Abstract
The enantioresolution of zolmitriptan was performed using cyclodextrin (CD)-modified capillary zone electrophoresis (CZE) with hydroxypropyl-β-CD (HP-β-CD) as the chiral selector. The influence of experimental conditions on the enantioseparation of zolmitriptan, such as pH, temperature, applied voltage, and concentrations of running electrolyte and CD, was systematically investigated, obtaining a baseline separation of two enantiomers by the use of a 25 mM sodium dihydrogen phosphate (SDPH) running electrolyte (pH 2.4) containing 30 mM HP-β-CD at 15 °C. Binding constants for each enantiomer–HP-β-CD pair at different temperatures, as well as thermodynamic parameters for binding, were calculated. A nonlinear van’t Hoff plot was obtained, indicating that the thermodynamic parameters of complexation were temperature-dependent for zolmitriptan enantiomers. The significant contribution of the enthalpy difference to the Gibbs free energy change suggested a stereomeric barrier mechanism for chiral recognition.

Resolution of zolmitriptan enantiomers was achieved by using CD-modified CZE
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
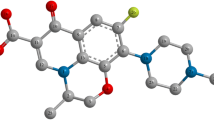
Migraine affects 18% of women and 6% of men. The significant impact of migraine results in a huge burden for the individual, health services, and society. Successful treatment of acute migraine attacks can reduce the use of healthcare resources and improve health-related quality of life [1]. The introduction of the triptans in the 1990s revolutionized the treatment of migraine, and a second-generation triptan, zolmitriptan, is highly effective in the oral treatment of acute migraine with or without aura. Zolmitriptan is a novel serotonin 5-hydroxytryptamine receptor agonist that inhibits the peripheral trigeminovascular system and is able to access central sites in the brainstem involved in processing cranial pain [2, 3]. Zolmitriptan is synthesized as the (S)-stereoisomer, because it is pharmacologically more potent than the toxic (R)-stereoisomer. So separation of zolmitriptan enantiomers (Fig. 1) is necessary for quality control of this drug and its related pharmaceutical and biological study.
High-performance liquid chromatography (HPLC) has been widely used for the quantitative determination of triptans with UV [4–6], fluorescence [7–10], coulometric detection [11, 12], and mass spectrometry (MS) [13–19]. Until now, HPLC [6, 10, 20], capillary electrochromatography (CEC) [21], and capillary zone electrophoresis (CZE) methods with sulfated β-CD as chiral selector [22, 23] have been employed for enantioseparation of zolmitriptan enantiomers.
Capillary electrophoresis (CE) is a powerful technique for enantioseparation, because of high separation efficiency, low reagent consumption, and the simplicity of the system used. Inclusion into the cavity of cyclodextrins (CDs) represents the most frequently used approach for enantioseparation by CE. The chiral recognition mechanism is based on inclusion of a bulky hydrophobic part of the molecules into the hydrophobic cavity of the CD. Secondary interactions are additionally required, including dipole–dipole interactions or hydrogen bonds between secondary hydroxyl groups around the cone opening and polar substituents close to the chiral center of the analyte.
CE is also a convenient technique for studying binding constants in separation systems without any adaptations and approximations, allowing the measurement of the binding constants under the exact conditions of enantioseparation [24–35]. CE also allows one to determine thermodynamic parameters [31, 32, 36–43] and to observe enantioselective recognition in the selector–selectand pairs in which other techniques do not provide any indications [40–43].
The inclusion complexation eventually leads to the improvement of drug solubility and stability, the enhancement of drug absorption, and the alleviation of local and systemic toxicity [44]. Chiral recognition of zolmitriptan with pyrrolidinylidenesulfamido-modified β-CDs has been studied by fluorescence-spectral titrations [45]. Hydroxypropyl-β-CD (HP-β-CD), a kind of water-soluble, safe, and bioadaptable drug carrier, is more desirable than other chemically modified CDs and has considerable pharmaceutical potential. The knowledge of binding constants and thermodynamic parameters of drug–HP-β-CD complexes helps to controlthe complexation equilibrium and thus to enhance drug bioavailability.
In this work, a CZE method with HP-β-CD as selector, instead of sulfated β-CD [23], was developed for the determination of zolmitriptan enantiomers and their binding constants with the chiral selector. Three linear plotting methods, which are actually applicable to zolmitriptan because of its completely ionized status under the operating conditions used (pK a = 9.6), were used and the van’t Hoff relationship was applied to compute the temperature dependence of the thermodynamic parameters. To investigate the chiral recognition mechanism, the differences between the molar Gibbs energy, enthalpy and entropy of the two enantiomers were determined. In addition, some related parameters were calculated, including the optimum CD concentration for enantioseparation, electrophoretic mobility of the fully complexed analyte, and the isoenantioselective temperature.
Experimental
Apparatus and conditions
All experiments were performed with an Agilent 3D CE system with air-cooling and diode array detector (Agilent Technologies, Palo Alto, CA, USA). A 48.5-cm (40.0 cm to the detector) × 50-μm-id uncoated fused silica capillary (Sino Sumtech, Handan City, Hebei, China) was utilized. Other conditions were capillary temperature 15 °C, applied voltage 25 kV, UV detection at 220 nm, and sample injection at 50 mbar for 5 s. The new capillary was washed with 1.0 M sodium hydroxide (15 min), water (20 min), and the running electrolyte (10 min) in turn. Between consecutive analyses, the capillary was flushed with 1.0 M sodium hydroxide (0.5 min), water (5 min) and the running electrolyte (5 min) sequentially to guarantee good reproducibility.
Chemicals
Zolmitriptan and (R)-enantiomer standards were kindly donated by Orient Lüyuan Co., Ltd. (Beijing, China). HP-β-CD was purchased from Acros Organics (97%, New Jersey, USA). Sodium dihydrogen phosphate dihydrate, phosphoric acid, and formamide were analytical grade from Beijing Chemical Factory (Beijing, China). Redistilled water was used for the preparation of solutions.
For the preparation of running electrolytes and standard solutions, please see Electronic supplementary material.
Results and discussion
Optimization of CD-modified CZE conditions
Optimization was performed to find operational conditions leading to satisfactory enantioseparations in short run times and permitting the study of the compound behavior. The experimental conditions were systematically optimized by investigating the effects of main CE parameters on separation as below.
Influence of pH
The pH of running electrolyte is the most important factor to optimize for the chiral resolution. A series of sodium dihydrogen phosphate (SDHP) running electrolytes at pH ranging from 2.0 to 4.5 were investigated in detail with 20 mM HP-β-CD in the running electrolyte. With increasing pH, the resolution (Rs) of enantiomers first increased and then decreased, while the resolution was not good enough at pH 2.0. The pH is responsible for the stabilities of the diastereomeric complexes formed between enantiomers and the chiral selector. It also affects the degree of ionization of the analytes and electroosmotic flow (EOF). Considering inaccuracies of the measurements and the time to achieve equilibrium on the silica, pH 2.4 afforded the maximum experimental resolution and is suitable for this chiral separation.
Effect of CD type and concentration
β-CD and HP-β-CD, which are both cheaper than sulfated β-CD, were tested under the same experimental conditions. HP-β-CD was chosen as the chiral selector because of better solubility and higher resolution (Fig. 2) than those of β-CD. The influence of HP-β-CD concentration, in the range 5–40 mM, on enantioseparation was investigated, showing that the resolution reached a maximum (2.21) at 30 mM and then decreased as shown in Fig. 3.
Enantioseparation comparison of β- and HP-β-CD as the chiral selector. Separation conditions: a 50-μm-id × 48.5-cm (40.0-cm effective) fused silica capillary, 25 mM SDHP running electrolyte containing 20 mM β-CD, applied voltage 20 kV, capillary temperature 20 °C, UV detection at 220 nm, injection at 50 mbar for 5 s; b same as a, except for 20 mM HP-β-CD; c same as a, except for 30 mM HP-β-CD, applied voltage 25 kV, capillary temperature 15 °C
Effect of SDHP running electrolyte
Experimental results showed that the chiral resolution and the migration time increased with increasing SDHP running electrolyte concentration from 15 to 50 mM. The baseline at 75 mM SDPH running electrolyte was unstable and there were no peaks eluted within 20 min. In terms of the current, peak shape, and migration time, 25 mM SDHP running electrolyte was considered to be the best (Fig. S1, Electronic supplementary material).
Effects of applied voltage and temperature
The effect of applied voltage on the separation was tested in the range 15–30 kV, indicating that higher applied voltage afforded lower resolution and separation efficiency, and 25 kV resulted in a better separation within reasonable analysis time.
Because the host–guest complexation mechanism is a kinetically driven process and there is potential Joule heating within the capillary under the usual voltage settings, temperature effects are crucial for CD-modified CZE. The optimization of capillary temperature was studied in the range 10–30 °C, showing that the resolution and separation efficiency increased with decreasing temperature. Considering the preferred high Rs and easy manipulation, a capillary temperature of 15 °C was chosen, at which the number of theoretical plates (N) was larger than 42,000. The dependence of enantioseparation on temperature is discussed in detail below.
Altogether, the most suitable CZE conditions for separation were 25 mM SDHP running electrolyte containing 30 mM HP-β-CD at pH 2.4, 50-μm-id × 48.5-cm (40.0-cm effective) fused silica capillary, applied voltage 25 kV, and capillary temperature 15 °C. A typical electropherogram obtained under these conditions is shown in Fig. 2c.
Quantitation of zolmitriptan enantiomers
Linear regression equations relating the concentration to peak area of zolmitriptan enantiomers using CZE are listed in Table 1, together with their correlation coefficients (R 2), which were higher than 0.99. The limit of detection (LOD) of each analyte is also listed in Table 1: the LODs were both 0.3 μg/ml. The method repeatability was proven by analyzing a mixed solution of 21 μg/ml for zolmitriptan and 19 μg/ml for the (R)-enantiomer, and relative standard deviations (RSDs) (n = 6) of migration time and peak area are shown in Table 1, illustrating that the method repeatability was acceptable.
Determination of enantiomer binding constants
Knowledge of binding constants of the inclusion complexes can help to elucidate the extent of analyte inclusion into the cavity of a CD. A theoretical model relating mobility to the concentration of the CD selector has been developed by Wren and Rowe [24, 25]. Chiral recognition depends on the difference between the electrophoretic mobilities of each free and complexed enantiomer. Binding constants can be determined by the following expression:
where K is the binding constant, [C] is the equilibrium concentration of uncomplexed ligand, μ f and μ c are the electrophoretic mobilities of the free and complexed solute, and μ i is the solute mobility at the ligand concentration [C].
Two procedures should be completed: (1) measuring of the mobilities for enantiomers at different concentrations of chiral selector; (2) using three linear plotting methods listed below to calculate the binding constants.
Double reciprocal plot of \(\frac{1}{{\mu _{\text{i}} - \mu _{\text{f}} }}\) versus \(\frac{1}{{\left[ {\text{C}} \right]}}\):
Y-reciprocal plot of \(\frac{{\left[ {\text{C}} \right]}}{{\mu _{\text{i}} - \mu _{\text{f}} }}\) versus [C]:
X-reciprocal plot of \(\frac{{\mu _{\text{i}} - \mu _{\text{f}} }}{{\left[ {\text{C}} \right]}}\) versus (μ i − μ f):
The mobilities of zolmitriptan enantiomers were measured at different temperatures. Formamide was used as a neutral marker to correct EOF changes.
Under these plotting forms, the direct measurement of μ c, which is difficult or impossible due to the difficulty of finding suitable CD markers and reaching saturated conditions, is not required. This approach also enables the calculation of μ c from the regression equations. As shown in Table 2, μ c increased with temperature, because the viscosity of the medium decreases and the frictional forces of the complexed species reduce.
Plots showing the effect of HP-β-CD concentration on solute mobilities at several temperatures are shown in Fig. 3. The lowest CD concentration needed for the measurement of solute mobilities at higher temperatures had to be enhanced for sufficient resolution. At the five temperatures checked for zolmitriptan enantiomers, electrophoretic mobilities decreased with increasing CD concentrations in all ranges tested. On the other hand, differences in electrophoretic mobilities of enantiomers, Δμ i, initially increased with increasing CD concentrations and decreased after a maximum was reached. These differences at 25 °C and 30 °C were larger than those at lower temperatures.
From the rearrangement of Eq. (1), μ i for each analyte can be calculated using the following expression:
As a result, theoretical Δμ i can be obtained. Maximum Δμ i between enantiomers occurs at the optimal CD concentration [C]opt [27, 39]. It is calculated by:
where K S and K R are the binding constants for both enantiomers. The effects of HP-β-CD concentration on experimental Δμ i, calculated Δμ i, and experimental resolution could be studied at different temperatures as shown in Fig. 3. Considering the isoelectric point of silica, all of experimental Δμ i values were smaller than the calculated values and the maximum of experimental Δμ i occurred at lower HP-β-CD concentration than [C]opt at 15 °C in agreement with the viscosity change caused by the variations in CD concentration [46]. On the other hand, maximal resolution was observed at higher CD concentration than maximal experimental and calculated Δμ i. Resolution is more complex because it must also consider EOF, band broadening due to diffusion, and other factors such as injection concentration and detector path length. So maximal resolution will not occur at the same concentration as those where maximal Δμ i values occur. Besides, a part of the observed variations must come from the inaccuracy of the measurements.
Table 2 lists the binding constants, enantioselectivities of complexation α (calculated as α = K R /K S ) at different temperatures using these linear plotting forms. Acceptable enantioselectivities of complexation were achieved (α ≥ 1.15). The double reciprocal fit for each complexation showed better linearity than those of Y- and X-reciprocal ones, because it masked deviations from linearity at low ligand concentrations [47]. So the double reciprocal fit was more reliable to assess analyte–ligand interaction. The thermodynamic parameters in this work were deduced from it.
Thermodynamic study of the complexation
Temperature dependence of the binding constants can be described as:
where ΔH° is the enthalpy change associated with inclusion complex formation, ΔS° is the corresponding entropy change, T is the absolute temperature, and R is the gas constant.
Usually, the Gibbs energy is known to be mainly dependent on the enthalpy of the complex formation, showing a linear relationship in the van’t Hoff plot. Nonlinearity indicates temperature dependence of the partitioning of analyte from the aqueous phase to the pseudostationary phase and entropy-controlled factors involved in complexation [36, 37].
At temperatures between 13 and 30 °C, distinct deviation from linearity in the van’t Hoff plot was observed for zolmitriptan enantiomers (data not shown). So the migration behavior of the complex was influenced significantly by the contribution of entropy to the CD complex formation. The entropy-controlled factors including the disarrangement of the solvation status of the CD and the loss of translational and rotational degrees of freedom during complex formation are attributed to temperature-controlled changes in the bulk liquid structure and viscosity [36, 40].
The Gibbs free energy ΔG° at different temperatures can be calculated by:
The negative ΔG° indicated that the complexation was thermodynamically favored. Besides, the inclusion into the cavity of HP-β-CD was favored for the (R)-enantiomer with more negative ΔG° in agreement with the migration order (data not shown).
Enantioselectivity (α) is dependent on the enthalpy difference (ΔΔH°) and the entropy difference (ΔΔS°) of the inclusion interaction following Eq. (6):
where ΔΔG° is the difference in the molar Gibbs energy of the two enantiomers.
A van’t Hoff plot of lnα versus 1/T (Fig. 4) showed an approximately linear relationship in the temperature range from 13 to 30 °C (R 2 = 0.8). An increase of temperature caused a decrease of separation factor. The aberrant values obtained at 15 °C for both K and µ c (S and R) explain this low value of the correlation coefficient. Table 3 summarizes the thermodynamic data from Fig. 3 according to Eqs. (6) and (7).
Usually, the Gibbs free energy of the inclusion complex is associated with a favorable proportion of the enthalpy and an unfavorable entropy proportion, as was applicable to the complex formation of HP-β-CD with zolmitriptan enantiomers. Its large negative TΔΔS° term represented an unfavorable influence of the entropy on ΔΔG° and thus on the separation. The entropy value is influenced by two main counteracting effects: (1) hydrate water surrounding host and guest becomes disordered in particular if lateral hydrogen bonds are formed during complexation; (2) there are losses in the translational and rotational degrees of freedom because of the association of host and guest [40]. For the complexation of HP-β-CD and zolmitriptan enantiomers, the loss of a degree of freedom dominated over the release of hydrate water. It also indicated that enantiomers formed only weak lateral hydrogen bonds with the host.
For enantiorecognition based on two mechanisms, namely, steric barrier and coulometric interactions, thermodynamic parameters of complexation should differ significantly [40]. Because negative ΔΔG° was mainly controlled by the contribution of ΔΔH°, the chiral separation of zolmitriptan enantiomers should be based on a steric barrier mechanism, not the electrostatic interaction characterized by the positive TΔΔS° value [40].
ΔΔH° and ΔΔS° are both negative which means that an isoenantioselective temperature (T iso) exits [42, 48]. T iso is the particular temperature at which ΔΔG° is nil with no enantiomer resolution. T iso is expressed by:
At temperatures higher than T iso, the enantiomer elution order should be reversed. Although the elution reversal was not observed, enantiometric resolution at temperatures close to the calculated T iso listed in Table 3 disappeared.
Conclusions
In this work, a CD-modified CZE method using relatively cheap HP-β-CD was established for the separation of zolmitriptan enantiomers. The developed method is simple, environmentally friendly, and involves low reagent consumption. Moreover, because all measurements are performed under defined conditions and in pure aqueous solutions, the method could be used to estimate the thermodynamic values with more precision. The binding constants of enantiomers were obtained using three linear plotting methods. The double reciprocal fit provided better reliability. The nonlinear plot of lnK versus 1/T suggested that the entropy-controlled factors dependent on temperature were involved in the complexation between zolmitriptan enantiomers and HP-β-CD. Because of the overwhelming contribution of ΔΔH° to ΔΔG°, the chiral recognition of zolmiotriptan enantiomers was based on a barrier mechanism. The calculations of related parameters such as μ c, [C]opt, and T iso have proven particularly useful to permit an additional insight into the enantioseparation.
References
Oldman AD, Smith LA, McQuay HJ, Moore RA (2002) Pain 97:247–257
Goadsby PJ, Hoskin KL (1996) Pain 67:355–359
Ahn AH, Basbaum AI (2005) Pain 115:1–4
Cooper JDH, Muirhead DC, Taylor JE (1999) J Pharm Biomed Anal 21:787–796
Srinivasu MK, Rao BM, Sridhar G, Chandrasekhar KB, Kumar PR (2005) J Pharm Biomed Anal 39:796–800
Srinivasu MK, Rao BM, Sridhar G, Kumar PR, Chandrasekhar KB, Islam A (2005) J Pharm Biomed Anal 37:453–460
Chen J, Jiang XG, Jiang WM, Mei N, Gao XL, Zhang QZ (2004) J Chromatogr B 805:169–173
Ge Z, Tessier E, Neirinck L, Zhu Z (2004) J Chromatogr B 806:299–303
Chen J, Jiang XG, Jiang WM, Mei N, Gao XL, Zhang QZ (2004) J Pharm Biomed Anal 35:639–645
Yu LS, Yao TW, Ni SQ, Zeng S (2005) Biomed Chromatogr 19:191–195
Franklin M, Odontiadis J, Clement EM (1996) J Chromatogr B 681:416–420
Clement EM, Franklin M (2002) J Chromatogr B 766:339–343
Seaber EJ, Peck RW, Smith DA, Allanson J, Hefting NR, van Lier JJ, Sollie FAE, Wemer J, Jonkman JHG (1998) Br J Cli Pharmacol 46:433–439
Vishwanathan K, Bartlett MG, Stewart JT (2000) Rapid Commun Mass Spectrom 14:168–172
Zhang ZJ, Xu FG, Tian Y, Li W, Mao GG (2004) J Chromatogr B 813:227–233
Boulton DW, Duncan GF, Vachharajani NN (2003) Biomed Chromatogr 17:48–52
Guo JF, Zhang AJ, Zhao L, Sun XH, Zhao YM, Gao HZ, Liu ZY, Qiao SY (2006) Biomed Chromatogr 20:61–66
Chen XY, Liu D, Luan Y, Jin FD, Zhong DF (2006) J Chromatogr B 832:30–35
Kiliç B, Özden T, Toptan S, Özilhan S (2007) Chromatographia 66:S129–S133
Zhang DF, Xu XZ, Ma SS (2005) J Sep Sci 28:2501–2504
Wang HF, Zhu YZ, Lin JP, Yan XP (2008) Electrophoresis 29:952–959
He M, Xia ZN, Liu Y, Li SF, Xiang GR (2002) Chin J Phar Anal 22:438–440
Yang GL, Liu Y, Song XR, Chen Y (2003) Chin J Anal Chem 31:1149
Wren SAC, Rowe RC (1992) J Chromatogr A 603:235–241
Wren SAC, Rowe RC (1992) J Chromatogr A 609:363–367
Rundlett KL, Armstrong DW (1996) J Chromatogr A 721:173–186
Van Eeckhaut A, Boonkerd S, Detaevernier MR, Michotte Y (2000) J Chromatogr A 903:245–254
Martín-Biosca Y, García-Ruiz C, Marina ML (2000) Electrophoresis 21:3240–3248
Bellini MS, Deyl Z, Manetto G, Kohlíčková M (2001) J Chromatogr A 924:483–491
Shakalisava Y, Regan F (2006) Electrophoresis 27:3048–3056
Hsiao JY, Wu SH, Ding WH (2006) Talanta 68:1252–1258
Wu SH, Ding WH (2005) Electrophoresis 26:3528–3537
Rawjee YY, Staerk DU, Vigh G (1993) J Chromatogr A 635:291–306
Rawjee YY, Williams RL, Vigh G (1993) J Chromatogr A 652:233–245
Chankvetadze B (1997) Capillary electrophoresis in chiral analysis. Wiley, Chichester
Lindner W, Böhs B, Seidel V (1995) J Chromatogr A 697:549–560
Akbay C, Gill NL, Warner IM (2007) Electrophoresis 28:1752–1761
Sadlej-Sosnowska N (1996) J Chromatogr A 728:89–95
Penn SG, Bergström ET, Goodall DM, Loran JS (1994) Anal Chem 66:2866–2873
Kuhn R, Erni F, Bereuter T, Häeusler J (1992) Anal Chem 64:2815–2820
Rekharsky MV, Inoue Y (1998) Chem Rev 98:1875–1918
Danel C, Lipka E, Bonte JP, Goossens JF, Vaccher C, Foulon C (2005) Electrophoresis 26:3824–3832
Cabrera K, Lubda D (1994) J Chromatogr A 666:433–438
Uekama K, Hirayama F, Irie T (1998) Chem Rev 98:2045–2076
Kumar VP, Kumar PA, Suryanarayana I, Venkata Y, Nageswar D, Rao KR (2007) Helv Chim Acta 90:1697–1704
Vespalec R, Boček P (2000) J Chromatogr A 875:431–445
Rundlett KL, Armstrong DW (1997) Electrophoresis 18:2194–2202
Berthod A, Li W, Armstrong DW (1992) Anal Chem 64:873–879
Acknowledgement
This work is partially supported by NSFC, Grand No 20575003, 20675004 and Sino-German Cooperation Research Project, Grand No GZ364.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM
(PDF 182 KB)
Rights and permissions
About this article
Cite this article
Pang, N., Zhang, Z., Bai, Y. et al. A study of the interaction between enantiomers of zolmitriptan and hydroxypropyl-beta-cyclodextrin by capillary electrophoresis. Anal Bioanal Chem 393, 313–320 (2009). https://doi.org/10.1007/s00216-008-2446-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2446-5