Abstract
Drug use by pregnant women has been extensively associated with adverse mental, physical, and psychological outcomes in their exposed children. This manuscript reviews bioanalytical methods for in utero drug exposure monitoring for common drugs of abuse in urine, hair, oral fluid, blood, sweat, meconium, amniotic fluid, umbilical cord tissue, nails, and vernix caseosa; neonatal matrices are particularly emphasized. Advantages and limitations of testing different maternal and neonatal biological specimens including ease and invasiveness of collection, and detection time frames, sensitivities, and specificities are described, and specific references for available analytical methods included. Future research involves identifying metabolites unique to fetal drug metabolism to improve detection rates of in utero drug exposure and determining relationships between the amount, frequency, and timing of drug exposure and drug concentrations in infant biological fluids and tissues. Accurate bioanalytical procedures are vital to defining the scope of and resolving this important public health problem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug use by pregnant women has been extensively associated with adverse mental, physical, and psychological outcomes in their exposed children [1–5]. With mounting evidence of drug-induced short- and long-term developmental effects, drug use during gestation is a significant public health concern. The National Survey on Drug Use and Health reported in 2005 that 9.8, 18.0, and 4.3% of pregnant women questioned admitted to drinking alcohol and using tobacco and illicit drugs within the last month, respectively [6]. Perhaps because of feelings of guilt, embarrassment, or fear of prosecution, mothers frequently underreport drug ingestion. Interviewing methods have been improved to better identify women at high risk for substance abuse during gestation and to generate more accurate responses to interview questions [7–9]. In spite of these efforts, underreporting remains a problem. One study has shown maternal interviews to be the least sensitive method of identifying drug use in pregnancy when compared to maternal hair and meconium drug testing [10]. Another recent study showed that 17% of women denying cocaine use had a positive maternal or newborn biological specimen [11]. Consequently, sensitive and specific bioanalytical methods are necessary to accurately measure biomarkers of in utero drug exposure. This review discusses advantages and limitations of monitoring various maternal and/or neonatal biological specimens for in utero drug detection, and briefly highlights the most commonly employed analytical techniques and instrumentation to isolate compounds of interest (Table 1). Analytical procedures specific for in utero drug detection in meconium, amniotic fluid, and umbilical cord blood and tissue will be described. Other maternal and neonatal specimens, i.e., blood, hair, oral fluid, sweat, and urine, may be analyzed by the same techniques and instrumentation as for clinical and workplace testing and post-mortem analysis.
Specimens for in utero drug exposure detection
Maternal monitoring
In utero drug exposure can be identified by testing biological specimens from the mother during gestation or the neonate shortly after birth. Identifying maternal drug use history has several advantages. Healthcare providers can prepare resources before delivery to handle withdrawal symptoms or effects that may manifest at birth and to extend hospitalization to detect neonatal withdrawal. For example, the average time to onset of withdrawal symptoms in a study of infants at a Dublin hospital was 2.8 days [12], while the average hospital stay for childbirth in 2003 was 2.6 days [13]; therefore, it is possible that babies are released prior to withdrawal onset. Further, social service resources to aid the mother in caring for her newborn at home can be established in advance. In addition, identifying women who are using drugs while pregnant provides the opportunity for enrollment in drug treatment. Methadone and buprenorphine pharmacotherapy and behavioral modification programs are associated with positive outcomes for both the mother and child [14, 15].
Currently, data correlating maternal drug use during pregnancy with concentrations of drug in maternal and fetal specimens and outcome measures after birth have not been available. Thus, we do not know if such correlations exist. Due to the highly complex nature of the maternal–fetal interaction, maternal drug and/or metabolite concentrations may not reflect the degree of fetal drug exposure. Notably, Boskovic et al. reported that a set of monozygotic twins had nearly identical concentrations of cocaine in hair, while in dizygotic twins, each twin had distinctly different hair concentrations [16]. Moreover, in three of six sets of dizygotic twins, one twin had hair concentrations below the detection limit while its sibling’s hair concentration was well above the limit of detection.
Several biological specimens of maternal origin are available for testing; however, each has advantages and disadvantages. Table 2 describes characteristics of each matrix including ease and invasiveness of collection, window of drug detection, and specimen preparation requirements.
While not indicative of in utero drug exposure, neonates may be exposed to drugs in infancy through breastfeeding, if the mother uses drugs while lactating. Recently, the United States’ National Library of Medicine released LactMed, a free online peer-reviewed database of drugs and other chemicals to which breastfeeding mothers may be exposed (http://toxnet.nlm.nih.gov). Fully referenced information derived from the scientific literature includes drug concentrations in breast milk and exposed infants’ blood, if data are available. Potential adverse effects in the nursing infant also are included. Analyzing drug concentrations in breast milk after controlled drug administration provides data for the development of pharmacokinetic models to estimate the amount of drug exposure for the child from breastfeeding [17].
Neonatal monitoring
Many neonatal specimens can be non-invasively collected shortly after birth to reveal in utero drug exposure. This is particularly important if the mother did not receive prenatal care. Analysis of neonatal specimens can identify type and perhaps the amount of drug exposure. Fetal metabolism is poorly understood, may be different than in adults, and unique fetal metabolites may not yet have been identified.
Urine
Urine was traditionally the specimen of choice for neonatal drug testing, although collection is difficult. The adhesive for the collection bag causes skin irritation and frequently fails to adhere. Another disadvantage is the short detection window; urine provides maternal drug use data only for a few days prior to delivery.
Meconium
Recently, meconium became the specimen of choice for detecting drug exposure in neonates. Meconium is primarily composed of mucopolysaccharides, water, bile salts, bile acids, epithelial cells, and other lipids. It begins to form around the twelfth week of gestation and accumulates until birth. Meconium acts as a drug reservoir and provides a long window of detection. Meconium usually is passed within the first one to three days of life, but defecation may be delayed in premature infants. Collection from diapers is easy and non-invasive. Ostrea et al. suggested using serial analyses to determine timing of exposure of xenobiotics [66]; however, this may be practical only in special circumstances (i.e., post-mortem examination). Drugs also may diffuse through the meconium in utero making interpretation difficult. Drug concentrations in meconium generally are higher than in urine because of accumulation over several months of gestation. Meconium is one of the most sensitive matrices to detect in utero drug exposure [10, 11, 67].
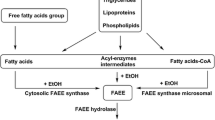
Frequently, meconium is contaminated with neonatal urine from the diaper, potentially complicating interpretation. As meconium is not homogenous, specimens should be mixed thoroughly before analysis. Meconium is complex and recovery of drugs is highly dependent on extraction technique. For the analysis of fatty acid ethyl esters (FAEEs) to identify ethanol consumption, freezing of meconium immediately after collection and shipping to the analytical laboratory on ice is recommended to slow degradation of FAEEs [68]. Improper storage or handling of specimens may produce false-negative results, particularly for the more susceptible long chain FAEEs. Generally, drug concentrations in meconium remain stable when stored at −20 °C; amphetamine and methamphetamine levels were stable when stored over one year [69] and cannabis-derived compounds for at least six months [70].
Another disadvantage of meconium analysis is the high false-positive rate of screening techniques, particularly immunoassay methods. Immunoassay techniques designed for urine have been modified for use with meconium, many times without extensive validation or confirmation. Of specimens screening positive for Δ9-tetrahydrocannabinol (THC) metabolites, cocaine metabolites, or opiates by immunoassay screening, 56–59% were confirmed by gas chromatography/mass spectrometry (GC/MS) [71]. Only 26% of samples screening positive for amphetamines were confirmed. Possibly there was cross-reactivity with other sympathomimetic amines in meconium or there may be as yet undetermined fetal amphetamine metabolites. Steele et al. demonstrated that m-hydroxybenzoylecgonine (mOHBE) is a major cocaine metabolite in meconium that is detected by immunoassay screens [72]. Later, it was shown that p-hydroxybenzoylecognine (pOHBE) also contributes to cocaine metabolite immunoassay reactivity [73]. A common nicotine metabolite detected in smokers’ plasma, urine, and oral fluid and in nicotine-exposed neonatal urine, trans-3′-hydroxycotinine, was not detected in meconium, illustrating that metabolic profiles in different neonatal matrices are not necessarily the same [74, 75]. Immunoassay results should be carefully interpreted and confirmed by a more specific method like GC/MS, liquid chromatography/mass spectrometry (LC/MS), or MS/MS.
Enzyme multiplied immunoassay test (EMIT) is the most commonly employed screening assay, but fluorescence polarization immunoassay (FPIA), radioimmunoassay (RIA), and enzyme-linked immunosorbent assay (ELISA) have also been utilized. Prior to immunoassay meconium screening, extraction of drugs must be performed to reduce endogenous specimen interferences. Moore et al. compared several extraction techniques including methanol, acidified water, phosphate buffer with methanol, and glacial acetic acid and diphenylamine in acetone [71]. The more complex glacial acetic acid and diphenylamine in acetone extraction yielded the best sensitivity; the other methods varied in ability to extract opiates, cocaine, and cannabis metabolites. Another procedure described using chloroform/isopropanol (3:1) to extract benzoylecgonine (BE), methamphetamine, morphine, and phencyclidine (PCP) for detection with EMIT [76]. Hydrolyzing meconium to cleave reversible Schiff base bonds that may form between cotinine and amino acids significantly increased cotinine detection by enzyme immunoassay from 33.3% without hydrolysis to 79.4% with hydrolysis [77].
Table 3 lists GC/MS, high-performance liquid chromatography (HPLC), LC/MS, and LC/MS/MS procedures for detection of cocaine and metabolites, cannabinoids, nicotine and metabolites, FAEEs, amphetamines, methadone, and PCP in meconium.
BE, ecgonine methyl ester (EME), mOHBE, and cocaine are the major cocaine analytes detected in meconium by GC/MS [73]. An LC/MS/MS method detected cocaine, anhydroecgonine methyl ester, BE, EME, cocaethylene, ecgonine, norcocaine, pOHBE, and p-hydroxycocaine in nearly all meconium specimens of cocaine-exposed neonates; ecgonine, though difficult to isolate, was present in higher concentrations than most other metabolites and may be an important analyte for monitoring in utero cocaine exposure.
Analysis of the THC metabolite 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) requires enzymatic hydrolysis to cleave the 11-OH-THC–glucuronide bond. Incorporating hydrolysis and 11-OH-THC and 8β,11-dihydroxy-Δ9-tetrahydrocannabinol analytes increased confirmation rates of positive-screening meconium specimens from 26 to 100% [78]. Recently, a method for THC, 11-OH-THC, and THCCOOH noted that enzymatic hydrolysis and three liquid–liquid extractions at different pHs were required to maximize recovery of analytes [70].
In utero tobacco exposure can be documented by identification of nicotine and metabolites in meconium. Ostrea et al. found statistically significant differences in mean cotinine concentrations between children of nonsmokers and active or passive smokers [74].
The incidence of women ingesting alcohol during pregnancy is high, despite the fact that exposure can cause a host of deleterious effects on the child commonly termed fetal alcohol spectrum disorder (FASD). Because alcohol is metabolized quickly, fetal urine only reflects maternal ingestion on the day or two before delivery. A more stable and longer detectable biomarker of ethanol exposure was needed to identify neonates at risk for FASD. The FAEEs ethyl linoate, ethyl palmitate, ethyl linoleate, ethyl stearate, and ethyl arachidonate have been suggested as biomarkers in meconium [79–81]. The sum of FAEEs above a cutoff of 2 nmol/g or 50 ng/g meconium was recommended as evidence of maternal alcohol use [68, 82]. FAEE is detected by organic solvent homogenization of meconium, SPE, and GC/MS or GC/flame ionization detection (FID) analysis [81, 83].
To date, there is no consensus on which FAEEs should be included in testing for ethanol exposure in meconium and no definitive cutoff concentrations to indicate positivity. It appears that there is fetal endogenous FAEE production, but the factors involved and quantities produced are unknown [82]. Klein et al. reported finding three FAEEs (ethyl laurate, ethyl myristate, and ethyl palmitoleate) in meconium of neonates whose mothers abstained from alcohol during pregnancy and in a neonate whose mother admitted to drinking beer throughout pregnancy. They also observed production of ethyl linoleate after spiking blank meconium with ethanol [80].
Choo et al. used LC/MS/MS to quantify methadone and its major metabolites in meconium; this method achieved a fivefold increase in sensitivity of methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, and 2-ethyl-5-methyl-3,3-diphenylpyraline compared to GC/MS [84].Additional procedures, described in Table 3, exist for the measurement of the opiates, oxycodone and hydrocodone in meconium [85, 86]. Hydrolyzing specimens significantly increases detected concentrations of codeine, hydrocodone, and hydromorphone, suggesting these analytes are significantly glucuronide-bound in meconium [86]. Morphine glucuronides, on the other hand, are present in low concentrations, prompting investigators to forego difficult and time-consuming secondary extractions or hydrolysis steps for morphine analysis [87, 88]. Hydrolysis of meconium for opiate analysis potentially interferes with identification of the heroin metabolite, 6-acetylmorphine (6-AM), by converting 6-AM to morphine. Meconium analysis has been used for other neurotoxicants including pesticides, metals, and xenobiotics [89].
Hair
Neonatal hair testing also can identify prenatal drug exposure. Hair begins to form at approximately six months gestational age; a positive result indicates use during the last trimester. Hair testing is advantageous because the specimen can be collected at any point during the first three months of life, after which time infant hair replaces neonatal hair. Specimens are easily and non-invasively collected and can be stored at room temperature. Drugs in hair could originate from deposition from fetal blood into the growing hair shaft or from contamination of hair by amniotic fluid. In either case, external contamination is not an issue for monitoring drug exposure in neonatal hair because the only source of drug is from maternal ingestion.
One major disadvantage of hair testing is the unwillingness of mothers to consent to hair collection from the babies for cosmetic or cultural reasons. Additionally, the neonate may not have sufficient hair for testing. As with maternal hair analysis, there is potential for color bias with basic drugs, and specimen preparation is complex.
Hair testing has identified alcohol, tobacco, cocaine, opioid, cannabinoid, benzodiazepine, barbiturate, and methamphetamine exposure [10, 16, 67, 97–101]. Hair cotinine concentrations have distinguished babies born to active and passive smokers, and nonsmokers [99, 102]. Methamphetamine, nicotine, and cotinine hair concentrations in paired maternal and neonatal specimens were well correlated [100, 102].
Recently, measurement of FAEE concentrations in hair has been suggested to identify in utero alcohol exposure. Caprara et al. found no statistically significant difference in the sum of ethyl laurate, ethyl myristate, ethyl palmitoleate, ethyl palmitate, ethyl oleate, and ethyl stearate concentrations in hair of newborns of alcohol-abstaining mothers and light social drinkers [103]. Unfortunately, babies of chronic and heavy drinkers were not included to compare FAEE concentrations in these groups. To date, there has been no large-scale study of hair FAEE concentrations from neonates of heavy drinkers.
Umbilical cord tissue and umbilical cord blood
A new alternative matrix for monitoring in utero drug exposure is umbilical cord tissue [104, 105]. Testing umbilical cord enables analysis to occur immediately after birth, in contrast to meconium testing that is delayed up to three days prior to specimen availability. Umbilical cord is easily and non-invasively collected and may reflect a long window of drug detection; however, because there have been few studies of cord tissue to date, it is difficult to interpret results.
A recent study monitored the presence of amphetamines, opiates, cocaine, and cannabinoids in umbilical cord and meconium [105]. Receiver-operating characteristic plots were constructed to determine cutoff values for the umbilical cord screening assay; cutoff levels for each analyte and specimen were not reported. Cord tissue appeared to be more sensitive for detecting amphetamines than meconium. Meconium analytical methods were adapted for umbilical cord. The method included homogenization in methanol, centrifugation, evaporation of supernatant, reconstitution in 0.1 M hydrochloric acid with 10% methoxyamine hydrochloride, addition of phosphate buffer, SPE with a mixed mode column, derivatization with BSTFA + 1% TMCS, and analysis by GC/MS [42]. Analysis of umbilical cord tissue for cocaine and its major metabolite benzoylecgonine was performed by homogenizing cord tissue, isolating analytes by SPE and detection by a HPLC/UV system [104]. Table 4 summarizes umbilical cord analytical methods.
Umbilical cord blood has identified in utero cotinine, cocaine, antidepressant, and cannabinoid exposure [11, 104, 106–108]. When compared to maternal serum, prescribed antidepressant parent compound and metabolite concentrations in cord serum were lower [106, 107]. To date, there are few data comparing umbilical cord blood to other matrices to guide interpretation of results. It is expected that the window of drug detection in cord blood will be short, as with maternal blood specimens.
Amniotic fluid
At the early stages of pregnancy, amniotic fluid consists of a filtrate of maternal blood. Drugs can enter amniotic fluid by diffusion across the placenta and from excretion of fetal urine in the latter stages of gestation. Jauniaux et al. reports detectable cotinine concentrations in amniotic fluid collected as early as seven weeks gestation in passive and active smokers [109]. Amniotic fluid acts as a fetal excretion reservoir, accumulating drugs throughout gestation. The fetus is potentially re-exposed to drugs excreted in urine due to continuous swallowing of amniotic fluid. Another possible route of exposure via amniotic fluid is transdermal diffusion, early in pregnancy when the skin is poorly developed and late in pregnancy when the production of vernix caseosa decreases. In a study comparing the presence of cocaine in amniotic fluid and meconium, comparable sensitivity was achieved for the two matrices at a cutoff of 2.5–50 ng/g, depending on the analyte, although the number of amniotic fluid specimens collected was too small to provide statistically significant results [11]. Another study reported detectable drug concentrations, but at lower levels than seen in maternal and umbilical cord blood [107]. Table 4 outlines analytical procedures for drugs of abuse in amniotic fluid.
The major disadvantage of amniotic fluid testing is collection. Amniotic fluid can only be non-invasively collected at birth or as excess specimen from another necessary medical procedure (i.e., amniocentesis). In general, amniotic fluid would not be collected for monitoring in utero drug exposure alone.
Fingernails and toenails
There is only one published report of analyzing fingernails and toenails specifically for prenatal drug exposure [110], although adult nail clippings are used for drugs of abuse testing [111]. Nails could reflect drug ingestion during the last trimester of pregnancy. Analysis of nails is complex because of the need to pulverize the specimen prior to extraction.
Vernix caseosa
Vernix caseosa is a thick, white lipid and cell mixture that covers the fetus starting at about 24 weeks gestational age. This coating prevents direct contact of the forming fetal skin with amniotic fluid. Vernix can easily be removed from a newborn’s skin with gauze prior to its first bath. Moore et al. described a procedure for analyzing cocaine and metabolites from vernix obtained from five neonates with positive cord serum concentrations; a summary of this procedure is reviewed in Table 4. Of the five babies, three vernix caseosa specimens were positive for cocaine and/or metabolites [112]. Mechanisms of drug deposition into vernix are unknown, but it is possible that the drug is deposited from drug present in amniotic fluid. A limited amount of vernix caseosa is available to collect, particularly in post-term babies, making weighing of specimens, and therefore, quantitative measurements difficult.
Future research
It is essential that the relationships between the amount, frequency, and timing of drug exposure and drug concentrations in infant biological fluids and tissues be defined. This will resolve whether quantification of drug analytes in neonatal tissues reliably predict degree of prior drug exposure or neonatal outcomes. If not, qualitative analysis may be sufficient. Controlled dosing with methadone and/or buprenorphine in opioid maintenance treatment provides a unique opportunity to investigate the disposition of drugs and metabolites in the drug-exposed infant and the relationship of in utero drug exposure and maternal and neonatal outcome measures.
To date, it is unknown if magnitude or chronicity of drug exposure during gestation correlates with maternal or neonatal outcome measures including obstetrical complications, withdrawal symptoms, low birth weight, and physical defects. It may be necessary to monitor illicit drug exposure and/or licit pharmacotherapies throughout pregnancy to determine if there is a correlation between total drug exposure or exposure during the third trimester and drug concentrations in neonatal fluids and tissues and developmental abnormalities. Our laboratory is currently investigating the efficacy of urine and oral fluid collected thrice weekly, weekly sweat patches, and monthly hair specimens to best predict the extent of maternal drug use during gestation and maternal and neonatal outcome measures.
Additional research into windows of drug detection, mechanisms of drug deposition, and optimal analytical procedures for analysis of umbilical cord, cord blood, and vernix caseosa must be completed before these alternative matrices can be considered for widespread monitoring. In addition, metabolites unique to fetal drug metabolism should be identified to improve detection rates of in utero drug exposure. More research is needed to determine if a single FAEE or the sum of specific FAEEs will produce a sensitive and selective biomarker of ethanol exposure in meconium testing. Additional research areas include whether individual or total FAEE concentrations correlate with maternal ethanol consumption in the last trimester, frequency of alcohol drinking, and chronic vs. binge drinking.
LC/MS and LC/MS/MS techniques are increasing in popularity as confirmation techniques because of high sensitivity and specificity, and the ability to handle complex matrices. Also, LC/MS techniques do not require derivatization common in GC/MS; however, ion suppression due to complex neonatal matrices is a frequent analytical complication [115].
Conclusion
Ultimately, the determination of the appropriate specimen to analyze for detecting in utero drug exposure will depend on the specific needs of the monitoring program and specimen availability. Each specimen has advantages and limitations, including ease and invasiveness of collection, and different detection time frames, sensitivities, and specificities. Regardless of specimen analyzed, confirmation of positive screening results is essential. It is hoped that there will be bioanalytical advancements in sensitivity and specificity and in identifying unique biomarkers of in utero drug exposure in order to connect drug exposure to toxicological outcomes. Accurate bioanalytical procedures are vital to defining the scope of and resolving this important public health problem.
References
Bauer CR, Shankaran S, Bada H, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J (2002) Am J Obstet Gynecol 186:487–495
D’Apolito K (1998) J Pediatr Nurs 13:307–316
Kaltenbach KA (1994) Drug Alcohol Depend 36:83–87
Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, Liu J, Lester BM (2006) Pediatrics 118:1149–1156
Arria AM, Derauf C, Lagasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Grotta SD, Liu J, Lester B (2006) Matern Child Health J 10:293–302
Office of Applied Studies (2004) Results from the 2003 national survey on drug use and health: national findings (DHHS Pub. No. SMA 04-3964, NSDUH Series H-25). Substance Abuse and Mental Health Services Administration, Rockville, MD
Clark KA, Dawson S, Martin SL (1999) Matern Child Health J 3:161–166
Chasnoff IJ, McGourty RF, Bailey GW, Hutchins E, Lightfoot SO, Pawson LL, Fahey C, May B, Brodie P, McCulley L, Campbell J (2005) J Perinatol 25:368–374
Richardson GA, Huestis MA, Day NL (2006) Assessing in utero exposure to cannabis and cocaine. In: Bellinger DC (ed) Human developmental neurotoxicology. Taylor and Francis Group, New York, pp 287–302
Ostrea EM Jr, Knapp DK, Tannenbaum L, Ostrea AR, Romero A, Salari V, Ager J (2001) J Pediatr 138:344–348
Eyler FD, Behnke M, Wobie K, Garvan CW, Tebbett I (2005) Neurotoxicol Teratol 27:677–687
Coghlan D, Milner M, Clarke T, Lambert I, McDermott C, McNally M, Beckett M, Matthews T (1999) Ir Med J 92:232–233, 236
Merrill C, Steiner C (2006) Agency for Healthcare Research and Quality
Sweeney PJ, Schwartz RM, Mattis NG, Vohr B (2000) J Perinatol 20:219–224
Nocon JJ (2006) Addiction 101:608
Boskovic R, Klein J, Woodland C, Karaskov C, Koren G (2001) Can J Physiol Pharmacol 79:942–945
Anderson GD (2006) Expert Opin Drug Metab Toxicol 2:947–960
Dams R, Murphy CM, Lambert WE, Huestis MA (2003) Rapid Commun Mass Spectrom 17:1665–1670
Paul BD, Lalani S, Bosy T, Jacobs AJ, Huestis MA (2005) Biomed Chromatogr 19:677–688
Weinmann W, Goerner M, Vogt S, Goerke R, Pollak S (2001) Forensic Sci Int 121:103–107
Cook E (1995) Alcohol Drugs Driv 2:79–91
Murphy CM, Huestis MA (2005) J Mass Spectrom 40:1412–1416
Meadway C, George S, Braithwaite R (2002) Forensic Sci Int 127:136–141
Pirnay SO, Abraham TT, Huestis MA (2006) Clin Chem 52:1728–1734
Soares ME, Carvalho M, Carmo H, Remiao F, Carvalho F, Bastos ML (2004) Biomed Chromatogr 18:125–131
Xu X, Iba MM, Weisel CP (2004) Clin Chem 50:2323–2330
Meger M, Meger-Kossien I, Schuler-Metz A, Janket D, Scherer G (2002) J Chromatogr B 778:251–261
Joseph RE Jr, Su TP, Cone EJ (1996) J Anal Toxicol 20:338–344
Romano G, Barbera N, Spadaro G, Valenti V (2003) Forensic Sci Int 131:98–102
Thorspecken J, Skopp G, Potsch L (2004) Clin Chem 50:1373–1379
Cone EJ, Yousefnejad D, Darwin WD, Maquire T (1991) J Anal Toxicol 15:250–255
Harkey MR, Henderson GL, Zhou C (1991) J Anal Toxicol 15:260–265
Baptista MJ, Mansanto PV, Marques EGP, Bermejo A, Avila S, Castanheira AM, Margalho C, Barroso M, Vieira DN (2002) Forensic Sci Int 128:66–78
Moore C, Guzaldo F, Donahue T (2001) J Anal Toxicol 25:555–558
Skender L, Karacic V, Brcic I, Bagaric A (2002) Forensic Sci Int 125:120–126
Scheidweiler KB, Huestis MA (2004) Anal Chem 76:4358–4363
Chetiyanukornkul T, Toriba A, Kizu R, Kimura K, Hayakawa K (2004) Biomed Chromatogr 18:655–661
Pichini S, Pacifici R, Altieri I, Passa A, Rose M, Zuccaro P (1995) Analysis of nicotine and cotinine in human hair by high-performance liquid chromatography and comparative determination with radioimmunoassay. In: Cone EJ, Welch MJ, Grigson Babecki MB (eds) Hair testing for drugs of abuse: international research on standards and technology. NIH Pub. No. 95-3727 National Institute on Drug Abuse, Rockville, MD, pp 212–224
Verstraete AG (2004) Ther Drug Monit 26:200–205
Schepers RJF, Oyler JM, Joseph RE Jr, Cone EJ, Moolchan ET, Huestis MA (2003) Clin Chem 49:121–132
Kato K, Hillsgrove M, Weinhold L, Gorelick DA, Darwin WD, Cone EJ (1993) J Anal Toxicol 17:338–341
Mortier KA, Maudens KE, Lambert WE, Clauwaert KM, Van Bocxlaer JF, Deforce DL, Van Peteghem CH, De Leenheer AP (2002) J Chromatogr B 779:321–330
Clauwaert K, Decaestecker T, Mortier K, Lambert W, Deforce D, Van Peteghem C, Van Bocxlaer J (2004) J Anal Toxicol 28:655–659
Niedbala S, Kardos K, Salamone S, Fritch D, Bronsgeest M, Cone EJ (2004) J Anal Toxicol 28:546–552
Kintz P, Cirimele V, Ludes B (2000) J Anal Toxicol 24:557–561
Toennes SW, Kauert GF, Steinmeyer S, Moeller MR (2005) Forensic Sci Int 152:149–155
Wood M, De Boeck G, Samyn N, Morris M, Cooper DP, Maes RA, De Bruijn EA (2003) J Anal Toxicol 27:78–87
Kim I, Wtsadik A, Choo RE, Jones HE, Huestis MA (2005) J Anal Toxicol 29:689–695
Kim I, Darwin D, Huestis M (2005) J Chromatogr B 814:233–240
Kolbrich E, Barnes A, Gorelick DA, Boyd SJ, Cone EJ, Huestis MA (2006) J Anal Toxicol 30:501–510
Lin SN, Moody DE, Bigelow GE, Foltz RL (2001) J Anal Toxicol 25:497–503
Nadulski T, Sporkert F, Schnelle M, Stadelmann AM, Roser P, Schefter T, Pragst F (2005) J Anal Toxicol 29:782–789
Huestis MA, Elsohly M, Nebro W, Barnes A, Gustafson RA, Smith ML (2006) Ther Drug Monit 28:540–544
Murphy CM, Huestis MA (2005) J Mass Spectrom 40:70–74
Bourquin D, Lehmann T, Hammig R, Buhrer M, Brenneisen R (1997) J Chromatogr Sci 694:233–238
Peters FT, Samyn N, Lamers CT, Riedel WJ, Kraemer T, de Boeck G, Maurer HH (2005) Clin Chem 51:1811–1822
Peters FT, Schaefer S, Staack RF, Kraemer T, Maurer HH (2003) J Mass Spectrom 38:659–676
Kim I, Huestis MA (2006) J Mass Spectrom 41:815–821
Shin HS, Kim JG, Shin YJ, Jee SH (2002) J Chromatogr B Anal Technol Biomed Life Sci 769:177–183
Kacinko SL, Barnes AJ, Schwilke EW, Cone EJ, Moolchan ET, Huestis MA (2005) Clin Chem 51:2085–2094
Follador MJ, Yonamine M, de Moraes Moreau RL, Silva OA (2004) J Chromatogr B 811:37–40
Saito T, Wtsadik A, Scheidweiler KB, Fortner N, Takeichi S, Huestis MA (2004) Clin Chem 50:2083–2090
Schwilke EW, Barnes AJ, Kacinko SL, Cone EJ, Moolchan ET, Huestis MA (2006) Clin Chem 52:1539–1545
Fay J, Fogerson R, Schoendorfer D, Niedbala RS, Spiehler V (1996) J Anal Toxicol 20:398–403
Kintz P, Henrich A, Cirimele V, Ludes B (1998) J Chromatogr B 705:357–361
Ostrea EM, Romero A, Knapp K, Ostrea AR, Lucena JE, Utarnachitt RB (1994) J Pediatr 124:477–479
Bar-Oz B, Klein J, Karaskov T, Koren G (2003) Arch Dis Child Fetal Neonatal Ed 88:F98–F100
Moore C, Jones J, Lewis D, Buchi K (2003) Clin Chem 49:133–136
Pichini S, Pacifici R, Pellegrini M, Marchei E, Lozano J, Murillo J, Vall O, Garcia-Algar O (2004) Anal Chem 76:2124–2132
Marchei E, Pellegrini M, Pacifici R, Palmi I, Lozano J, Garcia-Algar O, Pichini S (2006) Ther Drug Monit 28:700–706
Moore C, Lewis D, Leikin J (1995) Clin Chem 41(11):1614–1616
Steele BW, Bandstra ES, Wu NC, Hime GW, Hearn WL (1993) J Anal Toxicol 17:348–352
Oyler J, Darwin WD, Preston KL, Suess P, Cone EJ (1996) J Anal Toxicol 20:453–462
Ostrea EM, Knapp DK, Romero AI, Montes M, Ostrea AR (1994) J Pediatr 124:471–476
Kohler E, Bretschneider D, Rabsilber A, Weise W, Jorch G (2001) Hum Exp Toxicol 20:1–7
Moriya F, Chan K-M, Noguchi TT, Wu PYK (1994) J Anal Toxicol 18:41–45
Dempsey D, Moore C, Deitermann D, Lewis D, Feeley B, Niedbala RS (1999) Forensic Sci Int 102:167–171
ElSohly M, Feng S (1998) J Anal Toxicol 22:329–335
Bearer CF, Lee S, Salvator AE, Minnes S, Swick A, Yamashita T, Singer LT (1999) Alcohol Clin Exp Res 23:487–493
Klein J, Karaskov T, Korent G (1999) Ther Drug Monit 21:644–646
Ostrea EM Jr, Hernandez JD, Bielawski DM, Kan JM, Leonardo GM, Abela MB, Church MW, Hannigan JH, Janisse JJ, Ager JW, Sokol RJ (2006) Alcohol Clin Exp Res 30:1152–1159
Chan D, Bar-Oz B, Pellerin B, Paciorek C, Klein J, Kapur B, Farine D, Koren G (2003) Ther Drug Monit 25:271–278
Moore CM, Lewis D (2001) Clin Chim Acta 312:235–237
Choo RE, Murphy CM, Jones HE, Huestis M (2005) J Chromatogr B 814:369–373
Le NL, Reiter A, Tomlinson K, Jones J, Moore C (2005) J Anal Toxicol 29:54–57
Moore CM, Deitermann D, Lewis D, Leikin J (1995) J Anal Toxicol 19:514–518
Pichini S, Puig C, Zuccaro P, Marchei E, Pellegrini M, Murillo J, Vall O, Pacifici R, Garcia-Algar O (2005) Forensic Sci Int 153:59–65
Salem MY, Ross SA, Murphy TP, ElSohly MA (2001) J Anal Toxicol 25:93–98
Ostrea EM Jr, Bielawski DM, Posecion NC Jr (2006) Arch Dis Child 91:628–629
Clark GD, Rosenzweig IB, Raisys VA, Callahan CM, Grant TM, Streissguth AP (1992) J Anal Toxicol 16:261–263
Murphey LJ, Olsen GD, Konkol RJ (1993) J Chromatogr 613:330–335
Xia Y, Wang P, Bartlett MG, Solomon HM, Busch KL (2000) Anal Chem 72:764–771
Coles R, Clements TT, Nelson GJ, McMillin GA, Urry FM (2005) J Anal Toxicol 29:522–527
Baranowski J, Pochopien G, Baranowska I (1998) J Chromatogr B Biomed Sci App 707:317–321
Moore CM, Lewis DE, Leikin JB (1996) J Forensic Sci 41(6):1057–1059
Pichini S, Pacifici R, Pellegrini M, Marchei E, Perez-Alarcon E, Puig C, Vall O, Garcia-Algar O (2003) J Chromatogr B Anal Technol Biomed Life Sci 794:281–292
Chan D, Caprara D, Blanchette P, Klein J, Koren G (2004) Clin Biochem 37:429–438
Jacqz-Aigrain E, Zhang D, Maillard G, Luton D, Andre J, Oury JF (2002) Br J Gynaecol 109:909–911
Eliopoulos C, Klein J, Chitayat D, Greenwald M, Koren G (1996) Clin Invest Med 19:231–242
Garcia-Bournissen F, Rokach B, Karaskov T, Koren G (2006) Arch Dis Child Fetal Neonatal Ed 2006 Oct 31 (epub ahead of print)
Vinner E, Vignau J, Thibault D, Codaccioni X, Brassart C, Humbert L, Lhermitte M (2003) Forensic Sci Int 133:57–62
Klein J, Koren G (1999) Hum Exp Toxicol 18:279–282
Caprara DL, Klein J, Koren G (2005) Ther Drug Monit 27:811–815
Moore CM, Brown S, Negrusz A, Tebbett I, Meyer W, Jain L (1993) J Anal Toxicol 17:62
Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD (2006) J Perinatol 26:11–14
Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D (2003) Am J Psychiatry 160:993–996
Hostetter A, Ritchie JC, Stowe ZN (2000) Biol Psychiatry 48:1032–1034
Pichini S, Basagana XB, Pacifici R, Garcia O, Puig C, Vall O, Harris J, Zuccaro P, Segura J, Sunyer J (2000) Environ Health Perspect 108:1079–1083
Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C (1999) Obstet Gynecol 93:25–29
Skopp G, Potsch L (1997) Ther Drug Monit 19:386–389
Suzuki O, Hattori H, Asano M (1984) Forensic Sci Int 24:9–16
Moore C, Dempsey D, Deitermann D, Lewis D, Leikin J (1996) J Anal Toxicol 20:509–511
Winecker RE, Goldberger BA, Tebbett I, Behnke M, Eyler FD, Conlon M, Wobie K, Karlix J, Bertholf RL (1997) J Anal Toxicol 21:97–104
Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL, Stowe ZN (2006) Am J Psychiatry 163:145–147
Dams R, Huestis MA, Lambert WE, Murphy CM (2003) J Am Soc Mass Spec 14:1290–1294
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gray, T., Huestis, M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem 388, 1455–1465 (2007). https://doi.org/10.1007/s00216-007-1228-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1228-9