Abstract
The use of Vetiveria zizanioides (vetiver) was studied to evaluate its efficiency for the remediation of soils contaminated by heavy metals. Vetiver plants were tested for Cr, Cu, Pb and Zn. Phytoextraction and bioremediation experiments were carried out by irrigating the vetiver plants and the dry plants with solutions containing suitable amounts of Cr, Cu, Pd and Zn. The concentrations of the heavy metals were determined in both experiments in shoot and root parts of vetiver plants using inductively coupled plasma atomic emission spectroscopy after a mineralization step. Phytoextraction experiments showed a poor efficiency of vetiver for Cr and Cu uptake (both less than 0.1% in shoots and roots after 30 days), but a quite high capability of Pb and Zn uptake (0.4% in shoots and 1% in roots for Pb and 1% both in shoots and in roots for Zn, after 30 days). For these reasons the vetiver plant can be considered a quite good “hyperaccumulator” only for Pb and Zn. As for bioremediation experiments, the vetiver plant showed heavy metal uptake values significantly lower than those obtained with other biological substrates.

Vetiver plant
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils are generally contaminated with heavy metals as the result of numerous anthropogenic activities, primarily associated with industrial processes, manufacturing and disposal of industrial and domestic refuse and waste materials [1]. Soils and water contaminated with heavy metals represent a serious environmental and human health problem—owing to the possible transfer to the food chain—that needs an effective technological solution. Most current methods to remediate heavy metal contaminated soils consist of labor-intensive and costly excavation, landfilling and leaching technologies [2]. These methods are generally very expensive and cannot always be applied.
Phytoremediation is considered an innovative, economical and environmentally compatible method for heavy metal remediation [2, 3]. Phytoremediation is a general term which refers to the use of plants for environmental cleanup. It can be divided into (1) phytoextraction, in which heavy metal accumulating plants are used to transfer and concentrate metals from the soil into the roots and the above shoots [4]; (2) rhizofiltration, in which plant roots absorb and concentrate heavy metals from the soil [5]; (3) phytostabilization, in which plants stabilize the pollutants in soils, reducing their mobility into the soil and therefore the risk of leaching into ground water. Phytoextraction also includes bioremediation, in which plant roots together with their rhizospheric microorganisms are used to remediate contaminated soils with organics and the air-purifying uses of some plants [6]. The phytoremediation technique has a lot of advantages: it is a natural, in situ, low-cost and nondestructive technique which totally preserves the natural state of the environment [7–12].
Vetiver grass (Vetiveria zizanioides) is a perennial grass with strong ecological adaptability and large biomass. It is easy to manage and it grows in different soil conditions. Vetiver, owing to these unique morphological and physiological characteristics, is commonly known for its effectiveness in erosion and sediment control [13] and for its tolerance to extreme soil conditions, including heavy metal contamination [14]. Because of these characteristics, vetiver grass has, in principle, great potential in phytoremediation of heavy metal contaminated soils [15, 16].
The present study investigates the efficiency of vetiver grass in phytoextraction of Cr, Cu, Pb and Zn. The aim is to establish whether this plant can be considered a good hyperaccumulator of the above mentioned heavy metals and whether it can be successfully applied to the remediation of heavy metal contaminated soils.
Experimental
This study was performed as a laboratory experiment. Four vetiver plants were used to investigate the uptake of Cr, Cu, Pb and Zn.
CrCl3, CuCl2, PbCl2 and ZnCl2 were obtained from Carlo Erba (Milan, Italy). A proper amount of each salt was dissolved in distilled water to obtain the following heavy metal solutions: 623 ppm Cr, 190 ppm Cu, 621 ppm Pb and 653 ppm Zn. These concentrations were choosen because they are reported in the literature as the lowest toxic levels in soils [13]. Higher concentrations of heavy metals could cause toxicity symptoms, including death of the vetiver plants during experiments.
All solutions were prepared with high-purity water produced by a Milli-Q system.
Phytoextraction experiments
Vetiver plants in pots were irrigated daily with 250 ml of the heavy metal solutions for 30 days. The shoot and root parts of the vetiver plants were harvested every day during the first week (short-term experiment) and every 5 days up to 30 days (long-term experiment). Shoot and root samples (1 g each) were carefully rinsed with distilled water and successively cut in small pieces and dried in an oven at 70 °C for 2 h to obtain a stable dry weight. All dried parts were dissolved in 8 ml distilled water and digested with 2 ml HNO3 and 2 ml H2O2 using a microwave mineralizator (CEM-MARS 5). The digested liquid was filtered with a 0.5-μm nitrocellulose membrane filter and the filtrate was analyzed for heavy metals by inductively coupled plasma (ICP) spectrometry (Varian Vista-MPX CCD simultaneous ICP optical emission spectrometer). For the analytical determinations of the heavy metals the following wavelengths were used: 267 nm for Cr, 213 nm for Cu, 220 nm for Pb and 260 nm for Zn. Reference solutions were prepared by diluting the 1,000 mg/l standard solutions with 2% HNO3.
Heavy metal accumulation in each part of the plant was determined as the weight (milligrams) of heavy metal per kilogram of dry plant (parts per million).
Five samples of shoots and roots were prepared and analyzed.
Bioremediation experiments
Vetiver plants were cleaned with water, cut and separated into roots and shoots. These parts were immersed in 250 ml of heavy metal solutions for 30 days. Shoot and root samples (1 g each) were taken and analyzed every day during the first week (short-term experiment) and every 5 days up to 30 days (long-term experiment).
The samples were rinsed with distilled water, cut in small pieces, dried in an oven at 70 °C for 2 h and successively dissolved in 8 ml distilled water and digested with 2 ml HNO3 and 2 ml H2O2 and analyzed for their heavy metal content by ICP spectrometry, as previously mentioned.
Five samples of shoots and roots were prepared and analyzed.
Blank experiments were carried out for both phytoextraction and bioremediation studies. They consisted of the analysis of shoot and root samples of a vetiver plant not treated with the metal solution. Each measurement was repeated five times.
Desorption experiments
After the test at 30 days for the phytoextraction and bioremediation experiments, vetiver plants and the vetiver samples were rinsed with distilled water daily for 9 days and analyzed daily for heavy metals by ICP spectrometry.
Cyclic voltammetry experiments
The solutions of Cr, Cu, Pb and Zn in three different solvents, distilled water with 0.1 M sodium perchlorate as supporting electrolyte, extract of shoots and extract of roots, were analyzed by cyclic volatmmetry using a computer-controlled potentiostat (Amel 433, Milan, Italy), a graphite electrode as the working electrode (3-mm diameter), a platinum disk and a Ag/AgCl/KCl(sat) as counter and reference electrodes, respectively. The electrochemical cell was an one-compartment cell with an internal volume of 10 ml. All potentials are referred to the Ag/AgCl/KCl(sat) electrode. The cyclic voltammetry was carried out by varying the potential between -0.5 and -1.5 V. The graphite surface was polished with 0.003 μm alumina powder, sonicated in water for 10 min and then washed with water. All measurements were carried out at 20.0 ± 0.2 °C using a thermostatic bath. All solutions were carefully deaerated before analysis and maintained under a nitrogen atmosphere during the voltammetry experiments.
The extracts of shoots and roots were prepared as follows: 1 g of shoots or roots was carefully cut in small pieces, diluted to 250 ml with distilled water with 0.1 M sodium perchlorate and left at 4 °C for 10 days. The solutions were then filtered and used for the preparation of the heavy metal solutions.
Results and discussion
Phytoextraction experiments
Short-term experiments
Figure 1 shows the heavy metal uptake of vetiver plants both in shoots and roots when irrigated daily with solutions of 623 ppm Cr (Fig. 1a), 190 ppm Cu (Fig. 1b), 621 ppm Pb (Fig. 1c) and 653 ppm Zn (Fig. 1d). The results show that the vetiver plant can take up large amounts of Pb and Zn (Fig. 1c,d) but only very low amounts of Cr and Cu (Fig. 1a,b), both in the shoot and in the root parts of the plant. The Pb uptake by shoots is higher than 0.1% (dry weight) from the fourth day and about 3 times (approximately 0.3%) higher by roots after the same time. The Zn uptake is even higher, about 0.38 and 0.61% after 8 days in shoots and roots, respectively. As the term “hyperaccumulator” is used for a plant capable of accumulating in the shoots concentrations of trace metals of at least 0.1% (dry weight) with the exception of Zn, for which the concentration must be about 1%, from the results obtained it is possible to consider the vetiver plant as a quite good hyperaccumulator only for Pb and Zn.
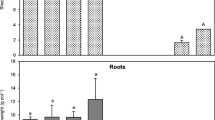
Figure 2 shows a comparison between the heavy metal uptake in shoots and roots. The results obtained clearly indicate that vetiver can take up heavy metals preferably in the roots, with the only exception being Cu.
Long-term experiments
Figure 3 show the heavy metal uptake of vetiver when irrigated daily with the same solutions used in the short-term experiments but harvested every 5 days for a period of 30 days.
As already observed in the short-term experiments, the vetiver plant is able to take up only very low concentrations of Cr and Cu but very high concentrations of Pb and Zn are accumulated both in shoots and in roots. The Cr uptake is much lower than 0.1% both in shoots and in roots after 30 days (Fig. 3a). Similarly, the Cu uptake is slightly higher but is always lower than 0.1% after 30 days both in shoots and in roots of the Vetiver plant (Fig. 3b). The other heavy metals studied showed the opposite behavior: the results indicate a very large Pb and Zn uptake, with values higher than 1% from day 25 in the roots for Pb (Fig. 3c) and both in the shoots and in the roots for Zn (Fig. 3d).
These results seem to confirm the efficiency of vetiver as a hyperaccumulator for Pb and Zn and not for Cr and Cu, as already reported for the short-term experiments.
Once again, the results showed that the total amount of heavy metals accumulated in roots is much higher then in shoots for all the heavy metals tested, with the exception of Cu. Figure 4 shows clearly the comparison between shoot and root uptake.
It is interesting to note that the plots of the heavy metal content versus time show a linear increase up to 8–10 days. After this period the increase of the heavy metal content both in shoots and in roots becomes progressively lower. This behavior is particularly evident starting from day 20.
For this reason the plots of the heavy metal uptake versus time were approximated by linear equations for the short-term experiments and by nonlinear equations, in particular logarithmic curves, for the long-term experiments. The equations are reported in Table 1. It is possible to observe that these approximations are quite good, as almost all R 2 values were higher than 0.98. Moreover, the values of the intercept of the linear curves were in very good agreement with the blank values calculated before heavy metal irrigation for both shoots and roots. The difference between the intercept values and the blank values was always less than 6%.
Bioremediation experiments
Short-term and long-term experiments
Figures 5 and 6 show the heavy metal uptake of vetiver shoot and root samples immersed in the heavy metal solutions and analyzed daily within the first 8 days (short-term experiment, Fig. 5) and every 5 days up to day 30 (long-term experiment, Fig. 6). Very low amounts of heavy metals were taken up both by the shoots and by the roots within the whole period studied. It is important to note that these concentrations are much lower than those accumulated by other substrates, such as bacteria, fungi and algae [17].
As already observed for the phytoextraction experiments, it seems that vetiver takes up heavy metals preferably in roots compared with shoots for all the heavy metals tested. Figures 7 and 8 show these results for the short-term and long-term experiments, respectively.
The trend of the heavy metal uptake in the bioremediation experiments is similar to that already reported for the phytoextraction experiments. The heavy metal uptake increases almost linearly within the first 8–10 days. Then the increase becomes progressively less and reaches a steady state between day 25 and day 30.
Table 2 shows the equations of the linear and logarithmic curves which better approximate the experimental curves. R 2 values are quite good, and almost all are between 0.97 and 0.99.
Desorption experiments
Table 3 shows the heavy metal desorption in vetiver shoots and roots rinsed daily with distilled water for 9 days. A fast desorption is observed for Cr, Cu and Pb, with desorption values after 9 days between 40 and 59% in shoots and between 44 and 56% in roots. The rapidity of the desorption seems to confirm the hypothesis of an uptake process based on a physical mechanism.
Different behavior is registered for Zn, with a desorption value of 42% in shoots and of only 32% in roots. This result seems to indicate a possible chemical nature of the metal uptake.
Cyclic voltammetry experiments
In order to better understand the nature of the heavy metal uptake, cyclic voltammograms of solutions containing the same amount of heavy metals in three different solvents, distilled water, extract of shoots and extract of roots, were recorded.
For each of the first three heavy metals studied, Cr, Cu and Pb, the cyclic voltammograms obtained in the three different solvents were absolutely identical, showing cathodic peaks at the same potentials and with the same current intensities, independently of the used solvent. This result seems to confirm the physical nature of the Cr, Cu and Pb adsorption.
In contrast, the voltammograms obtained with Zn showed different behavior, depending on the solvent employed. In distilled water the cyclic voltammogram showed an irreversible voltammogram with a cathodic peak with E p = -1.34 V versus Ag/AgCl and an intensity i p ≈ 35 μA. No anodic peak was observed in the reverse scan. Both in shoot and in root extracts, the shapes of the voltammograms are similar but the cathodic peaks appeared with a slight cathodic shift (E p = -1.36 V vs. Ag/AgCl) and much lower intensities (i p ≈ 13 μA).
A possible explanation of the results obtained is that the Zn uptake by vetiver shoots and roots is based also on a chemical mechanism involving the formation of a complex between the metal and some chelating molecules within the plants. This hypothesis is largely confirmed by the literature where is reported that metal-chelating molecules (phytosiderophores) can be secreted into the rhizosphere to chelate and solubilize the “soil-bound” metals, in particular Fe and Zn [2].
Conclusions
The vetiver plant has been tested to evaluate the possibility of its use for heavy metal soil remediation.
Phytoextraction experiments showed that vetiver can be considered a hyperaccumulator of Pb and Zn, but quite low concentrations of Cr and Cu were accumulated both in the shoot and in root parts of the plant.
In contrast, bioremediation experiments showed a very low efficiency of vetiver for accumulating all the heavy metals tested both in shoots and in roots.
In both experiments the heavy metal accumulation in roots was always higher than that observed in shoots.
From the results obtained, it is possible to conclude that vetiver could, in principle, be successfully used for remediation of Pb- and Zn-contaminated soils. However, further research should be done in order to enhance the uptake rate of these metals and to study the possibility of improving the uptake of other metals by utilizing, for example, chelating agents.
However, it is important to note that the phytoremediation process can show, in principle, a decrease of the biomass production because of the strong inhibition of numerous enzymes by heavy metals with a consequent negative effect on some important physiological processes of the plants, such as chlorophyllous photosynthesis.
References
Nriago JO (1979) Nature 279:409–411
Salt DE, Blaylock M, Kumar N, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Bio/technology 13:468–474
Baker AJM, Brooks RR (1989) Biorecovery 1:81–126
Kumar PBAN, Dushenkiv V, Motto H, Raskin I (1995) Environ Sci Technol 29:1232–1238
Dushenkov V, Kumar PBAN, Motto H, Raskin I (1995) Environ Sci Technol 29:1239–1245
Anderson TA, Guthrie EA, Walton BT (1993) Environ Sci Technol 27:2630–2636
Baker AJM, McGrath SP, Sidoli CMD, Reeves RD (1994) Resour Conserv Recycl 11:41–49
Raskin I, Smith RD, Salt DE (1998) Curr Opin Biotechnol 8:221–226
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemosphere 41:229–234
Nedelkoska TV, Doran PM (2000) Miner Eng 13:549–561
McIntyre T (2003) Adv Biochem Eng/Biotechnol 78:97–123
Knight B, Zhao FJ, McGrath SP, Shen, ZG (1997) Plant Soil 197:71–78
Truong PN (2000) Invited paper presented at the Pollution Solutions seminar, Clear Lake, California, 10 May 2000
Kasetsart J (2001) Nat Sci 35:46–50
Chantachon S, Kruatrachue M, Pokethitiyook P, Upatham S, Tantanasarit S, Soonthornsarathool V (2004) Water Air Soil Pollut 154:37–55
Wilde EW, Brigmon RL, Dunn DL, Heitkamp MA, Dagnan DC (2005) Chemosphere 61:1451–1457
Volesky B, Holan ZR (1995) Biotechnol Prog 11:235–250
Acknowledgements
The financial support of the EU and of the Italian Ministry of Education University and Scientific Research is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antiochia, R., Campanella, L., Ghezzi, P. et al. The use of vetiver for remediation of heavy metal soil contamination. Anal Bioanal Chem 388, 947–956 (2007). https://doi.org/10.1007/s00216-007-1268-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1268-1