Abstract
An amperometric immunosensor for the quantification of Staphylococcus aureus based on the coimmobilization of rabbit immunoglobulin G (RbIgG) and tyrosinase on a mercaptopropionic acid self-assembled monolayer modified gold electrode is reported. A competitive mode in which protein-A-bearing S. aureus cells and antiRbIgG labeled with alkaline phosphatase (AP) compete for the binding sites of immobilized RbIgG was used. Monitoring of the affinity reaction was carried out by the amperometric detection at –0.15 V of phenol generated in the enzyme reaction with AP, at the tyrosinase-modified electrode through the electrochemical reduction of the o-quinone formed. Optimization of the working variables, such as the immunosensor composition and incubation times, the applied potential, the working pH and the concentration of phenyl phosphate used as the AP substrate, was carried out. Under the optimized conditions, both the repeatability of the measurements and the reproducibility of the responses obtained with different immunosensors yielded relative standard deviation values for the steady-state current lower than 10%. The immunosensor showed a dynamic range from 4.4×105 to 1.8×107 S. aureus cells mL−1, with a detection limit of 1.7×105 cells mL−1. The limit of detection was remarkably improved by subjecting S. aureus cells to wall lysis by heat treatment. The value obtained was 2.3×103 cells mL−1, which is adequate for the monitoring of S. aureus contamination levels in some foodstuffs. As an application, milk samples spiked with bacteria at the 4.8×103 cells mL−1 level were analyzed.

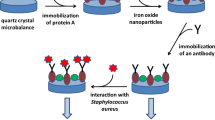
The immunosensor configuration. AP alkaline phosphatase, RbIgG rabbit immunoglobulin G, MPA mercaptopropionic acid
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus, one of the major pathogen organisms, constitutes the most common cause of suppurative infections such as boils, abscesses, wound infections, and even pneumonia, and toxic shock syndrome. It has also been reported as a major cause of food poisoning, accounting for 2.9% of hospitalizations due to foodborne pathogens in the USA during the 1990s. Therefore, detection of these bacterial contaminations in food is of high significance for public health protection [1, 2]. Because foodborne pathogens are related to health risks and economic losses, the development of rapid and sensitive detection systems is nowadays of great importance [3].
Traditional methods for bacterial detection have usually been based on plate incubation with the drawbacks of being time-consuming and the lack of precision. More recently, methods based on polymerase chain reaction (PCR), and in situ hybridization, have been proposed, allowing specific and rapid bacterial detection while eliminating the cultivation step. Still, PCR-based quantification shows low precision also involving extensive laboratory work. Moreover, many other innovative bacterial detection methods have been described but few have the potential for becoming standardized procedures [1].
Electrochemical immunosensors combine the high specificity of traditional immunochemical methods with the low detection limits that can be achieved with modern electrochemical systems. They are usually fabricated by immobilizing antibodies or antigens onto electrode surfaces through adsorption [4], covalent attachment [5] or polymer entrapment [6]. The crucial steps in their design are the choice of the electrode substrate and the immobilization of the immunoreagent onto the electrode surface [7]. Nowadays, enzyme-labeled immunoglobulins are typically used in the preparation of these sensors, the analyte quantification being achieved through either amperometric or potentiometric measurement of the enzyme-generated product. Alkaline phosphatase (AP) is a commonly used enzyme label in electrochemical immunoassays (EIAs) owing to its highly favorable characteristics such as high stability, high turnover, low cost, broad substrate specificity, and reactions basically free of interferences [8, 9]. Several electroinactive phosphate substrates like phenyl phosphate [10, 11], 1-naphthyl phosphate [12, 13] and 2-aminophenyl phosphate [14] have been used together with AP in EIAs to produce, respectively, phenol, 1-naphthol and 2-aminophenol following enzymatic hydrolysis [8]. Moreover, given the high potentials required for the direct oxidation of these compounds, tyrosinase electrodes have been proposed to enzymatically oxidize phenol and detect the quinone product at less positive potentials, also with the advantage of a further amplification of the signal via phenol recycling at the electrode surface [15].
On the other hand, self-assembled monolayers (SAMs) formed by the adsorption of short-chain alkanethiols such as mercaptopropionic acid (MPA) on gold electrodes have been shown to be useful for the design of robust enzyme [16, 17], and DNA [18, 19] electrochemical biosensors. Thiol-based SAMs have also been used for immobilization of antibodies, but when they are used in the detection of pathogen organisms, a low sensitivity is still obtained [20, 21]. Therefore, seeking novel immobilization strategies oriented to improve sensitivity and for application in real samples is highly desirable.
This paper reports on the development of an amperometric immunosensor for the quantification of S. aureus cells based on the coimmobilization of rabbit immunoglobulin G (RbIgG) and tyrosinase on a MPA-SAM-modified gold electrode. A competitive immunoreaction between protein-A-bearing S. aureus cells and antiRbIgG labeled with AP for the binding sites of immobilized RbIgG was accomplished, its monitoring being carried out by the electrochemical detection of phenol released upon hydrolysis of phenyl phosphate by AP.
Experimental
Apparatus and electrodes
Amperometric measurements were carried out with an ECO Chemie Autolab PSTAT 10 potentiostat (Herisau, Switzerland) using the software package GPES 4.9 (General Purpose Electrochemical System). A P-Selecta (Barcelona, Spain) ultrasonic bath and a P-Selecta agimatic magnetic stirrer were also used.
A XBAS-NS-AU gold disk electrode (diameter approximately 3 mm) was used as the electrode substrate to be modified with the MPA SAM. A BAS MF-2052 Ag/AgCl/KCl (3 M) reference electrode and a Pt wire counter electrode were also employed. A 5-mL glass electrochemical cell was used.
Reagents and solutions
A 2 M KOH (Panreac) solution, prepared in deionized water, was used for the pretreatment of the gold disk electrode. A 40 mM MPA (Research Chemicals) solution, prepared in a 75:25 v/v ethanol/water mixture, was employed for the formation of the SAMs. The solutions employed for the enzyme immobilization were a 46.8 U μL−1 tyrosinase (Sigma, EC 232-653-4 from mushroom, 5,370 U mg−1 solid) solution prepared in a 0.05 M phosphate buffer solution (pH 7.0), and a 25% glutaraldehyde solution (Scharlau). A 1 mg mL−1 IgG from rabbit serum (Sigma) solution was prepared in 150 mM NaCl. A 0.13 mg mL−1 antiRbIgG (γ-chain-specific)–AP conjugate (Sigma) and a 0.48% w/v S. aureus cells (Sigma; 10% wet w/v of essentially nonviable S. aureus Cowan strain cells in 0.04 M sodium phosphate buffer, pH 7.2, 0.15 M sodium chloride containing 0.05% sodium azide) solution were prepared in 0.05 M phosphate buffer solution (pH 7.0). More diluted S. aureus cells solutions were prepared in 5 mL of a 25 mM tris(hydroxymethyl)aminomethane (Tris) (Sigma, 99%) buffer solution containing 10 mM MgCl2·6 H2O (Merck), pH 8.5. A 1% bovine serum albumin (BSA) solution in 0.05 M NaH2PO4/Na2HPO4 containing 0.15 M NaCl and 0.05% Tween 20 (pH 7.0) was also used.
Phenyl phosphate was purchased from Sigma, and the solutions used as the enzyme substrate were prepared daily in the 25 mM Tris buffer solution containing 10 mM MgCl2·6 H2O (Merck) of pH 8.5. Semiskimmed milk samples were purchased in a local supermarket.
All chemicals used were of analytical-reagent grade, and water was obtained from a Millipore Milli-Q purification system.
Procedures
Preparation of the amperometric immunosensor
Firstly, the gold disk electrode was pretreated as described previously [22]. MPA SAMs were formed by immersion of the clean gold electrode in a 40 mM MPA solution in ethanol/water (75/25, v/v) for 15 h. Then, the modified electrode was rinsed with deionized water, and the surface was dried under a nitrogen stream. Coimmobilization of tyrosinase and the antibody was carried out by cross-linking with glutaraldehyde. The procedure was as follows. A 7-μL aliquot of the 46.8 U μL−1 tyrosinase solution was deposited on the MPA-modified gold electrode and left to dry at ambient temperature. Then two 5-μL drops of a 1 mg mL−1 RbIgG solution were also deposited on the electrode surface (waiting in-between drops for drying of the previous one at ambient temperature). Once the electrode surface had dried out at ambient temperature, it was immersed in a 25% glutaraldehyde solution for 1 h at 4 °C.
Immunoassay procedure
The immunoassay procedure is based on a competitive format involving different stages. The modification of the working electrode with tyrosinase and RbIgG was followed by a blocking treatment which consists of stirring the RbIgG–tyrosinase–MPA–gold electrode for 5 min in a 1% BSA solution in 0.05 M NaH2PO4/Na2HPO4 containing 0.15 M NaCl and 0.05% Tween 20 (pH 7.0) to prevent nonspecific adsorption of antiRbIgG–AP and S. aureus cells on the electrode surface. After that, the modified electrode was incubated for 20 min in 5 mL of a 25 mM Tris buffer solution containing 10 mM MgCl2·6 H2O (pH 8.5) and a standard solution with a variable quantity of S. aureus nonviable cells at concentrations ranging from 2.3×103 to 2.88×107 cells mL−1. Then the S. aureus–RbIgG–tyrosinase–MPA–gold electrode was stirred for 15 min at room temperature in a 5 mL solution of 25 mM Tris buffer solution containing 10 mM MgCl2·6 H2O (pH 8.5) and an aliquot (5 μL) of antiRbIgG–AP (0.13 mg mL−1).
Amperometric measurements
All measurements were carried out under room temperature. Amperometric measurements in stirred solutions were performed by applying the desired potential and allowing the steady-state current to be reached. Once prepared, the immunosensors were transferred to 5 mL of a 25 mM Tris buffer solution containing 10 mM MgCl2·6 H2O (pH 8.5) and the amperometric responses (E app = –0.15 V vs. Ag/AgCl) to the addition of 100 μL of a 0.01 M phenyl phosphate solution were recorded.
Staphylococcus aureus cells subjected to wall lysis by heat treatment
In order to partially extract protein A from the nonviable S. aureus cells, the appropriate portion of the standardized cell suspension, prepared in 25 mM Tris buffer solution containing 10 mM MgCl2·6 H2O (pH 8.5), was heated for 30 min in a boiling-water bath [23]. Then it was left to cool to ambient temperature and, after blocking with BSA as described previously, the RbIgG–tyrosinase–MPA–gold electrode was incubated in this solution for 20 min.
Milk analysis
S. aureus was determined in semiskimmed milk which was spiked with bacteria at the 4.8×103 cells mL−1 level. The spiked milk sample was heated for 30 min and left to cool to ambient temperature. After it had been blocked with BSA as described in “Immunoassay procedure,” the RbIgG–tyrosinase–MPA–gold electrode was incubated directly in the milk sample for 20 min.
Results and discussion
The proposed immunosensor design implies firstly coimmobilization of tyrosinase and RbIgG by cross-linking with glutaraldehyde on a MPA-modified gold electrode, which gave rise to three-dimensional biosensing aggregates. The immunosensor configuration is displayed in Fig. 1, where the relative sizes of the components are not drawn to scale in order to visualize all of them.
The immunochemical reaction is based on binding of protein-A-bearing S. aureus cells with the Fc regions of RbIgG [24]. The competitive immunosensing approach involved competition of S. aureus cells with the labeled derivative, antiRbIgG–AP, for the binding sites of the RbIgG immobilized on the electrode surface. Moreover, electrochemical monitoring of the immunological reaction was carried out using phenyl phosphate as the AP substrate. Phenol generated in the enzyme reaction was amperometrically measured at the tyrosinase-modified electrode through the electrochemical reduction of the o-quinone formed. Recycling between the tyrosinase substrate and the electroactive product allowed the amplification of the biosensor response, thus achieving a sensitive detection of AP [15] (Scheme 1).
Preliminary studies (not shown) demonstrated that nonspecific adsorption of antiRbIgG–AP and S. aureus cells occurred at a tyrosinase–MPA–gold electrode, leading to significant amperometric signals in the absence of RbIgG. Consequently, the electrode sites not modified with RbIgG were blocked with BSA. Both the BSA concentration (in the 0–1% range) and time of electrode immersion (0–5-min range) in this solution were optimized to minimize nonspecific adsorptions. The immersion of the modified electrode for 5 min in a 1% BSA solution was enough to achieve minimization of nonspecific adsorption onto the modified-electrode surface.
Optimization of the working variables
The influence of several parameters, such as the composition of the immunosensor, the applied potential and the working pH, was tested. Amperometry in stirred solutions was employed in all these studies.
Firstly, the tyrosinase loading was optimized taking as the criterion of selection the magnitude of the amperometric signals obtained at –0.15 V (vs. Ag/AgCl) after additions of 100 μL of a 0.01 M phenyl phosphate solution to 5 mL of a 25 mM Tris buffer solution containing 10 mM MgCl2·6 H2O (pH 8.5). These studies were carried out at a S. aureus cell level of 4.8×106 cells mL−1 with an incubation time of 5 min. As can be seen in Fig. 2, an increase of the amperometric signal with the tyrosinase loading occurred up to 327.6 U, after which a signal decrease was observed, which can be attributed to an increase of the resistance for high enzyme loadings giving rise to a sluggish electron transfer. Consequently, the above mentioned tyrosinase loading was selected for further work.
Effect of the tyrosinase loading in the integrated competitive immunosensor on the amperometric response for 2.0×10−4 M phenyl phosphate. Amount of immobilized RbIgG, 0.01 mg; Staphylococcus aureus cell level in the incubation solution, 4.8×106 cells mL−1; incubation time in the solution of S. aureus cells, 5 min; antiRbIgG–AP incubation solution concentration, 0.039 μg mL−1; incubation time in the antiRbIgG–AP solution, 30 min. Background solution, 25 mM tris(hydroxymethyl)aminomethane buffer containing 10 mM MgCl2·6 H2O (pH 8.5). E app = –0.15 V
Later, the dependence of the amperometric immunosensor response with the amount of antibody immobilized onto the electrode surface was evaluated. The current measured at –0.15 V increased with the antibody loading up to 0.010 mg (Fig. 3), after which it decreased significantly. This effect can be attributed to the nonconducting nature of RbIgG, which makes the electron transfer more difficult in the presence of large amounts of antibody on the electrode surface. Consequently, 0.010 mg RbIgG was always immobilized for subsequent work.
Effect of the RbIgG loading immobilized in the integrated competitive immunosensor on the amperometric response for 2.0×10−4 M phenyl phosphate. Tyrosinase loading, 327.6 U; other conditions as for Fig. 2
Furthermore, the influence of the incubation time in the solution of S. aureus cells was also optimized. This time must be enough to ensure that as many S. aureus cells as there are in the solution are bound to the immobilized antibody on the electrode surface. Figure 4 shows that, as expected, an increase in the loading of S. aureus cells produced a decrease in the amount of antiRbIgG–AP attached to the RbIgG immobilized onto the electrode surface. Accordingly, the longer the incubation time in the analyte solution, the lower the amperometric signals that were recorded, with a leveling off for incubation times longer than 20 min. This incubation time was then chosen for further studies because it is sufficient for binding of all S. aureus cells in solution.
Effect of the integrated immunosensor incubation time in the solution of S. aureus cells on the amperometric response for 2.0×10−4 M phenyl phosphate. Tyrosinase loading, 327.6 U; other conditions as for Fig. 2
The incubation time of the immunosensor in the antiRbIgG–AP solution as well as the concentration of this solution were also evaluated. The effect of the antiRbIgG–AP concentration was checked after immersion of the immunosensor in a 4.8×106 cells mL−1 S. aureus solution for 20 min. The current increased with the conjugate concentration, showing a leveling off for concentrations higher than 0.13 μg mL−1 (data not shown). Furthermore, when the effect of the incubation time in a 0.13 μg mL−1 antiRbIgG–AP solution was tested from 0 to 30 min, it was observed that the equilibrium was reached (i.e., longer incubation times did not result in any increase in the signal obtained) after 15 min, and, therefore, these incubation conditions were selected.
The immunosensor functioning implies the coupling of two enzyme reactions, one of them involving AP to generate phenol, and the other one the catalytic oxidation of phenol by tyrosinase. The optimum working pH values for these enzyme reactions are different. The highest activity of the AP reaction is found to occur around pH 10, while tyrosinase has been reported to show its higher activity for phenol between pH 5.5 and 6.5 [25]. Therefore, the immunosensor response was influenced by these two effects (Fig. 5). As can be observed, the highest response was obtained for pH 8.5, and this value was selected for further work as a compromise between high AP activity and high sensitivity for the detection of the phenol generated.
Effect pH on the amperometric response for 2.0×10−4 M phenyl phosphate. Tyrosinase loading, 327.6 U; incubation time in the solution of S. aureus cells, 20 min; antiRbIgG–AP incubation solution concentration, 0.13 μg mL−1; incubation time in the antiRbIgG–AP solution, 15 min; other conditions as for Fig. 2
The influence of the applied potential on the phenyl phosphate amperometric response in the −0.30 to +0.20 V range was tested. As can be seen in Fig. 6, the cathodic current for the o-quinone generated increased rapidly at the antiRbIgG–AP–S. aureus–RbIgG-tyrosinase–gold electrode as the applied potential varied from +0.20 to –0.15 V, then it almost reached a steady state for more negative potential values. A working potential of –0.15 V was chosen as a compromise between good sensitivity and minimization in the number of potential interferents able to be reduced at the electrode surface.
Effect of the applied potential on the amperometric response for 2.0×10−4 M phenyl phosphate. Tyrosinase loading, 327.6 U; incubation time in the solution of S. aureus cells, 20 min; antiRbIgG–AP incubation solution concentration, 0.13 μg mL−1; incubation time in the antiRbIgG–AP solution, 15 min; other conditions as for Fig. 2
Finally, the concentration of phenyl phosphate used as the AP substrate was optimized. As expected, the measured current increased with the substrate concentration, leading to a saturation region from 1.0×10−3 M. Although the use of a substrate concentration in the saturation region is desirable to ensure that the enzyme reaction rate depends only on the enzyme concentration, the use of high concentrations of phenyl phosphate produced significant background currents in the absence of AP. These currents have been attributed to traces of free phenol in the phenyl phosphate solution likely due to the spontaneous hydrolysis of this reagent [26]. Therefore, in order to obtain a high signal-to-background ratio, a phenyl phosphate concentration of 2×10−4 M was employed.
Immunosensor stability
Different aspects concerning the stability of the constructed immunosensors were considered. Firstly, the repeatability of the measurements was evaluated by performing ten successive amperometries under the optimized experimental conditions for 4.8×106 S. aureus cells mL−1 using the same immunosensor. The relative standard deviation (RSD) value for the steady state current of 8.4% showed an acceptable repeatability of the amperometric responses with no need to apply a cleaning or pretreatment procedure between measurements.
Furthermore, the reproducibility of the responses obtained with different immunosensors was also tested. Results for five different immunosensors yielded a RSD value for the amperometric responses of 9.1%, thus demonstrating that the immunosensor fabrication procedure was reliable, and that reproducible amperometric responses can be obtained with different immunosensors constructed in the same manner.
Analytical characteristics
Once the variables involved both in the immunosensor preparation and in the immunoassay procedure had been optimized, a calibration curve for S. aureus, expressed as the number of S. aureus cells per milliliter, was constructed. The responses obtained were normalized according to the expression
where i is the amperometric signal measured for a given analyte concentration, \(i_\infty \) is the current measured in the presence of an excess of S. aureus cells, and i 0 is the background current in the absence of analyte. The calibration curve obtained is displayed in Fig. 7 (filled circles). This calibration curve shows a decrease in the amperometric signal when the concentration of S. aureus increases, which corresponds to the competitive configuration used. An increase in the number of S. aureus cells results in less possibility for antiRbIgG–AP to compete efficiently for the antibody binding sites, thus resulting in a decrease in the amperometric signals obtained. The sigmoidal curve fitted to a four-parameter logistic equation:
where i max is the asymptotic maximum current in the absence of analyte, b is the slope at the inflection point, IC50 is the analyte concentration at the inflection point (concentration giving 50% decrease of i max), and i min is the asymptotic minimum. The IC50 value corresponded to 2.6×106 cells mL−1, and the limit of detection, calculated as the analyte concentration for which the tracer binding to the antibody was inhibited by 10%, was 1.7×105 cells mL−1. The immunosensor showed a dynamic range (normalized signal in the 20–80% range) from 4.4×105 to 1.8×107 cells mL−1.
Calibration curves obtained for S. aureus using the optimized competitive immunosensor without cell lysis (filled circles), and after cell lysis by heat treatment for standard solutions (open circles) and spiked semiskimmed milk samples (triangles). Tyrosinase loading, 327.6 U; incubation time in the solution of S. aureus cells, 20 min; antiRbIgG–AP incubation solution concentration, 0.13 μg mL−1; incubation time in the antiRbIgG–AP solution, 15 min; other conditions as for Fig. 2
The limit of detection obtained can be considered as rather good taking into account that neither amplification processes nor cell lysis is involved, and it is similar to that achieved with previous immunosensors [21]. However, the immunosensor design reported here exhibits a significantly broader concentration dynamic range.
In order to improve the sensitivity obtained, S. aureus cells were previously subjected to wall lysis by heat treatment (see “Experimental”). This process produced the breaking of bacteria and the availability of many more protein-A-bearing cell portions [27]. Moreover, their diffusion towards the antibodies immobilized on the sensing surface was also improved. These treated S. aureus cells were subsequently immobilized following the same procedure described above for nontreated cells. The calibration graph obtained with these lysed cells is displayed in Fig. 7 (open circles). In this case, a dynamic range from 4.0×103 to 4.6×104 cells mL−1 was obtained, as well as a limit of detection of 2.3×103 cells mL−1, and an IC50 value of 1.1×104 cells mL−1. As expected, the limit of detection was remarkably lower (2 orders of magnitude) than that obtained without cell lysis, which can be attributed to the lysis effects commented on above that also lead to a lower steric hindrance. Using this process, the limit of detection obtained is sufficient for the detection of contamination by S. aureus tolerated in many countries in most foodstuffs [28, 29].
The performance of the amperometric immunosensor developed was compared with those provided by other S. aureus sensors and immunoassay methods reported in the literature. As can be seen in Table 1, in general, electrochemical detection approaches offer a better sensitivity than piezoelectric and fluorimetric detection modes. Furthermore, the S. aureus immunosensor developed in this work can be favorably compared with other electrochemical immunosensors or immunoassays described in the literature. In fact, the immunosensor shows one of the lowest detection limits reported for this microorganism till now. However, given that the maximum acceptable levels legislated for S. aureus in some foodstuffs are lower than 103 cells mL−1 (absence, 10 or 102 cfu g−1, these values depending on the nature of the foodstuff [28]), research following these approaches in order to improve sensitivity is under progress in our laboratory to find applicability in real food samples.
Analysis of spiked milk
As commented on earlier, the determination of S. aureus in semiskimmed milk was accomplished using the immunosensor developed. In order to do this, milk samples were spiked at a 4.8×103 cells mL−1 concentration level. Then, the samples were heated as described in “Milk analysis” to achieve bacteria cell lysis and, consequently, to improve the sensitivity of the determination. Following the same method, a calibration graph for S. aureus in milk was constructed over the 1.2×103–4.8×106 cells mL−1 range (Fig. 7). As can be seen, this calibration plot is similar to that obtained with S. aureus standards prepared in buffer solution, thus indicating that direct S. aureus determination in milk, with no need for dilution or sample treatment, is feasible. The calculated limit of detection in milk, according to the criterion described above, was 1.9×103 cells mL−1, which is very similar to that obtained with standards. Analysis of three milk samples in triplicate, spiked as described earlier, yielded S. aureus contents of 5.3×103, 5.1×103 and 4.6×103 cells mL−1. The mean value is (5.0 ± 0.9)×103 cells mL−1, the confidence interval having been calculated for α = 0.05. The mean recovery is (105 ± 19)%, which, taking into account the type of analysis carried out, can be considered as a very good result, thus showing fairly well the usefulness of the immunosensor to be used in the analysis of real samples.
Conclusions
The new amperometric immunosensor developed for the quantification of S. aureus cells, based on the coimmobilization of RbIgG and tyrosinase on a MPA-SAM-modified gold electrode, exhibits a good analytical performance in terms of reproducibility, sensitivity and easiness of application. By implementation of cell wall lysis by heat treatment the limit of detection can be significantly improved (2 orders of magnitude lower), thus demonstrating the possibility to detect and quantify the target microorganism in moderate or heavily contaminated samples. This was proved by analyzing milk samples which were spiked at a 4.8×103 cells mL−1 concentration level. However, further improvement of sensitivity should be sought, if lower degrees of contamination in some foodstuffs are to be detected.
References
Chen L, Deng L, Liu L, Peng Z (2007) Biosens Bioelectron 22:1487–1492
Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV (1999) Emerg Infect Dis 5:607–625
Hassen WM, Abdelghani A, Vonna L, Cherif K, Boussaid M, Maaref MA (2007) Sens Actuators B 120:621–627
Dan D, Xiaoxing X, Shengfu W, Aidong Z (2007) Talanta 71:1257–1262
Subramanian A, Irudayaraj J, Ryan T (2006) Biosens Bioelectron 21:998–1006
Gupta R, Chaudhury NK (2007) Biosens Bioelectron 22:2387–2399
Díaz-González M, González-García MB, Costa-García A (2005) Biosens Bioelectron 20:2035–2043
Wilson MS, Rauh RD (2004) Biosens Bioelectron 20:276–283
Ruan C, Li Y (2001) Talanta 54:1095–1103
Santandreu M, Cespedes F, Alegret S, Martinez-Fabregas E (1997) Anal Chem 69:2080–2085
Hart JP, Pemberton RM, Luxton R, Wedge R (1997) Biosens Bioelectron 12:1113–1121
Wang J, Pamidi PV (1998) Anal Chem 70:1171–1175
Fernandez-Sanchez C, Costa-Garcia A (1997) Biosens Bioelectron 12:403–413
Pemberton RM, Hart JP, Foulkes JA (1998) Electrochim Acta 43:3567–3574
Bauer CG, Eremenko AV, Ehrentreich-Förster E, Bier FF, Makower A, Halsall HB, Heineman WR, Scheller FW (1996) Anal Chem 68:2453–2458
Campuzano S, Loaiza OA, Pedrero M, Manuel de Villena FJ, Pingarrón JM (2004) Bioelectrochem 63:199–206
Campuzano S, Pedrero M, Pingarrón JM (2005) Talanta 66:1310–1319
Loaiza OA, Campuzano S, López-Berlanga M, Pedrero M, Pingarrón JM (2005) Sensors 5:344–363
Loaiza OA, Campuzano S, Pedrero M, Pingarrón JM (2007) Talanta 73:838–844
Susmel S, Guilbault GG, O’Sullivan CK (2003) Biosens Bioelectron 18:881–889
Escamilla-Gómez V, Campuzano S, Pedrero M, Pingarrón JM (2007) Electroanal 19:1476–1482
Campuzano S, Gálvez R, Pedrero M, Manuel de Villena FJ, Pingarrón JM (2002) J Electroanal Chem 526:92–100
Mirhabibollahi B, Brooks JL, Kroll RG (1990) Appl Microbiol Biotechnol 34:242–247
Rishpon J, Ivnitski D (1997) Biosens Bioelectron 121:195–204
Serra B, Morales MD, Reviejo AJ, Hall EH, Pingarrón JM (2005) Anal Biochem 336:289–294
Pemberton RM, Hart JP, Mottram TT (2001) Biosens Bioelectron 16:715–723
Taylor AD, Yu Q, Chen S, Homola J, Jiang S (2005) Sens Actuators B 107:202–208
Le Loir Y, Baron F, Gautier M (2003) Genet Mol Res 2:63–76
Moragas-Encuentra M, Pablo-Busto MB (2007) Recopilación de mormas microbiológicas de los alimentos y asimilados y otros parámetros físico-químicos de interés sanitario. http://cvu.rediris.es/pub/bscw.cgi/d311175/Normicro/Recopila/normicro.htm
Le D, He FJ, Jiang TJ, Nie L, Yao S (1995) J Microbiol Methods 23:229–234
Su FY, Endo Y, Saiki H, Xing XH, Ohmura N (2007) Biosens Bioelectron 22:2500–2507
Mirhabibollahi B, Brooks JL, Kroll RG (1990) Lett App Microbiol 11:119–122
Mirhabibollahi B, Brooks JL, Kroll RG (1990) J App Bacteriol 68:577–585
Acknowledgements
The financial support of project CTQ2006-02743BQU is gratefully acknowledged. V.E.-G. acknowledges a pre-PhD fellowship from the Spanish Ministerio de Ciencia y Tecnología.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escamilla-Gómez, V., Campuzano, S., Pedrero, M. et al. Immunosensor for the determination of Staphylococcus aureus using a tyrosinase–mercaptopropionic acid modified electrode as an amperometric transducer. Anal Bioanal Chem 391, 837–845 (2008). https://doi.org/10.1007/s00216-007-1810-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1810-1