Abstract
Monitoring of cell cultures in microbioreactors is a crucial task in cell bioassays and toxicological tests. In this work a novel tool based on a miniaturized sensor array fabricated using low-temperature cofired ceramics (LTCC) technology is presented. The developed device is applied to the monitoring of cell-culture media change, detection of the growth of various species, and in toxicological studies performed with the use of cells. Noninvasive monitoring performed with the LTCC microelectrode array can be applied for future cell-engineering purposes.

Microelectrode array for monitoring of cell cultures
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultured mammalian cells under laboratory conditions have become indispensable research tool in understanding the unity of living systems, including the human body. Culture of mammalian cells enables the development of advanced cell biology, which offers the possibility of investigating the mechanisms of cell-cell interactions, cell-extracellular matrix (ECM) interactions, and transport phenomena [1]. The early stages of drug development most frequently involve the testing of compounds on cells, using defined in-vitro cell-based assays. Cultured mammalian cells play all-important roles as the fundamental components of tissue culture and tissue engineering [2, 3]. Moreover, cell cultures are fundamental tools in the manufacture of vaccines, enzymes, hormones, and monoclonal antibodies, etc.
Cells are permanently exposed to a multiplicity chemical or mechanical stimuli, which strongly influence their physiological functions and their properties. The complex intercellular communication network, which coordinates the proliferation, differentiation, and metabolism of the multitude of cells in diverse tissues and organs, is a necessary condition for the proper performance of a multicellular organism. Aberrations in signal transduction underlie many different diseases, including the majority of cancers [4]. Understanding the establishment of specific cell-cell and cell-extracellular matrix interactions has several advantages for research on fundamental aspects of cell biology. The tendency of animal cells in vivo to interact with one another and with surrounding extracellular matrix is mimicked in their growth in culture. It is recognized that for anchorage-dependent cells microenvironmental conditions such as the topology of the substrate, the composition of the extracellular matrix, and the signals of neighboring cells have significant effects on the results obtained from cell-based assays [5]. Chemical and mechanical signals in the cell microenvironment are sensed by cells and subsequently transformed into biochemical responses [6].
According to these facts, cellomics [7], i.e. the study of cells, is nowadays a rapidly developing branch of science. Usually, prokaryotic, eucariotic, and plant cells are grown in cell-culture flasks and maintained under controlled conditions at an appropriate temperature and with an appropriate atmosphere ensured by cell incubators.
There is tremendous requirement to create modern systems enabling 3D cell growth to realize cell functions successfully and offering possibilities of studying drug metabolism and toxicity, comparable with use of animal and human models. One proposed solution is to fuse advanced molecular biology and cell-culture techniques with micro and nanotechnology, to develop microfluidic cell-culture systems, called lab-on-a-chip, suitable for conducting high-throughput cell-based assays. Lab-on-a-chip systems unquestionably exhibit many advantages compared with 2D monolayer cell cultures, because they mimic more accurately the in vitro microenvironment [8–10]. They offer the possibility of controlling cell-cell and cell-extracellular interactions, the ability to distribute and remove soluble biochemical molecules in a controlled way, and the opportunity to monitor mechanical forces [9, 11]. Other benefits are connected with the integration of laboratory functions on a single chip, requirements for small amounts of reagents, short reaction time, high speed of analysis, and low fabrication costs. Moreover, the short diffusion distances, laminar flow linked with low Reynolds numbers, high interface-to-volume ratio, and small heat capacities that are obtained on the microscale are further performance advantages significant in cell culture [7, 12].
Nowadays, cell culture on microchips embraces various tasks from placing and handling, sampling, trapping, sorting, cell lysis, gene transfection, cell fusion, and analysis of cell viability [7, 12, 13]. The fully integrated lab-on-a-chip system should provide repeated cell growth/passage cycles, reagent introduction, and real-time cell-based analysis. The design and development of multi-property detection systems to monitor the cell events in real-time involve the combination of different intra- and extracellular detection techniques.
Optical detection is probably the most versatile and powerful method used to visualize cells and subcellular structures. The majority of standard cell viability tests and cytotoxicity assays utilizes exogenous fluorophores or transfected fluorescent reporters. Fluorescence-based methods enable easy determination of the viability of adherent or non-adherent cells. For example, a fluorogenic esterase substrate, calcein AM, which is hydrolyzed to a green-fluorescent product (calcein), is often used to indicate cells with esterase activity and an intact membrane. Propidium iodide is a red-fluorescent nucleic acid stain useful as a marker of dead cells, because of its ability to pass only through the damaged membranes of cells. Moreover, fluorescence-based methods provide possibilities of detecting specific proteins and organelles in fixed cells, to follow an expressed recombinant fluorescent protein, such as green fluorescent protein (GFP), in living cells. Potential cytotoxicity of fluorophores and photobleaching are substantial problems in fluorescence-based methods. Moreover, those assays cannot be used continuously on the same sample; therefore real-time monitoring is problematic [14]. Additionally, the use of fluorophores is complicated in microfluidic systems, because of the need for quick and uniform penetration of the cell culture. There is also a need for non-invasive detection methods enabling detection of cell biochemical responses to specified stimuli. Therefore nondestructive, minimally invasive methods for cell-viability estimation are of great interest, especially when they can be fully integrated with microscale cell bioreactors.
In this work, the first results from application of a potentiometric miniaturized sensor array fabricated using low-temperature cofired ceramics (LTCC) technology to the monitoring of cell cultures is presented. The developed tool was applied in preliminary research on the differentiation of the cell-culture media for future cell-engineering purposes. Use of the sensor array coupled with principal components analysis (PCA) enabled the detection of media changes linked with different media, different cell growth, and toxic effects caused by biologically active substances.
Experimental
LTCC structure fabrication
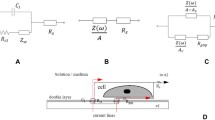
The ceramic structure of microelectrode array was formed by use of eight layers of green tape (DP 951, DuPont). To obtain the appropriate shape of each layer, laser beam cutting (Aurel NAVS 30 laser trimming and cutting system) was performed. Metallic electrodes and their conductive paths together with the contacts were screen-printed (PdAg conductive paste DP 6146, DuPont) on the 2nd ceramic layer (Figs. 1 and 2). Six layers with holes for membrane casting were then applied. All eight films were stacked into one module and then pressed in an isostatic press (10 min under 180 atm).
The last phase of LTCC structure preparation embraced cofiring with a typical temperature profile (maximum temperature 875°C). In the final device (thickness 1.1 mm), the wells formed for PVC membranes were ∼700 μm in depth. Before membrane casting, an AgCl layer was electrochemically formed on every electrode. Details of the LTCC structure dimensions, fabrication, and performance were the subject of our previous work [15].
Preparation of chemosensitive membranes
For each membrane composition two electrode specimens were prepared. A sensor array consisted of eight types of electrode—four ion-selective (NH4 +, Cl−, HCO3 −, Ca2+) and four partially selective (Na+/K+, “cation-selective”, F−/H2PO4 −, IL). This configuration previously enabled the recognition, classification, and differentiation of various foodstuff samples (mineral waters, juices, tonics [16], beers [17], milk [18], diet supplements [15]), and biosamples (dialysate fluids [19], plant homogenates [20]).
Preparation, composition, and deposition of membrane cocktails were described in detail in our previous works [15, 21]. Electrode arrays fabricated in LTCC technology and equipped with such polymeric membranes were the subject of our previous work [15]. It was shown that better adhesion is achieved when polyurethane is used as polymeric matrix. Selectivity patterns of the prepared electrodes were similar to those presented previously [15].
Potentiometric measurements
All measurements were carried out with cells of the type:
EMF measurements were performed with a 16-channel electrode monitor (EMF 16 Interface, Lawson Labs, Malvern, USA), without sample pretreatment-only dilution of samples with distilled water was applied (1:9 v/v). Data analysis was performed with MatLab (The MathWorks, Natick, USA). In the case of fresh media differentiation (in which cells were not cultured) raw EMF signals were analyzed. The rest of the experiments were conducted with correction of the signal based on the measurement of reference solution (baseline correction according to Ref. [15]). Cleaning solution was prepared according to Ref. [22] and consisted of 30% EtOH, 10 mmol L−1 KOH, and 100 mmol L−1 KCl.
Cell cultures and media preparation
The first experiment concerning media differentiation was conducted with “fresh” media in which cells were not cultured. Five types of cell-culture media were chosen for this study (Lonza, USA): 12-115F RPMI, 12-722F Iscove’s Modified Dulbecco’s Medium (IMDM), 12-136F MEM Eagle with Earle’s BSS (MEM), 12-761F William’s Medium E (WME), and 12-604F Dulbecco’s Modified Eagle’s (DMEM). In the rest of the experiments, two types of cell were cultured in Dulbecco’s Modified Eagle’s (DMEM).
A549 cells (human lung adenocarcinoma epithelial cell line; American Type Culture Collection) and Vero cells (a green monkey kidney continuous cell line, American Type Culture Collection) were cultured in DMEM medium (Lonza) supplemented with 10% fetal bovine serum (FBS; Gibco), 2 mmol L−1 l-glutamine (Gibco), 100 U mL−1 penicillin (Sigma), and 100 μg mL−1 streptomycin (Sigma), and grown until confluent, following which cells were subcultured by trypsinization. Cell cultures were plated in flasks (Techno Plastic Products; 25 cm2 of growth surface) at a density of 106 cells per flask. After 48 h, when the cells reached 80% confluence, the medium was changed to fresh supplemented medium (DMEM), and experiments were started 2 h later. If it was necessary, cells were preincubated with 1,4-dioxane for 30 min. After 24 h the culture medium from each flask was taken as the biological sample and was investigated by use of the LTCC structure based miniaturized electrode array.
Visualization of cells was performed by utilizing fluorescent microscopy. Propidium iodide (PI) (Sigma), a red-fluorescent nucleic acid stain, was used as a marker of dead cells. To indicate living cells, fluorescein dibutyrate (FDB) (Sigma) which is hydrolyzed to green-fluorescent product (fluorescein) was applied. Cell counting was performed using a hemocytometer.
Results and discussion
Cell-culture medium can be a potential source of information about cell-culture condition. By changing the culture medium periodically, cells can be kept in good conditions and regular growth is observed. When cell-culture medium is not changed, cells die rapidly because of shortage of essential ingredients. Alterations of the cell-culture medium are detected by cells and converted by them into biochemical responses, which result in changes of their microenvironment. In addition to microscopy, arrays of chemical sensors and biosensors could provide information about cell attachment, migration, growth, proliferation, and differentiation in real time, according to the ability to monitor important nutrients and metabolic components. Usually, the factors of interest are oxygen concentration, pH, carbon dioxide, amino acids, and glucose concentration, which are correlated with cell growth and can be monitored either noninvasively or minimally invasively [14]. Quantitative information about the metabolic activity of some cells can also be obtained by monitoring urea and albumin production, calcium, and pH variation [14].
These factors can be monitored with electrochemical detection techniques, which in combination with recent progress in molecular biology and microtechnology show the potency of the development of non-invasive multifunctional biosensors [23, 24]. However, it is necessary to choose appropriate sensor and transducer materials, enabling miniaturization and compatibility with cell-culturing microsystems. LTCC technology has been used widely in microelectronics, because it offers good mechanical and electrical properties, high reliability, and stability. Moreover, the multilayer architecture offers the possibility of the construction of 3D structures. These advantages can be used in the development of miniaturized analytical devices, and were presented previously [15, 25]. In this work, an integrated microelectrode array based on LTCC technology was tested. Dimensions and architecture of the device (see Figs. 1 and 2 and details in Ref. [15]) were compatible with its future application as a microfluidic flow-through module for cell-media monitoring.
Typical conditions for mammalian cell culture are 37°C and 5% CO2, which are maintained by cell incubators. The next factor influencing cell culture is the growth medium, which can vary in pH, glucose concentration, growth factors, and many other nutrient components. Supplementation of media with various blood-derived ingredients is a commonly used practice. Culture maintenance conditions are different for different kinds of cell, and, for particular types of cell, changing the composition of the medium can cause various phenotypes to be expressed and cell differentiation to occur. There is also a possibility of stimulating selected domains inside cells by using multiple laminar streams of various media [12]. Therefore appropriate media composition should be controlled. Differentiation between various cell-culture media was chosen in this study as a model task.
The developed sensor array based on LTCC technology was applied to potentiometric tests of five cell-culture media. From each “fresh” medium (i.e. medium in which samples were not cultured) four samples were tested. When the steady-state response of the microelectrodes was reached, 10 outputs were recorded from each sensor (i.e. 10 measurements were collected for each sensor in intervals of 5 s). The data matrix consisted of 200 cases; each case was characterized by 16 features. After autoscaling, principal components analysis (PCA) was performed. The visualization of electrode array responses is presented on the PCA plot in Fig. 3. This led to evident differentiation between various media—clusters of WME, DMEM, and MEM were easily separable on the PCA plot. Also further kinds of sample—RPMI and IMDM—were differentiated from each other when additional principal components were taken into account. These results show that the presented microelectrode array is capable of cell-culture media classification, which can be exploited in future applications of this device as a module for controlling media composition in cell-culture bioreactors.
Media changes and passaging of cells are common manipulations in cell cultures. Although they are performed relying on sterile techniques, there is possibility of contamination with bacteria, yeast, and other cell lines. Some cells exhibit intense growth, and are therefore able to cross-contaminate other cell-line cultures. After some time, the number of contaminating cells in comparison with the original is overgrowing, and the cultured cells start to be displaced by the contaminating ones. As long as general properties of cells are to be investigated, that effect is not problematic, however in medical research focused on specific types of cell it usually leads to inappropriate conclusions. Therefore the LTCC microelectrode array should also be able to detect changes in media composition linked with cell growth. Differentiation between media in which two different cell lines were cultured served as a model experiment. The scheme of the analysis is presented in Fig. 4. Cells were separated from the cell medium and their viability was observed under a fluorescence microscope because of the PI and FDB markers. In the case of the adherent cells used in this work, media were removed by aspiration and were then prepared for measurement with the LTCC sensor array. Cells for fluorescence microscopy study were obtained by detachment from the flask surface by trypsinization.
The media poured from the cell-culture flask were analyzed by use of the LTCC microelectrode array (sampling and data collection were applied as previously). Before each measurement of the sample, reference solution signals were recorded (Fig. 4). The raw signals obtained in the following measurements were corrected according to raw signals obtained from the “fresh” medium (the procedure has been described in Ref. [15]). Therefore only differences in the signals connected with the cell growth could have been recorded and the influence of drift could be omitted. After autoscaling, the outputs obtained were used in PCA, which resulted in the visualization of the chemical images presented in Fig. 5. The effect of media change during cell growth is clearly visible—clusters of media in which A549 and Vero cells were cultured are separated from the cluster obtained for the “fresh” medium. Moreover, it is possible to differentiate between two cell lines, which could be helpful in assessment of contamination of the cell culture with unwanted species. However, the objects in clusters are not uniformly disposed—this is probably caused by the difference in the number of cells in various flasks. Although they are fabricated uniformly, they can vary from flask to flask, which influences the adhesion properties of cells. This, in turn causes the differences in signals needed for growth and differentiation. Therefore the number of cells in flasks after a particular time is not strictly determined and it vary within a range.
PCA plot of DMEM media obtained from two kind of cell culture—human lung adenocarcinoma epithelial cells, A549 (a, b, c) and Vero green monkey kidney cells (d, e, f). Both type of cell were cultured routinely in three different cell-culture flasks before taking samples of cell-culture medium for measurement. Both types of cells exhibit the appropriate morphology
The last experiments were devoted to investigation of the ability of the LTCC sensor array to monitor toxic effects in cell cultures. As a model substance with toxic activity, 1,4-dioxane was chosen. The procedure of measurement was the same as presented above (Fig. 4)—the same samples were investigated by fluorescence microscopy (A549 and Vero cells) and the LTCC sensor array (media in which the cells were cultured). Properly cultured cells continue to divide and they grow to fill available space, which is linked with nutrient depletion in growth media, accumulation of dead cells, and growing amounts of metabolic products. When exposed to toxic substances, part of the cell population is not able to continue life functions and they die, which results in cell destruction and rapid changes in the cell microenvironment. In the case of both kinds of cell, various amounts of dead cells were observed—between 0 and 15% in the case of A549 cells and between 0 and 100% in the case of Vero cells. The ability of the LTCC sensor array to differentiate between media obtained from cell cultures incubated with 1,4-dioxane is presented in Figs. 6 and 7. Because separations of clusters related to samples that differ by a smaller number of dead cells are less visible, slightly worse differentiation ability is visible in Fig. 6 compared with Fig. 7. However, it must be noticed that those results are affected by the problem mentioned above—the number of cells in various flasks is not strictly determined, and, as a consequence, changes in the distinct volume of medium can differ from each other, resulting in non-uniform cluster formation on PCA plots.
PCA plot of toxic effect of 1,4-dioxane on A549 cells: (a) A549 cells cultured routinely; (b, c, d) A549 cells treated with different concentrations of 1,4-dioxane. The application of PI (marker of dead cells) and FDB (marker of living cells) showed nearly 100% (a), 95% (b), 90% (c), and 85% (d) of living cells
PCA plot of toxic effect of 1,4-dioxane on Vero cells: (a) Vero cells cultured routinely; (b, c, d) Vero cells treated with different concentrations of 1,4-dioxane. The application of PI (marker of dead cells) and FDB (marker of living cells) showed nearly 100% (a), 70% (b), 40% (c), and 0% (d) of living cells
In the last experiment we wanted to prove that the changes observed by use of the LTCC sensor array in toxically treated cell cultures are not only because of the presence of the toxic substance but are also associated with cell death causing changes in media composition. Therefore PCA images of samples of “fresh” medium were compared with samples of “fresh” media with addition of toxic substance and media in which cells were treated with this toxic substance. One can see from Fig. 8 that the presence of the toxic substance (1,4-dioxane) does not change the PCA images as significantly as when cell death influenced the change in media composition. Moreover, it can be observed, that the effect of rapid death caused by toxic treatment with 1,4-dioxane can be differentiated from the effect of death caused by the depletion of nutrients and accumulation of metabolic products when the medium was not changed (Fig. 8). The results presented can be treated as preliminary and should be further studied in detail.
PCA plot of media in which A549 cells were (a) cultured routinely, (b) treated with 1,4-dioxane, (c) left without medium change. PI was used as a marker of dead cells and use of FDB reveals living cells. In (b) and (c) almost 100% dead cells were observed. Addition of the toxic substance to the medium without cells does not change the PCA image of the medium significantly in comparison with (b) and (c)
Summary
Sensor technologies are helpful tools in investigation of cellular functions, monitoring of biochemical microreactors, analysis of bioassays systems, controlling of hybrid bio/artificial tissue engineered organs, and controlling bio-microactuators [12, 14]. In tissue engineering, sensor technologies also offer spatial and time resolution, real-time monitoring [14], and thus complex information on the 3D matrix is accessible nondestructively.
The major restriction of the use of cell-culture experiments for scientists in biomedical research and drug testing is closely associated with the fact that the growth, proliferation, and function of cells as multicellular in-vivo 3D structures is different from their growth as conventional in-vitro 2D monolayer structures. Microscale culture of the cells in lab-on-a-chip devices enables observation of the effects occurring when 3D structure of the cells can be obtained, therefore cell-culture bioreactors will support high-throughput experimentation in the study of complex biological processes and in drug testing in the future [13]. In such systems microenvironmental changes in culture media can be monitored to obtain indirect information about the condition of the cultured cells. There is a need to develop tools capable of monitoring changes in the composition of the medium. Miniaturized sensor arrays can be helpful in this task, because of their compatibility with cell-culture bioreactors.
However, direct measurements in cell bioreactors determine the limitation of sensor attributes—they have to use non-toxic materials not prone to adsorption of proteins, to be sterilizable (by one or, better, two sterilization techniques), and to be able to provide the result of the analysis without sample pretreatment and use of additional reagents [14]. The method of cell culture monitoring based on the investigation of media from which cells have already been separated could be helpful in flow-cell bioreactors, when media can be analyzed in an additional module enabling eventual sample pretreatment, sensor calibration, and sensor cleaning. The LTCC sensor array presented in this work is an example of such a device. It was shown that it is capable of monitoring media change, and detection of the growth of various species, and that it can be used in toxicological studies performed in microsystems.
In the future, the presented device will serve as a parallel module followed by a cell-culture bioreactor and its performance under flow conditions will be investigated. Following integration of (bio)sensor arrays with physical sensors for additional monitoring of flow rate, pressure, and temperature variations would be useful for full characterization of microenvironmental changes in cell cultures which would be advantageous for various purposes.
References
Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN (2006) Nat Methods 3:369–375
Fernyhough ME, Hausman GJ, Guan LL, Okine E, Moore SS, Dodson MV (2008) Biochem Biophys Res Commun 368:455–457
Trounson A, Elefanty A (2007) Curr Opin Biotechnol 18:432–433
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell JE (1999) Molecular cell biology. W.H, 4th edn. Freeman, New York
Giselbrecht S, Gietzelt T, Gottwald E, Trautmann C, Truckenmuller R, Weibezahn KF, Welle A (2006) Biomed Microdevices 8:191–199
Girard PP, Cavalcanti-Adam EA, Kemkemer R, Spatz JP (2007) Soft Matter 3:307–326
Andersson H, Van den Berg A (2003) Sens Actuators B 92:315–325
Griffith LG, Swartz MA (2006) Nature Rev Mol Cell Biol 7:211–224
Kim L, Toh YC, Voldman J, Yu H (2007) Lab Chip 7:681–694
Leclerc E, Sakai Y, Fujii T (2004) Biotechnol Prog 20:750–755
Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL (2005) Lab Chip 5:401–406
Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T (2007) Biosens Bioelectron 23:449–458
Yi C, Yi CW, Ji S, Yang M (2006) Anal Chim Acta 560:1–23
Starly B, Choubey A (2008) Ann Biomed Eng 36:30–40
Ciosek P, Zawadzki K, Stadnik D, Bembnowicz P, Golonka L, Wróblewski W (2009) J Solid State Electrochem 13:129–135
Ciosek P, Augustyniak E, Wróblewski W (2004) Analyst 129:639–644
Ciosek P, Wróblewski W (2006) Talanta 69:1156–1161
Ciosek P, Wróblewski W (2008) Talanta 76:548–556
Ciosek P, Grabowska I, Brzózka Z, Wróblewski W (2008) Microchim Acta 163:139–145
Ciosek P, Pokorska B, Romanowska E, Wróblewski W (2006) Electroanalysis 18:1266–1272
Ciosek P, Mamińska R, Dybko A, Wróblewski W (2007) Sens Actuators B 127:8–14
Habara M, Ikezaki H, Toko K (2004) Biosens Bioelectron 19:1559–1563
Bao N, Wang J, Lu C (2008) Anal Bioanal Chem 391:933–942
Ehrfeld W (2003) Electrochim Acta 48:2857–2868
Golonka LJ, Roguszczak H, Zawada T, Radojewska J, Grabowska I, Chudy M, Dybko A, Brzozka Z, Stadnik D (2005) Sens Actuators B 111/112:396–402
Acknowledgment
This work was financially supported by the Ministry of Science and Higher Education, project no. N204 106 31/2448 (2006–2008). The authors thank Mr Piotr Żbikowski for assistance in LTCC structure fabrication. Patrycja Ciosek wishes to thank The Foundation for Polish Science for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciosek, P., Zawadzki, K., Łopacińska, J. et al. Monitoring of cell cultures with LTCC microelectrode array. Anal Bioanal Chem 393, 2029–2038 (2009). https://doi.org/10.1007/s00216-009-2651-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2651-x