Abstract
The extraction of six sulfonamides (sulfadiazine, sulfadimidine, sulfathiazole, sulfachloropiridazine, sulfadimethoxine, and sulfaquinoxaline) from soils with different physicochemical characteristics and at several aging times was investigated. Conventional mechanical shaking, microwave-assisted extraction, ultrasound probe-assisted extraction and pressurized liquid extraction techniques were evaluated. The four techniques provided similar results when applied to freshly contaminated soils. However, microwave-assisted extraction was the most suitable to extract sulfonamide aged residues from soils. Microwave-assisted extraction was applied to eight soils aged for 3 months, using acetonitrile:buffer pH 9 (20:80) as the extraction solvent, and recoveries ranged from 15–25% for STZ to 42–64% for SDM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are widely used in veterinary practices as therapeutic and prophylactic agents against infectious animal diseases and sometimes for growth promotion purposes. However, since January 2006, the European Union banned the feeding of antibiotics and related drugs to livestock growth promotion [1]. Antibiotics excreted by treated animals, as well as their metabolites, reach the environment, mainly when manure is used as fertilizer and spread on agricultural soils.
Antibiotics, which are considered emerging pollutants, are specifically designed to act against microorganisms and they are among the most controversial contaminants since, in addition to ecological effects, they can enhance the generation and spread of drug resistant bacterial strains [2, 3]. In European countries, sulfonamides (SAs) are one of the most widely administered groups of antibiotics in animal husbandry [4] and it has been estimated that between 40% and 90% of the parent SA is excreted by the animal [5]. Studies to evaluate their impact and fate on the environment include the characterization of sorption processes of SAs to soils [6–18], which finally determine the bioavailability of the pollutants and their mobility by leaching, run-off, or migration to the aquatic system. Another key issue for environmental studies is the quantification of SAs in water and soil samples, to evaluate their presence in the environment, which requires suitable analytical methodology. From the last decade, methods for the quantification of SAs in the water compartment have been developed [19–31]. They are mostly based on liquid chromatography (LC) with mass spectrometry detection, and a previous solid-phase extraction step for preconcentration and clean-up purposes is applied. However, methods for the analysis in soil samples are still scarce [4, 11, 27, 32–36].
Soil analysis methods consist of the extraction of the analytes from the soil matrix, usually followed by a SPE clean-up and LC determination. To this date, the extraction from the soil matrix is the most critical step. Methanol [4, 11, 33, 35] or acetonitrile [27, 32, 34, 36], either with or without a buffer mixture, are the most used extraction media. Different extraction techniques have been applied, from the simple mechanical shaking (MECS) to microwave-assisted extraction (MAE) or pressurized liquid extraction (PLE). Table 1 summarizes methods reported in the literature for the extraction of SAs in soils.
Sulfadiazine (SDZ), which is the most investigated SA, can be considered as a representative compound for this family of antibiotics. Extraction recoveries reported for SDZ range from around 10% to 95%. The differences in the extraction efficiency can be due to the methodology applied, to soil physicochemical characteristics and to the residence time of the SAs on the soil [32, 34, 36, 37]. Most studies are usually restricted to one soil or to a small set of soils, which makes difficult to compare the efficiency of the different extraction systems and their behavior with regard to soil characteristics and aging.
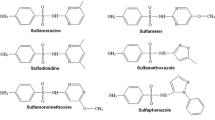
In this paper, a study on the extraction of six SAs (see Fig. 1), widely used as veterinary drugs is presented. Soils covering a wide range of physicochemical properties and different aging times have been considered, as well as different extraction techniques.
Experimental
Chemicals and solutions
Sulfadiazine (SDZ), sulfathiazole (STZ), sulfadimidine (SDD), sulfamethoxidiazine (SMZ), sulfachloropiridazine (SCP), sulfadimethoxine (SDM), and sulfaquinoxaline (SQ) were purchased from Sigma-Aldrich (St Louis, MO, USA). Individual primary stock standard solutions (50 mg l−1) of all SAs were prepared monthly by dissolving the compounds in methanol (Merck) and stored in dark glass bottles at 4 °C. Secondary stock standard solutions containing 2 mg l−1 of each SA were prepared weekly by mixing the primary stock solutions of the seven SAs and diluting with methanol. Working solutions were prepared daily by dilution of stock standard solutions with acetonitrile (ACN) or water.
Fluorescamine was purchased from Fluka (Buchs, Switzerland). Fluorescamine solutions 0.2% were prepared in ACN. The reagents were used without further purification.
Acetonitrile HPLC gradient grade (Merck, DarMecStadt, Germany) and doubly de-ionized water (Milli-Q, Millipore, Molheim, France) with a resistivity of 18.2 MΩ cm−1 were used throughout. All other reagents were of analytical reagent grade.
The mobile phase consisted of ACN and aqueous formic acid/ammonium formiate buffer solution pH 3.4 previously filtered through a 0.22-μm nylon filter.
Concentrations of 0.1 M buffer solutions at pH 3.4, 9.0, 10.5, and 12 were prepared from formic acid/sodium formiate, ammonium chloride/ammonia, sodium hydrogen carbonate/sodium carbonate and sodium hydrogen phosphate/sodium phosphate, respectively.
The SPE cartridges used in this study were Oasis HLB (30 mg; Waters, Milford, MA, USA).
Glassware used for experiments was previously soaked in 10% nitric acid for 24 h and rinsed with ultrapure water.
Samples
Soil samples were collected from different locations in Spain. They were air-dried and sieved through 2-mm mesh. Soils were sterilized using γCo60 radiation, stored in the dark, and were passed through a riffle splitter before analysis. Table 2 shows parameters referring to texture, chemical composition, and other properties of the soil samples.
Apparatus
Chromatographic analysis was performed in an Agilent 1100 system (Palo Alto, CA, USA), consisting of an HP 1100 quaternary pump, an automatic injector Agilent 1100 G1313A, and an Agilent 1100 fluorimetric detector. The analytical column was a 5 μm LiChrospher 100 RP-18, 250 × 4 mm I.D. (Merck), equipped with a 5 μm LiChrospher 100 RP-18 guard column.
Microwave-assisted extraction was carried out using an ETHOS E closed-vessel system (1,000 W) supplied by Milestone (Sorisole, Italy). The system is designed for extraction using organic solvents and is able to hold twelve extraction vessels.
Pressurized liquid extraction was performed by an ASE 100 (Dionex, Sunnyvale,CA, USA).
An ultrasound probe MS 72 (Bandelin Electronic, Berlin, Germany) coupled into a ultrasonic homogeneizer HD 3200 (Bandelin ELECTRONIC, Berlin, Germany) was used to carry ultrasonic extractions.
A rotary mixer 34526 (Breda Scientific, Breda, Netherlands) was used in conventional mechanical shaking extraction experiments.
For SPE preconcentration, a Rapid Trace Workstation (Caliper LifeSciences, Inc. MA, USA) was used.
pH was measured using a Crison GLP 21 pH-meter (Alella, Spain), equipped with an Ag/AgCl combined glass electrode, Crison 52-02.
A vortex mixer SA 8 (Afora, Barcelona, Spain), a Jones microsplitter riffle (Sepor Inc., CA, USA), and a Heraeus Christ centrifuge (Osterode am Harz, Germany) were also used.
Procedures
Spiking
For recovery studies, soil samples were spiked by adding an appropriate volume of a standard aqueous solution containing the six SAs to dry soil. Spiking levels were 150–300 ng g−1.The mixture was vortexed and then left to stand from overnight (17–20 h) at room temperature up to several months at 4 °C.
Extraction and clean-up
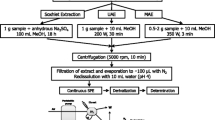
Mechanical shaking
Six milliliters of ACN:buffer pH 9 (20:80) was added to 2 g spiked soil sample in a 30-ml centrifuge tube. The mixture was end-over-end shaken for 30 min and then centrifuged (1,507×g, 15 min). The extract was transferred to a 25-ml volumetric flask. The residue was rinsed with 1 ml of the extraction solvent and centrifuged again. The supernatant was combined with the previous extract, SMZ was added as internal standard and the volume adjusted to 25 ml with 0.1 M formic/formiate buffer pH 3.4. Then, 10 ml of this solution was loaded in a 30 mg Oasis HLB SPE cartridge, which was previously conditioned with 5 ml methanol followed by 5 ml formic/formiate buffer pH 3.4. After loading, the cartridge was rinsed with 10 ml water, air-dried, and eluted with 1 ml ACN at a flow rate of 0.5 ml min−1. The extract was then derivatized as mentioned in the “Derivatization-LC-Fluorescence” section and injected into the chromatographic system.
Microwave-assisted extraction
Six milliliters of ACN:buffer pH 9 (20:80) was added to 2 g spiked soil sample in a 35 ml PTFE extraction vessel. After microwave irradiation (15 min, 115 °C), the vessels were air-cooled and the content transferred to a 30-ml centrifugation glass tube. Then, it was centrifuged and proceeded as above.
Pressurized liquid extraction
Ten grams of spiked soil sample was mixed with diatomaceous earth and introduced into a 33-ml stainless cell. The working conditions were 130 °C, 5 min preheating and 13 min static temperature, one cycle and 150 s purging time and the extraction solvent was ACN:buffer pH 9 (20:80). The extract was transferred to a 100 ml volumetric flask, SMZ was added as internal standard, the volume adjusted with 0.1 M formic/formiate buffer pH 3.4 and then it was proceeded as above.
Ultrasonic probe-assisted extraction
Six milliliters of ACN:buffer pH 9 (20:80) was added to 2 g spiked soil sample in a 30-ml centrifuge tube. After sonicating (5 min, 50% amplitude (78 W)), it was centrifuged and proceeded as above.
MAE and PLE experimental conditions were adapted from those described in the literature [27, 34], whereas the optimization of the USPE conditions were performed in this work.
Derivatization-LC-Fluorescence
One milliliter of solution containing SAs in ACN (that is, standard or sample eluate from SPE) was mixed in a 5-ml volumetric flask with 2 ml of aqueous formic/formiate buffer solution at pH 3.4 and 1 ml of 0.2% fluorescamine solution. The mixture was left to stand for at least 2 h at room temperature, diluted to volume with ACN/buffer (1:1) and filtered through a 0.45-μm Nylon membrane before injection (50 μl) into the chromatographic system kept at 40 °C. The fluorescence of the derivatized compounds remains constant for about 8 h. The derivatized SAs were separated using binary gradient elution. Mobile phase A was 10 mM formic/formiate buffer at pH 3.4 and mobile phase B acetonitrile. The elution profile was as follows: 36% ACN for 10 min, gradient elution from 36% to 40% acetonitrile in 10 min, kept at 40% ACN to min 24, return to 36% ACN in 2 min and kept 36% ACN to min 30. The mobile phase flow rate was set at 1.2 ml min−1 and the separation was carried out at a temperature of 40 °C. The excitation/emission wavelengths selected were 405 and 485 nm, respectively. Quantification was performed by internal standard calibration by measuring peak areas. Figure 2 shows a chromatogram in the working conditions.
Results and discussion
Selection of the extraction solvent
Preliminary extraction experiments were performed with overnight spiked soils (i.e., about 17–20 h of contact time of the spiking solution with the soil matrix before extraction) and using conventional end-over-end mechanical shaking. At this stage, four soils (S-A, S-B, S-C, and S-D) were used (see Table 2 for soil characteristics). Since SAs are relatively polar, with octanol–water partition coefficients in the range 0.8–6.3 [38, 39], solvents such as acetonitrile (ACN), acetone and methanol, as well as hydroorganic mixtures of these solvents were assayed. For the six SAs and four soils, hydroorganic mixtures provided higher recoveries than the corresponding pure organic solvent, with this effect being more noticeable for the most polar compounds, i.e., SDZ and STZ.
SAs are amphoteric compounds, and their interaction with the soil matrix depends on the pH of the medium. The pK a values of the SAs are in the ranges 1.2–2.4 and 5.1–7.4 for pK a1 and pK a2, respectively. Therefore, the non-ionized form is dominant in neutral and mild acid conditions, whereas the anionic form prevails at basic pH, and this negatively charged species experience weaker interaction with soils than the neutral form [10, 15, 17]. This is in accordance with results obtained in the preliminary screening of extraction solvents: hydroorganic mixtures buffered at pH 9 provided higher extraction efficiencies than the equivalent mixtures without pH adjustment, being this behavior common to the four soils.
Regarding to the organic solvent nature, no remarkable differences between recoveries provided by the three solvents were observed when considering overall SAs extraction. However, noticeable differences were observed for each individual SA. Extraction recoveries corresponding to soil D and mixtures consisting of organic solvent/aqueous buffer pH 9 (20:80) are shown in Fig. 3. For the other assayed soils, a similar pattern was observed. As can be seen, methanol-based mixtures were more efficient than ACN or acetone for SDD, whereas SDT and SQ recoveries were higher with ACN, and for STZ and SCP the three solvents provided similar results. ACN–water was selected for further studies, and the effect of ACN content (20–80%) and pH of the aqueous buffer (9–12) on recovery rates was investigated. No relevant effects of pH or % ACN on SAs extraction recoveries were found in the explored ranges, but obviously as pH increased a higher matrix load was obtained, due to humic organic matter solubilization. Finally, ACN:aqueous buffer pH 9: (20:80) was chosen as extracting system, since extracts were cleaner and the low content of ACN facilitated the SPE clean-up step of the soil extract before the chromatographic determination.
Extraction recoveries obtained with the selected solvent for different soils are shown in Fig. 4. As can be seen, for most SAs, recoveries were soil dependent. SDZ, STZ, SDD, and SCP recoveries for soil A were similar to those for soil B, but higher than those obtained for soils C and D. Regardless of soil characteristics, SDM was the most extracted SA, whereas STZ was the least. The relatively low organic carbon content in soils A and B could account for the higher recoveries observed.
As an alternative to mechanical shaking, more exhaustive extraction techniques, such as PLE, MAE, or ultrasound probe-assisted extraction (USPE) were assayed. Experiments were performed with soils A and D. ACN:aqueous buffer pH 9 (20:80) was used as extraction solvent. Extraction recoveries obtained with overnight spiked soils proved that all these extraction techniques led to similar recoveries than mechanical shaking.
Extraction of aged sulfonamide residues
Extraction of SAs from aged spiked soils was then investigated. When mechanical shaking extraction was applied to spiked soils A and D, the obtained results showed that recoveries drastically decreased from overnight spiked samples to those extracted 1 month after spiking. The average decrease of SAs recoveries was about 60–70%. A further decrease around 20–30% was observed from 1 to 2 months, leading to recovery rates as low as 5–20% for 2-month aged soils. This relationship between extraction efficiency and aging processes in soil is well-known and has been reported for a wide range of compounds [40–42], including sulfonamides [11, 32, 34]. Aging may be related to processes of a very different nature, such as physical aging, e.g., diffusion throughout soil micropores [43] or chemical aging, due to the slow formation of covalent bindings with some soil components. Compounds having amino or phenolic groups are prone to the formation of bound residues, through the coupling to humic matter [44, 45]. Bialk and Pedersen [46, 47] demonstrated the formation of covalent bonds between SAs and soil humic substances through the formation of Michael adducts via the anilinic nitrogen.
PLE, MAE, and USPE techniques, which can be advantageous when the analytes strongly interacts with the solid matrix, were then applied to these soils. Figure 5 shows data obtained when the four extraction techniques were applied to 1-month aged spiked soil D. As can be seen, extraction recoveries provided by PLE and USPE are similar to those obtained with mechanical shaking. In contrast, MAE resulted in significant higher recoveries for all analytes, except SQ. Results obtained with soil A were similar. These results agree with data reported by Foster et al. [32] when extracting aged SDZ residues. Although MAE provided the highest recoveries for SAs extraction, the aging effect was still observed: for soil D the average decrease of SAs recoveries was about 35%, in contrast to the 65% overall decrease observed for mechanical shaking. From these assays, MAE was selected as the most suitable approach for the SAs extraction from soils. The technique was applied to a series of eight different soils covering a wide range of physicochemical properties.
The soils were spiked with the analytes, aged for 3 months and then extracted under microwave conditions. Results, which are presented in Fig. 6, pointed out that extraction efficiency depended on the SA. The pattern observed with freshly spiked soils was reproduced in aged soils, i.e., STZ was the most difficult to extract, whereas the highest recoveries were obtained for SDM. SAs recovery values were usually in the 30–50% range, except for STZ that were below 30%, and for SDM, that were between 40-65%.
The effect of soil characteristics on extraction recovery was also observed for these aged soils. Although differences depended on the SA, some general trends were observed. For almost all the compounds the lowest recoveries were those corresponding to soils D and E, whereas soils J and K provided the highest values. Not only the organic carbon content [7, 13, 17, 18], but also the clay content [6, 12] have been identified in the literature as key parameters for SAs sorption to soils. However, the results obtained in this study cannot be explained just in terms of quantitative data of organic carbon or clay content. For instance, although both clay and organic carbon content of soil F were higher than those of soil D, SAs recoveries for soil F were much higher than for soil D.
A more detailed look through the obtained data revealed that for soils having low organic carbon content, recoveries decreased as clay percentage increased; this can be seen by comparing soils I and K or soils G and H. Nonetheless, this trend was not observed for soils with higher organic carbon. This was probably because the accessibility of SAs to minerals in the clay fraction was reduced, and thus the effect of the mineral fraction of the soil is only relevant when the content in organic carbon is low [6, 17].
Partial least squares regression was applied to analyze SAs recoveries as a function of soil characteristics but no conclusive results about the factors affecting extraction were obtained from the multivariate data analysis. As previously mentioned, according to literature, organic matter plays a decisive role in the interaction of SAs with the soil. However, organic matter refers to a complex heterogeneous mixture of organic molecules, and, in addition to the partition of the compounds to the organic phase, chemical reactions can occur. Therefore, the %OC bulk parameter would not be functional enough to describe contribution of organic matter, but its chemical composition would determine the extent of the SAs to soil interactions
Conclusions
Recoveries for the extraction of SAs with acetonitrile:buffer pH 9 (20:80) from overnight spiked soils ranged from 40% to 80%, depending on the SA and soil. The extraction technique (MECS, MAE, PLE, and USPE) had no noticeable effect. Recoveries obtained from aged SAs residues were lower, and depended on the extraction technique applied, with MAE being the most efficient. Recoveries obtained with MAE from soils aged for 3 months were in the range of 15–64%.
Since extraction recoveries are generally established from freshly spiked soils, the extraction efficiency is usually overestimated. Therefore, if applied to aged SAs residues quantification, it would lead to lower SAs concentration values in the soil than the total. However, the labile SAs fraction that is quantified has more relevance to environmental fate and bioavailability.
References
Comission Regulation (EC) No 1831/2003. Additives for use in animal nutrition. O.J. of European Communities L268/29
Nwosu VC (2001) Res Microbiol 152:421–430
Boxall AB, Kolpin DW, Halling-Sorensen B, Tolls J (2003) Environ Sci Technol 37:286A–294A
Jacobsen AM, Halling-Sorensen B, Ingerslev F, Hansen SH (2004) J Chromatogr A 1038:157–170
Langhammer JP (1989) PhD diss. University of Bonn
Kahle M, Stamm C (2007) Chemosphere 68:1224–1231
Kahle M, Stamm C (2007) Environ Sci Technol 41:132–138
Accinelli C, Koskinen WC, Becker JM, Sadowsky MJ (2007) J Agric Food Chem 55:2677–2682
Kurwadkar ST, Adams CD, Meyer MT, Kolpin DW (2007) J Agric Food Chem 55:1370–1376
Ter Laak TL, Wouter AG, Tolls J (2006) Environ Toxicol Chem 25:904–911
Hamscher G, Pawelzick HT, Hoper H, Nau H (2005) Environ Toxicol Chem 24:861–868
Gao J, Pedersen JA (2005) Environ Sci Technol 39:9509–9516
Thiele-Bruhn S, Seibicke T, Schulten HR, Leinweber P (2004) J Environ Qual 33:1331–1342
Thiele-Bruhn S, Aust MO (2004) Arch Environ Contam Toxicol 47:31–39
Boxall AB, Blackwell P, Carvallo R, Kay P, Tolls J (2002) Toxicol Lett 131:19–28
Tolls J (2001) Environ Sci Technol 35:3397–3406
Lertpaitoonpan W, Ong SK, Moorman TB (2009) Chemosphere 76:558–564
Sukul P, Lamshöft M, Zühlke S, Spiteller M (2008) Chemosphere 73:1344–1350
Senta I, Terzić S, Ahel M (2008) Chromatographia 68:747–758
Ben W, Qiang Z, Adams C, Zhang H, Chen L (2008) J Chromatogr A 1202:173–180
Pedrouzo M, Borrull F, Marce RM, Pocurull E (2008) J Sep Sci 31:2182–2188
Diaz-Cruz MS, Garcia-Galan MJ, Barcelo D (2008) J Chromatogr A 1193:50–59
Chang H, Hu J, Asami M, Kunikane S (2008) J Chromatogr A 1190:390–393
Lin CY, Huang SD (2008) Anal Chim Acta 612:37–43
Peng X, Tan J, Tang C, Yu Y, Wang Z (2008) Environ Toxicol Chem 27:73–79
Ye S, Yao Z, Na G, Wang J, Ma D (2007) J Sep Sci 30:2360–2369
Raich-Montiu J, Folch J, Compano R, Granados M, Prat MD (2007) J Chromatogr A 1172:186–193
Richter D, Dünnbier U, Massmann G, Pekdeger A (2007) J Chromatogr A 1157:115–121
Balakrishnan VK, Terry KA, Toito J (2006) J Chromatogr A 1131:1–10
Yang S, Cha J, Carlson K (2004) Rapid Commun Mass Spectrom 18:2131–2145
Renew JE, Huang C-H (2004) J Chromatogr A 1042:113–121
Forster M, Laabs V, Lamshoft M, Putz T, Amelung W (2008) Anal Bioanal Chem 391:1029–1038
Martinez-Carballo E, Gonzalez-Barreiro C, Scharf S, Gans O (2007) Environ Pollut 148:570–579
Stoob K, Singer HP, Stettler S, Hartmann N, Mueller SR, Stamm CH (2006) J Chromatogr A 1128:1–9
KarcI A, BalcIoglu IA (2009) Sci Total Environ 407:4652–4664
Chen L, Zeng Q, Wang H, Su R, Xu Y, Zhang X, Yu A, Zhang H, Ding L (2009) Anal Chim Acta 648:200–206
Heise J, Holtge S, Schrader S, Kreuzig R (2006) Chemosphere 65:2352–2357
Congliang Z, Yan W, Fuan W (2007) Bull Korean Soc 28:1183–1186
Martínez F, Gómez A (2002) Phys Chem Liq 40:411–420
Hawthorne SB, Grabanski CB, Martin E, Miller DJ (2000) J Chromatogr A 892:421–433
Langenfeld JJ, Hawthorne SB, Miller DJ, Pawliszyn J (1995) Anal Chem 67:1727–1736
Song J, Zhao FJ, McGrath SP, Luo YM (2006) Environ Toxicol Chem 25:1663–1670
Pignatello JJ, Xing B (1996) Environ Sci Technol 30:1–11
Dec J, Bollag JM (1997) Soil Sci 161:854–874
Bollag JM, Myers CJ, Minard RD (1992) Sci Tot Environ 123/124:205–217
Bialk HM, Simpson AJ, Pedersen JA (2005) Environ Sci Technol 39:4463–4473
Bialk HM, Pedersen JA (2008) Environ Sci Technol 42:106–112
Acknowledgments
Financial support from the Spanish Ministerio de Ciencia y Tecnología (CiCYT Project CTQ2007-62773) is gratefully acknowledged. The authors acknowledge Professor F. Macías from Universidad de Santiago de Compostela and M. Aran from Applus+Agroambiental (Sidamon, Lleida, Spain) for providing soil samples. J. Raich also thanks the Universitat de Barcelona (Spain) for a BRD grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raich-Montiu, J., Beltrán, J.L., Prat, M.D. et al. Studies on the extraction of sulfonamides from agricultural soils. Anal Bioanal Chem 397, 807–814 (2010). https://doi.org/10.1007/s00216-010-3580-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3580-4