Abstract
This study demonstrates the use of solid-phase microextraction (SPME) to extract and pre-concentrate volatile signatures from static air above plastic explosive samples followed by detection using ion mobility spectrometry (IMS) optimized to detect the volatile, non-energetic components rather than the energetic materials. Currently, sample collection for detection by commercial IMS analyzers is conducted through swiping of suspected surfaces for explosive particles and vapor sampling. The first method is not suitable for sampling inside large volume areas, and the latter method is not effective because the low vapor pressure of some explosives such as RDX and PETN make them not readily available in the air for headspace sampling under ambient conditions. For the first time, headspace sampling and detection of Detasheet, Semtex H, and C-4 is reported using SPME-IMS operating under one universal setting with limits of detection ranging from 1.5 to 2.5 ng for the target volatile signatures. The target signature compounds n-butyl acetate and the taggant DMNB are associated with untagged and tagged Detasheet explosives, respectively. Cyclohexanone and DMNB are associated with tagged C-4 explosives. DMNB is associated with tagged Semtex H explosives. Within 10 to 60 s of sampling, the headspace inside a glass vial containing 1 g of explosive, more than 20 ng of the target signatures can be extracted by the SPME fiber followed by IMS detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Of the common explosives used in terrorist bombings, plastic explosives have been frequently used because they can be easily molded for concealment [1]. Researchers and manufacturers are encouraged to develop and commercialize systems that can detect plastic explosives at security checkpoints. Recently, RedXDefense commercialized a portable plastic explosive detection kit, which is a luminescent polymer spray and UV light for visualization [2, 3]. A vapor detector based on monolayer-coated microcantilevers was developed with the aim to also detect plastic explosives [4]. Other vapor-detection technologies include: single-compound detectors such as mass spectrometers [5, 6], ion mobility spectrometers [7, 8], biological detectors such as canines and chemical-based sensors such as metal oxide (MOX) [9], surface acoustic wave (SAW) [10, 11], conducting and conjugated polymer sensors [12, 13]. At present, canines and ion mobility spectrometry are still the most commonly used trace explosive detection systems employed at security checkpoints [14–16]. The one feature that the above explosives vapor-detection technologies (excluding canine detectors) have in common is that their detection channels are configured only to detect the energetic materials of explosives, which in many cases are non-volatiles. This is the case in particular for explosive mixtures that contain cyclotrimethylene trinitramine (RDX), cyclotetramethylene tetranitramine (HMX), tetryl, and pentaerythritol tetranitrate (PETN). These compounds have extremely low vapor pressures, ranging from 10−9 to 10−14 torr at 20 °C [13], and thus are not readily available in vapor form for air sampling at atmospheric pressure and temperature. Figure 1 shows the vapor pressure of the above explosives in comparison to some of the volatile compounds detected from headspace of plastic explosives by solid-phase microextraction coupled to gas chromatography mass spectrometry (SPME-GC/MS) [13].
This study takes a different approach as compared to current vapor-detection systems. That is, instead of focusing on the detection of the energetic materials, the IMS detector is optimized and configured at new operating conditions to detect the volatile components extracted in the headspace air above the plastic explosives. The volatiles emitted from the explosive samples can be compounds such as impurities, solvents, by-products, degradation products, and/or raw materials. Table 1 presents the general compositions of plastic explosives Detasheet, Composition 4 (C-4) and Semtex. Harper et al. reported 2-ethyl-1-hexanol, 1-butanol acetic acid ester, and 2-ethyl-1-hexanol acetic acid as the volatiles emitted from untagged Detasheet (Flex X) explosive extracted and detected by headspace SPME-GC/MS [14]. Furton et al reported cyclohexanone, and 2-ethyl-1-hexanol as the volatiles emanating from untagged C-4 explosive extracted and detected by headspace SPME-GC/MS [15, 16]. In both studies, only one explosive sample was analyzed. In addition to the volatiles, tagged plastic explosives contain taggants such as dimethyl dinitro butane (DMNB), ethylene glycol dinitrate (EGDN), p-mononitrotoluene (MNT), and o-MNT. IMS detection of the taggants listed above has been reported in the literature, mostly as neat standards [17, 18] or from C-4 bulk explosive [7]. In addition to being present in the explosives, these volatile compounds are also present in other household or industrial products as listed in Table 2.
IMS detectors presumptively detect the presence of an analyte based on the ion’s mobility coefficient (K, cm2·V−1·s−1), defined as K = v d·E −1, where v d is the ion-drift velocity, and E is the drift electric field. The mobility of an ion under the influence of an electric field is governed by the size-to-charge ratio and the reduced mass of the ion in the supporting atmosphere. When normalized to temperature (T) and pressure (P), the mobility coefficient can be expressed as the reduced mobility (K 0) shown in Eq. 1.
Literature reduced mobility values (K 0) for DMNB and EGDN range from 1.39 to 1.49 and 1.43 to 1.66 (cm2·V−1·s−1), respectively [7, 17]. K 0 for p-MNT and o-MNT are 1.45 and 1.47, respectively [17]. This research aims to detect the taggant DMNB as well as the volatile signatures emitted from Detasheet, C-4, and Semtex H explosives under one IMS universal setting such that when an untagged explosive is encountered, IMS would be capable of detecting the presence of the explosive based on the associated target volatiles. Multiple samples of the plastic explosives were analyzed for better generalization of the volatiles’ presence.
Preconcentration of analytes
Although IMS analyzers have low detection limits, at the picogram-to-low-nanogram level for most compounds [8, 19], detection from the air inside a large room or container is a challenge even for compounds with high vapor pressures, hence requiring sample preconcentration prior to detection. A preconcentrator is a standard front end that can be adapted to any analytical system of interest, including ion mobility spectrometry, gas chromatography, mass spectrometry, and sensor arrays, etc. [20]. Commonly used preconcentrators for an IMS detector are charcoal beds, membrane filters, or solid surfaces to trap analyte vapors. These preconcentrators offer little or no selectivity for explosives and are often mechanically weak for high flow rate sampling [21]. Several preconcentrator designs with stronger mechanical properties are available for high-volume air sampling, such as porous metallic filter meshes, woven wire meshes, or sheets of metallic felt [19]. These preconcentrators tend to allow the high vapor pressure analytes to pass through without adsorbing onto the wires [22, 23]. Other preconcentrator designs with improved selectivity and sensitivity are those with a coated polymer phase. Examples are the sorbent-coated microfabricated devices from the US Naval Research Laboratory (NRL) [24], and SPME devices, with the latter being a much more mature technology developed by the Pawliszyn research group in the 1990s. SPME preconcentration has been shown to offer many advantages for the analysis of volatile and semi-volatile components from the headspace of a sample [25]. The Almirall research group had successfully coupled the SPME fiber device to a commercial ion mobility spectrometer using an in-house-designed SPME-IMS interface [26]. Headspace sampling and detection of low-vapor-pressure illicit drugs such as cocaine and methylenedioxymethylamphetamine (MDMA) have been reported using SPME fiber to pre-concentrate the volatile signatures followed by IMS detection [27]. To further improve the sensitivity of the SPME device, Guerra et al. developed a planar SPME geometry, which provides a much larger surface area (100×) and capacity for absorption or adsorption. The planar geometry sol–gel SPME device was reported to have an improvement in sensitivity by a factor of 4 over the commercial SPME fiber geometry using IMS as a detector with TNT as the test compound [28]. Cotte-Rodríguez et al. introduced the use of FI-MS (SPME fiber introduction mass spectrometry) as the second preconcentration stage after SS-MIMS (single-sided membrane introduction mass spectrometry). This technique improves sensitivity by factor of 23 and LOD (S/N = 3) by 3.5 over the FI-MS alone [21].
This research uses commercially available SPME fibers of different coating chemistries to pre-concentrate the volatile components in the headspace of the plastic explosives Detasheet, Semtex H, and Composition C-4. The extracted analytes are then desorbed, separated, and identified by GC/MS. Once the volatile compounds are identified, some are selected as target compounds depending on whether or not they serve well as signatures for detection of the explosives. Individual standards are used to optimize the IMS operating conditions to detect these newly identified volatile signature compounds since they are currently not detectable at the manufacturer’s default settings. Once a universal setting is found, the IMS is configured to analyze samples extracted by the SPME fiber from the headspace of actual plastic explosive samples originating from different sources.
Materials and methods
Instrumentation and operating conditions
The General Electric Ion Track (Wilmington, MA) Itemiser 2 ion mobility spectrometer was used in this study along with the SPME interface designed by the Almirall research group (patent pending) [26] to allow headspace sampling and preconcentration of the volatiles in the headspace of plastic explosives. Details on the SPME interface design have been reported elsewhere [26, 29]. The operating conditions of the Itemiser 2 at the manufacturer default settings are not optimal for the detection of the volatiles under investigation [30]. Hence, the instrument operating conditions were optimized to one (universal) setting that allows for the detection of all the volatiles of interest. Table 3 lists the IMS instrumental conditions, SPME-IMS interface conditions, and GC/MS conditions. A detailed description of the systematic optimization method for identifying the optimal operating conditions for new compounds of interest has been reported in previous work [30].
Chemical and explosive samples
Prior to the detection by IMS, standard chemicals were used to calibrate the instrument to determine the expected drift time of corresponding product ion peaks. The following standards were purchased: n-Butyl acetate (Acros Organics, New Jersey), cyclohexanone (Fisher Scientific, New Jersey), and DMNB (Aldrich Chemical Company, Wisconsin). Nicotinamide was used as a dopant gas for the analyses in positive operating mode. Empty permeation tubes purchased from VICI Metronics Inc. (Poulsbo, WA) were filled with nicotinamide obtained from Acros Organics (New Jersey). Methanol was used as a solvent for GC/MS liquid calibration. The SPME-GC/MS and SPME-IMS analyses of the plastic explosive C-4 were performed at Florida International University, while Detasheet and Semtex H samples were performed at a law enforcement laboratory.
Preparation of explosive samples
There were three cases of Detasheet explosive provided for this study. Two (2) were Flex X-untagged explosives that originated from the same lot but different cuts, and the other was Primasheet 1000 tagged explosive. Three cases of Semtex H were also provided, of which all three were tagged; the first two cases were different cuts from the same lot and the third case was from a different lot. Weights of 1.0 g ± 0.02 of these explosives were cut out from the center of the bulk to avoid surface contamination. The 1 g samples were contained in a 15 mL glass vial (Supelco, Bellefonte PA) and sealed 1 week prior to analysis. One set of vials was sealed 24 h prior to analysis. See Table 4 for information on the explosives’ origin and years of manufacture.
SPME fiber chemistry study
Five different fiber chemistries were used in the study to extract volatiles from the headspace of each of the explosives, Detasheet (untagged, case 2), Semtex H (tagged, case 2), and C-4 (tagged) followed by analysis using GC/MS. Carboxen/polydimethylsiloxane (CAR/PDMS, StableFlex, 85 μm), polydimethylsiloxane (PDMS, 100 μm), divinylbenzene/Carboxen/polydimethylsiloxane (DVB/CAR/PDMS, StableFlex, 50/30 μm), Carbowax/divinylbenzene (CW/DVB, 70 μm), and polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 μm) were the chemistry coatings used in the C-4 study, three replicates each with a blank run in between. The CW/DVB fiber was replaced with PDMS/DVB (StableFlex 65 μm) in the Detasheet and Semtex H studies because the CW/DVB coating was very easily damaged. Only one analysis with a blank in between samples was performed on each of these two explosives. The mass of each of the analytes extracted on the fiber was quantified using a calibration curve prepared from liquid injection of the standards into the GC/MS using methanol as the solvent.
SPME extraction quantification and confirmation
The mass of analytes extracted on the SPME fiber from the headspace of the glass vial containing the explosives for each extraction time was calculated from the intensity responses obtained from the IMS analysis using response curves of the standards. The SPME-IMS response curves were prepared by exposing the SPME fiber to the headspace inside a glass vial containing the standard solution for different extraction times. The fiber was then analyzed using the GC/MS and the mass extracted on the fiber was then determined from the GC/MS calibration curves. The same extraction procedure was repeated for analysis by the IMS to generate a response curve indicating mass vs. intensity. The SPME-IMS response equations are as follows: y = 14x + 1,064, R 2 = 0.945 for n-butyl acetate; y = 9x + 344, R 2 = 0.983 for DMNB, and y = 55x + 945, R 2 = 0.919 for cyclohexanone. The unit for x is in nanograms (ng), and y is in millivolts (mV). In order to confirm if the pre-concentrated analytes on the SPME fiber were the volatile constituents of the explosives and not the explosive particles, GC/MS analysis was performed.
SPME extraction equilibrium
Vials containing Detasheet Flex X explosive from Case 2 (untagged) were used in the SPME-IMS extraction time experiment for sampling times of 5 s, 10 s, 20 s, 1 min, 5 min, 20 min, and 30 min. A minimum waiting period of 30 min was allowed between each extraction to avoid diminishing the headspace concentrations of the vials in use. The vials containing Semtex H explosive from Case 2 were used for sampling times of 2, 5, 10 15, 23, 60, and 120 min. For C-4 explosive, sampling times were 10 s, 30 s, 1 min, 2 min, 3 min, 4 min, 5 min, and 10 min. The sampling time was measured from the start of the extraction, when the SPME needle punctured the septum of the glass vial and the fiber was exposed, up until the fiber was withdrawn. The mass of analytes recovered corresponding to each extraction time were plotted to form the equilibrium extraction time profile for each of the explosives. All SPME extractions were performed at room temperature, using PDMS/DVB fiber for C-4 and Detasheet Flex X explosives, and PDMS/DVB StableFlex fiber for Semtex H.
Results and discussion
Headspace SPME-GC/MS analysis of Detasheet, Semtex H, and C-4
In order to target the non-energetic signatures from plastic explosives for headspace air sampling, SPME fibers were used to extract and pre-concentrate analytes that are readily present in the headspace of the explosive samples under atmospheric conditions, followed by thermal desorption into a GC/MS. Signature compounds are those that can be associated with the target compound of interest, such as a degradation product, a starting material or an impurity. There are some considerations when choosing a signature to presumptively detect a target substance. The signature compounds must be differentiated from the background and present in high-enough concentration to be detected by the analytical instrument. Detection algorithms with more than one compound or ion peak for the target substance would reduce false-positive alarms. False-positive alarms are one of the concerns when operating the IMS instrument in positive polarity and at low temperature.
The results in Fig. 2a show acetic acid, 1-butanol, toluene, and n-butyl acetate as being the most dominant peaks detected by SPME-GC/MS in untagged Flex X explosives. In the analysis of tagged Primasheet 1000 (results shown in Fig. 2b), three compounds (acetic acid, toluene, and 1-butanol), along with DMNB as the taggant were detected, however, n-butyl acetate was not detectable and only a very small peak for acetic acid was detected. It is possible that when the DMNB taggant is present, it competes with other trace volatile components in the headspace of the explosive. A side experiment was performed to confirm this competition theory. Neat standards (to avoid solvent effects) of DMNB, and n-butyl acetate were placed into a gallon-sized metal can until headspace equilibrium was reached and extracted using fiber SPME followed by GC/MS analysis. For a short extraction time (1 min), the intensity of n-butyl acetate was three times higher than that of DMNB. While for a longer extraction time (20 min, as in the case of the real explosive study), the DMNB intensity was three times higher than n-butyl acetate. These observations suggest that the n-butyl acetate may be present in the tagged Primasheet explosive in very low concentrations and not detectable when the displacement effect by DMNB occurred. Although 1-butanol and toluene were present in high concentrations in all Detasheet explosive samples, these compounds were not chosen because of their lower discrimination power [31, 32]. Thus, in the cases where Detasheet explosives were manufactured without the addition of the DMNB taggant [8], an analytical technique such as IMS can aim to detect n-butyl acetate. Future experiments will investigate Detasheet explosives manufactured more recently but without taggant added in order to determine if n-butyl acetate is introduced in the explosive from the manufacturing process or formed from the reaction between acetic acid and 1-butanol as the explosive sample ages. A summary of the compounds detected for each of the explosive cases by GC/MS is presented in Table 5.
For Semtex H explosives, samples from cases 1 and 2 show acetone, undecane, dodecane, and DMNB as the four most dominant peaks extracted and detected by GC/MS. In case 3, undecane and dodecane were not present, hence eliminated as possible candidate signatures for Semtex H explosive; case 3 was from a different lot of explosive than cases 1 and 2. Although acetone was consistently present along with DMNB, it does not serve well as a signature compound since it is ubiquitous in commodities. That leaves only DMNB as the good target compound. Future sampling of aged and untagged Semtex and new Semtex samples will be studied to identify other possible volatile signatures.
For C-4, cyclohexanone, DMNB, and 2-ethyl-1-hexanol were the three most dominant compounds extracted. Cyclohexanone is a solvent that is used in the production of RDX explosive but is primarily used in the production of nylon. 2-ethyl-1-hexanol is primarily used in the manufacture of the diester bis(2-ethylhexyl) phthalate (DEHP) plasticizer, which are present in plastics at up to 40% by mass. Both cyclohexanone and 2-ethyl-1-hexanol can serve as candidate signature compounds for the detection of C-4 explosive. However, the latter is more likely to produce more false positives due to the higher use of plastics in everyday commodities. Hence, if detection of C-4 can be based on both compounds in the case of untagged explosive and one of the two compounds along with DMNB taggant in the case of tagged explosive, the false-alarm rate can be reduced.
Fiber chemistry study by GC/MS
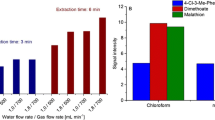
Product ion formation within the IMS analyzer is based on the ability to compete for reactant ions, which mostly depends on the compound’s proton affinity [8] but the initial mass of the compound available is also critical. Therefore, different SPME fiber chemistries were tested to determine which would provide the best extracted mass ratio for compounds of interest in the detection of the target explosive. The objective was to detect multiple peaks representing multiple target compounds, because the capability to detect multiple signatures would provide a second dimension for detection, thus lowering the false-alarm rate [19]. Figure 3 shows the comparison for five different fiber chemistries, CAR/PDMS, PDMS, DVB/CAR/PDMS (StableFlex), PDMS/DVB (StableFlex), and PDMS/DVB by GC/MS for the extraction of the explosives. Figure 3a is the result for the extraction of Flex X-untagged explosive. The fiber chemistry study was performed on the untagged explosive rather than the tagged explosive because it is a greater concern that the extraction of n-butyl acetate be optimized since it is already apparent from Fig. 3b and c that DMNB is very well extracted by all of the five fiber chemistries. The best chemistry of choice for the extraction of Flex X-untagged explosive was determined to be PDMS/DVB since more of n-butyl acetate was extracted than acetic acid and 1-butanol. Except for PDMS, all of the five coating chemistries provided very high extraction efficiency for n-butyl acetate. For Semtex H explosive shown in Fig. 3b, both PDMS/DVB and PDMS/DVB (StableFlex) provided better extraction ratios between DMNB and unwanted volatiles compared to the other three fiber chemistries. The extraction ratio between cyclohexanone, 2-ethyl-1-hexanol and DMNB from C-4 explosive was almost equivalent for five types of fiber chemistry as shown in Fig. 3c; hence, any of the fiber chemistries can be used except for PDMS, which has a slightly lower total extraction efficiency.
Headspace SPME-IMS analysis of Detasheet, Semtex H, and C-4
Detasheet, Semtex H, and C-4 explosive samples were successfully detected using IMS by means of headspace air sampling, with detection algorithms based on the volatile chemical signatures associated with the targeted explosives. Figure 4a shows the overlaid spectra of a 20 min SPME extraction in the headspace of a 15 mL glass vial containing 1 g of Flex X explosive and n-butyl acetate standard. Two peaks were presumptively identified as monomer and dimer of n-butyl acetate at the drift time 8.7 and 11.4 ms, respectively. Multiple peak detection, indicating multiple compounds and/or monomer and dimer formation would provide higher discrimination power for the detection of the target substance. Figure 4b shows the overlaid spectra of a 20 min SPME extraction of Semtex H, where DMNB was detected at a drift time of 10.2 ms. The explosive C-4 was tested at a temperature 30 °C higher than the universal drift tube temperature determined in this study. The associated DMNB peak was still detectable, but at a shorter drift time, 8.3 ms, as shown in Fig. 4c. A 5 min extraction of C-4 explosive yielded a strong peak for DMNB, and a very small peak for cyclohexanone at 7.6 ms. Table 5 also shows the summary of the compounds detected by IMS from each of the explosive cases, and Table 6 reports the limits of detection for each of the compounds on the GE Itemiser 2 in the positive operating mode, and their reduced mobilities (K 0) determined using the Smiths Detection IonScan 400B.
The positive operating mode in IMS is prone to higher false-positive alarm rates compared to the negative operating mode, due to the ease with which neutral molecules cluster with H+(H2O) n as compared to O −2 (H2O) n reactant ions [8]. The rate of false-positive alarms can be reduced for IMS in positive polarity if the detection algorithms can be based on multiple target peaks. For the plastic explosives in this study, multiple channel detection algorithms can be implemented on Flex X, and C-4, since the headspace of these two explosives contains compound(s) that produced two or more peaks in the IMS.
Minimum SPME extraction time (mass) for a positive alarm
The SPME extraction time profiles shown in Fig. 5a, b, and c are for Flex X, Semtex H, and C-4 explosives, respectively. The extraction from the headspace of Flex X and C-4 explosive quickly reached the equilibrium extraction time after 5 and 4 min, respectively. After this equilibrium time, extended SPME sampling time no longer increased the observed intensity in the IMS. Although SPME fiber can extract more analyte if sampled for longer time, the mass extracted with the above extraction time was already at the saturation point on the IMS instrument. It was also observed that instantaneous (<1 s of fiber exposure) extraction of Flex X explosive already extracted enough n-butyl acetate to form dimer product ions. On the other hand, the Semtex H extraction took much longer (1 h) to achieve SPME extraction time equilibrium.
The minimum SPME sampling time required for a true positive alarm from a sample containing 1 g of explosives sealed for 1 week in a 15 mL glass was also determined to provide a reference point for future researchers when conducting field sampling of real sample size. The headspace of Flex X-untagged explosive is saturated with n-butyl acetate, thus for only 5 s of sampling time, 25 ng of n-butyl acetate was detected, which is 11 times the LOD of the IMS instrument. For C-4 explosive, 20 ng of DMNB was detected in a 10 s sampling time, which is 12 times the detection limit. Semtex H explosive requires a slightly longer sampling time; 2 min were required to detect 25 ng of DMNB. These same explosives were also sealed for a shorter time period, 24 h instead of 1 week. The peak intensities observed were similar in both cases, which suggest shorter sealing times are possible because of fast build-up of the headspace concentrations of the volatile signatures being targeted. Air sampling in open environments is expected to be much more challenging due to the large volume and fast diffusion of volatiles in the air. Hence, the headspace concentrations of the volatiles would be much more dilute as compared to a sealed environment, even at positions close to the emitting source. It is expected that longer extraction times may be necessary than the times reported in this study, and improvement of SPME capacity may also be needed to further increase extraction efficiency of the analytes.
Conclusion
Air sampling and detection of plastic explosive vapors has always been a challenge due to the low vapor pressures of the energetic materials, RDX and PETN that commercial IMS instruments are currently programmed to detect. This research study demonstrates an approach that targets the more volatile, non-energetic compounds, such as taggants, decomposition products and/or impurities, rather than the explosive itself. Successful vapor detection of plastic explosives was proven possible using IMS with operating conditions optimized to detect the volatile signatures of the explosives. A SPME device was used to extract and pre-concentrate the target volatile markers with sampling times in seconds to minutes as sufficient to extract and detect ∼20 ng of the target analyte(s), which is ten times the amount required for a reliable IMS response. SPME-IMS can greatly simplify the field sampling and detection process because SPME allows for remote air sampling without the need for additional cumbersome equipment and IMS provides rapid analysis at atmospheric pressure. Therefore, SPME-IMS has great potential to be a non-invasive, non-surface contact method for screening of low-vapor-pressure hidden explosives. Commercial dual-tube IMS analyzers can be set to have one drift tube operating at conditions to detect the energetic explosives while the other drift tube can be set at an alternate operating condition to detect the non-energetic volatiles.
References
Pinnaduwage LA, Boiadjiev V, Hawk JE, Thundat T (2003) Appl Phys Lett 83:1471–1473
Sanchez JC, Trogler WC (2008) J Mater Chem 18:3143–3156
Bourzac K (2008) Technology review-portable plastic explosives detector
Pinnaduwage LA, Hedden DL, Gehl A, Boiadjiev VI, Hawk JE, Farahi RH, Thundat T (2004) Rev Sci Instrum 75:4554–4557
Jehuda Y (1995) Forensic applications of mass spectrometry, vol. 3, 2nd edn. CRC, Boca Raton
Röck F, Barsan N, Weimar U (2008) Chem Rev 108:705–725
Ewing RG, Miller CJ (2001) Field Anal Chem Technol 5:215–221
Eiceman GA, Karpas Z (2005) Ion mobility spectrometry, 2nd edn. CRC, Boca Raton
Gui Y, Xie C, Xu J, Wang G (2009) J Hazard Mater 164:1030–1035
Kapoor JC, Kannan GK (2007) Defense Science Journal 57:797–810
Yang X, Du XX, Shi J, Swanson B (2001) Talanta 63:439–445
Toal SJ, Trogler WC (2006) J Mater Chem 16:2871–2883
Singh S (2007) J Hazard Mater 144:15–28
Harper RJ, Almirall JR, Furton KG (2005) Talanta 67:313–327
Furton KG, Myers LJ (2001) Talanta 54:487–500
Lorenzo N, Wan T, Harper RJ, Hsu YL, Chow M, Rose S, Furton KG (2003) Anal Bioanal Chem 376:1212–1224
Jehuda Y (1993) Advances in analysis and detection of explosives. Kluwer, Norwell, MA
Lawrence AH, Neudorfi P (1988) Anal Chem 60:104–109
Oxley JC, Waggoner LP (2009) Aspects of explosives detection. Elsevier, Kidlington, Oxford
Voiculescu I, McGill RA, Zaghloul ME, Mott D, Stepnowski J, Stepnowski S, Summers H, Nguyen V, Ross S, Walsh K, Martin M (2006) IEEE Sens J 6:1094–1102
Cotte-Rodríguez I, Handberg E, Noll RJ, Kilgourb DPA, Cooks RG (2005) Analyst 130:679–686
Linker KL, Conrad FJ, Custer CA, Rhykerd CL Jr (2005) U.S. Patent, Sandia National Laboratories: USA
Linker KL, Bouchier FA, Theisen L, Arakaki LH (2007) U.S. Patent, Sandia: USA
Senesac L, Thundat TG (2008) Materials Today 11:28–36
Pawliszyn J (1997) Solid phase microextraction: theory and practice. Wiley, New York, NY
Perr JM, Furton KG, Almirall JR (2005) J Sep Sci 28:177–183
Lai H, Corbin I, Almirall RJ (2008) Anal Bioanal Chem 392:105–113
Guerra P, Lai H, Almirall RJ (2008) J Sep Sci 31:2891–2898
Perr JM, Furton KG, Almirall JR (2005) Pro. SPIE – The Int. Soc. for Optical Engineering, Carapezza EM, Ed
Lai H, Guerra P, Joshi M, Almirall RJ (2008) J Sep Sci 31:402–412
World Health Organization (1987) Butanols: Four isomers 65: Environmental Health Criteria
Merory J (1968) Food flavorings: composition, manufacture and use, 2nd edn. AVI, Westport
Acknowledgments
The authors would like to acknowledge Anna Whitehead and Richard Lareau from the Transportation Security Laboratory of the Science and Technology Directorate, US Department of Homeland Security for permitting the experiments to be conducted in their laboratory. Some portions of this work were possible through funding from the National Institute of Justice (2006-DN-BX-K027). Funding for Hanh Lai is acknowledged from the Dissertation Year Fellowship by the Florida International University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, H., Leung, A., Magee, M. et al. Identification of volatile chemical signatures from plastic explosives by SPME-GC/MS and detection by ion mobility spectrometry. Anal Bioanal Chem 396, 2997–3007 (2010). https://doi.org/10.1007/s00216-010-3501-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3501-6