a microchannel. Biosens Bioelectron 24:1870–1876
Yang SY, Lien KY, Huang KJ, Lei HY, Lee GB (2008) Micro flow cytometry utilizing a magnetic bead-based immunoassay for rapid virus detection. Biosens Bioelectron 24:861–868
Lee YF, Lien KY, Lei HY, Lee GB (2009) An integrated microfluidic system for rapid diagnosis of dengue virus infection. Biosens Bioelectron 25:745–752
Acknowledgements
JSK was a postdoctoral fellow of the American Society for Engineering Education. The work presented here was performed under NIH grant UO1 A1075489 and ONR/NRL 6.2 work unit 6336. The views presented here are those of the authors and do not represent the opinion or policy of the National Institutes of Health, Department of Health and Human Services, the US Navy, or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue on Focus on Bioanalysis with Guest Editors Antje J. Baeumner, Günter Gauglitz, Frieder W. Scheller.
Rights and permissions
About this article
Cite this article
Kim, J.S., Ligler, F.S. Utilization of microparticles in next-generation assays for microflow cytometers. Anal Bioanal Chem 398, 2373–2382 (2010). https://doi.org/10.1007/s00216-010-3848-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3848-8