Abstract
Microwave-assisted thermal desorption (MAD) coupled to headspace solid-phase microextraction (HS-SPME) has been studied for in-situ, one-step, sample preparation for PAHs collected on XAD-2 adsorbent, before gas chromatography with mass spectrometric detection. The PAHs on XAD-2 were desorbed into the extraction solution, evaporated into the headspace by use of microwave irradiation, and absorbed directly on a solid-phase microextraction fiber in the headspace. After desorption from the SPME fiber in the hot GC injection port, PAHs were analyzed by GC–MS. Conditions affecting extraction efficiency, for example extraction solution, addition of salt, stirring speed, SPME fiber coating, sampling temperature, microwave power and irradiation time, and desorption conditions were investigated. Experimental results indicated that extraction of 275 mg XAD-2, containing 10–200 ng PAHs, with 10-mL ethylene glycol–1 mol L−1 NaCl solution, 7:3, by irradiation with 120 W for 40 min (the same as the extraction time), and collection with a PDMS–DVB fiber at 35 °C, resulted in the best extraction efficiency. Recovery was more than 80% and RSD was less than 14%. Optimum desorption was achieved by heating at 290 °C for 5 min. Detection limits varied from 0.02 to 1.0 ng for different PAHs. A real sample was obtained by using XAD-2 to collect smoke from indoor burning of joss sticks. The amounts of PAHs measured varied from 0.795 to 2.53 ng. The method is a simple and rapid procedure for determination of PAHs on XAD-2 absorbent, and is free from toxic organic solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatics hydrocarbons (PAHs) are well known toxic and hazardous pollutants and highly potent carcinogens that can cause tumors in some organisms. PAHs are produced by incomplete combustion or pyrolysis of organic materials containing carbon and hydrogen [1]. Occupational exposure to PAHs has been shown to be associated with an increased risk of cancer in coke oven, foundry, and aluminium production workers [2, 3]. There has also recently been concern about interference of PAHs with hormone systems, their potential effect on reproduction, and their ability to depress immune function [4].
When monitoring workplace or indoor air, XAD-2 is commonly used as adsorbent for sampling non-polar pollutants, for example PAHs [5–8]. After sampling, appropriate pretreatment of the XAD-2 sample is required, including desorption with organic solvent (e.g. cyclohexane), cleaning, and concentration by purging evaporation, before chromatographic analysis [6, 9–15]. Although such pretreatment efficiently yields precise results, it is tedious, time consuming, and requires use of toxic organic solvents and expensive equipment.
Methods of pretreatment that take a short time with little or no use of organic solvents have recently been developed. Solid-phase microextraction (SPME) has several advantages over conventional extraction techniques [16–19]. It has been used for successful extraction of many volatile and semivolatile organic compounds, including PAHs, from different matrixes [20–26]. Headspace SPME (HS-SPME) was developed later and has been successfully used to eliminate interference problems [27–29]. HS-SPME has been reported to be efficient only for the analytes with high and medium Henry coefficients, however [30]. Although HS-SPME has been used to extract PAHs, with preheating of the samples to enhance extraction of PAH, it was, however, still time-consuming [31–34].
We have recently investigated use of microwave heating to enhance the evaporation of semi-volatile analytes for HS-SPME sampling before GC analysis [35–39]. Microwave-assisted desorption (MAD) coupled with HS-SPME sampling has also been successfully used for determination of aniline in silica gel [40]. MAD coupled to MA-HS-SPME has the potential to become an alternative pretreatment step for isolation of PAHs from XAD adsorbent. Because the optimum conditions for MAD-HS-SPME depend on the characteristics of the adsorbent and the desorption solution and the boiling point and polarity of analyte, it is necessary to investigate the MAD-HS-SPME technique for a wide range of high-boiling-point PAHs.
In this study we have systematically investigated the applicability of microwave-assisted desorption (MAD) and in-situ HS-SPME (MAD-HS-SPME) coupled to GC–MS for simple, rapid, sensitive, and solvent-free analysis PAHs on XAD-2 adsorbent.
Experimental
Chemicals and reagents
Eight PAHs standards including naphthalene (NaP), acenaphthylene (AcPy), acenaphthene (AcP), fluorene (Flu), phenanthrene (PhA), anthracene (AnT), fluoranthene (FluA), and pyrene (Pyr), and d 10-anthracene (d 10-AnT) as internal standard, were purchased from Dr Ehrenstorfer (Augsburg, Germany) and stored at 4 °C. Working solutions (10 μg mL−1 in acetone) were prepared weekly by appropriately dilution of the stock solutions with acetone (Mallinckrodt, Paris, Kentucky, USA). All standards and working solutions were stored at 4 °C in silanized brown glass bottles with Teflon-lined caps.
XAD-2 adsorbent tubes were purchased from SKC (Model No 226-30-6, front 270 mg, back 140 mg). Amberlite XAD-2 resin (surface area 370 m2 g−1, mean particle size 551 μm) were from Supelco (Bellefonte, PA, USA). They were pre-cleaned with dichloromethane by Soxhlet extraction for 20 h before use. Deionized water for all aqueous solutions was produced by use of a Barnstead (New York, USA) Nanopure water system. All chemicals and solvents were of ACS reagent grade.
GC–MS
GC–MS was performed with a Varian 3800 system equipped with Saturn 2000 mass detector (MS) and a split/splitless injector. Compounds were separated on a fused silica DB-5 capillary column (30 m × 0.25 mm i.d., 1.0 μm film thickness; J&W Scientific, Folsom, CA, USA). Helium was used as carrier gas and purge gas at flow rates of 1.0 and 20 mL min−1, respectively. The gas chromatograph was operated in splitless mode with a desorption time of 5 min; the injector temperature was 300 °C. The oven temperature was maintained at 50 °C for 1 min then programmed at 10° min to 300 °C which was held for 5 min. The ion trap, manifold, and transfer line temperatures were 230, 60, and 300 °C, respectively. Mass spectra in EI mode was obtained at an electron energy of 70 eV. Saturn GC–MS Workstation 5.5 software was used for data acquisition.
MAD/HS-SPME
The microwave oven used in this work was a modified version of the domestic NE-V32 inverter system (2450 MHz, Panasonic, Canada) with a maximum power of 720 Watts, equipped with a temperature-control cooling system (YIH DER BL-720, Taiwan). A home-made microwave stirrer was used to stir the samples at 2400 rpm during extraction. After modification, microwave power of 78, 94, 102, 120, 137, and 154 W was used in this study. The sampling system was set up as shown in Fig. 1. Aluminium foil was attached to the inner and outer wall of the microwave oven at the interface between the microwave body and the headspace sampling apparatus to prevent leakage of irradiation. During the experiments a microwave leak detector (MD-2000; Less EMF, NY, USA) was used to check safety aspects of the equipment.
The SPME device, consisting of the holder and fiber assembly for manual sampling, was obtained from Supelco (Bellefonte, PA, USA) and was used without modification. The fibers selected in this study were 1 cm long and coated with different materials (65-μm PDMS–DVB, 85-μm PA, and 100-μm PDMS). Before use the fibers were conditioned under nitrogen in the hot injection port, as recommended by the manufacturer. The needle on the SPME holder was set at its maximum length of 4 cm in the GC injector port. A desorption temperature of 290 °C held for 5 min was used to achieve the highest sensitivity for PAHs. All analyses were performed with a 25-mL flask with ground-glass joints containing 275 mg XAD-2 and 10 mL extraction solution.
Preparation of XAD-2 spiked sample
XAD-2 samples spiked with different quantities of PAHs were prepared by adding 27.5 g XAD-2 adsorbent to 100 mL of standard solutions of different PAHs (in acetone) at concentrations of NaP (0.01 μg mL−1), AcPy and AcP (0.02 μg mL−1), Flu (0.03 μg mL−1), PhA and AnT (0.1 μg mL−1), FluA and Pyr (0.2 μg mL−1), individually. After the solutions had been thoroughly mixed, acetone was removed to dryness by use of an evaporator. The XAD-2 adsorbents spiked with 10 ng NaP, 20 ng AcPy and Acp, 30 ng Flu, 100 ng PhA and AnT, and 200 ng FluA and Pyr per 275 mg, individually, were obtained.
Procedure
Spiked or unspiked XAD-2 samples (275 mg) were mixed with 10 mL extraction solution (ethylene glycol–1 mol L−1 NaCl, 7:3) in a 25-mL flask with ground-glass joint. After swirling, the flask was placed in the microwave oven and connected to the HS-SPME system. An SPME device with a fiber was inserted into the hollow part of the condenser which was connected to a circulated cooling water system to control the sampling temperature. The PAHs were desorbed into the extraction solution, with stirring at 2400 rpm, then vaporized and absorbed on the SPME fiber in the HS by direct microwave irradiation at 120 W for 40 min. After collection of the PAHs the fiber was desorbed in the GC injector for GC–MS analysis.
Real sample collection
Samples of smoke from indoor burning of joss sticks were collected with an air sampling device containing a filter and an adsorbent tube connected by silicone rubber tubing to a battery-powered pump (SKC Model 222) at a flow rate of 1.4 L min−1 for 5 h. The samples were stored at 4 °C until analysis.
Results and dscussion
Fiber selection
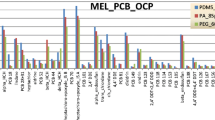
The mixed-polymer bipolar PDMS–DVB (65-μm) fiber was evaluated for headspace sampling of PAHs in addition to the polyacrylate (PA, 85-μm) and polydimethylsiloxane (PDMS, 100-μm) fibers regarded as most appropriate for PAH [34–37, 41]. A spiked sample described in the section “Preparation of XAD-2 spiked sample” was analyzed in triplicate by use of each fiber. The efficiencies of extraction of PAHs (related to peak areas) by use of the different fibers under the action of microwave irradiation (120 W, 40 min) and HS-SPME at 35 °C are illustrated in Fig. 2. Efficiency of extraction of NaP, AcPy and AcP was higher for the bipolar PDMS–DVB fiber than for the PDMS fiber and almost the same for the other five PAHs. Efficiency of extraction of all eight PAHs by the PDMS–DVB fiber was higher than that by the polyacrylate fiber. The PDMS–DVB fiber was therefore selected for the studies.
Optimization of microwave irradiation conditions
Microwave irradiation was used to assist extraction of PAHs from the XAD-2 adsorbent and to promote vaporization of the PAHs into headspace for SPME sampling. Microwave irradiation at 78, 94, 102, 120, 137, or 154 W was used for 20–60 min. The experimental results indicated that efficiency of extraction of two and three-ring PAHs was best when low-power irradiation was used for a short time whereas four-ring PAHs were best extracted by use of high-power irradiation for longer times. The overall optimum efficiency of extraction of PAHs from XAD-2 was achieved by use of 120 W for 40 min.
Effect of temperature of the circulating water system
Because absorption is an exothermic process, absorption of PAHs on to the fiber was not favored by high temperature. Although use of a low temperature was thermodynamically helpful for absorption of the PAHs it did not promote transport of PAHs vapor to the sampling zone. Circulating water was therefore used to control the sampling zone temperature. When temperatures from 25–50 °C were investigated optimum absorption was achieved at 35 °C. This temperature was thus used in the circulating water system.
Effect of extraction solution
In this study the sample was PAHs collected on or added to XAD-2 adsorbent. XAD-2 has strong affinity for PAHs, i.e. the partition ratio of PAHs between air and XAD-2 is extremely low, so it was difficult to achieve headspace SPME sampling for PAHs from XAD-2. Microwave irradiation was used to promote PAHs direct desorption from XAD-2 into the headspace but very few PAHs were detected after HS-SPME sampling. This indicated the microwave energy absorbed by the XAD-2 was not sufficient to desorb PAHs. Potential highly microwave-absorbing solutions (10 mL H2O, ethylene glycol, glycerol, and 7:3 and 5:5 solutions of ethylene glycol in water were thus examined for extraction of PAHs from XAD-2 into solution, and the PAHs were vaporized into the headspace, with the help of microwave irradiation. The experimental results showed that extraction with 7:3 ethylene glycol–H2O resulted in the best recovery. In our study the temperature of this ethylene glycol–H2O solution reached 130 °C during microwave irradiation. Although the temperature of MAD of PAHs with ethylene glycol or glycerol could exceed 130 °C, PAHs were barely collected on the SPME fiber. This was because the high viscosity of the extraction solution and the high solubility of the analytes in the extraction solution hindered evaporation of the analyte. Both factors should be considered when selecting the extraction solution for MAD-HS-SPME.
Salting-out effect
Salting-out is often used to enhance extraction of aqueous neutral organic species on to SPME fibers [17]. Aqueous NaCl (0–2.0 mol L−1) was added to the extraction solution to determine the effect on the efficiency of HS-SPME. The efficiency of extraction of most PAHs increased on addition of NaCl up to 1.0 mol L−1 but more than this had an adverse effect, especially for Nap. Thus salting-out with low concentrations of NaCl enhanced vaporization of the PAHs but high concentrations blocked desorption from XAD-2, because the PAHs were salted-out on to XAD-2 in water of high-salt content.
Effect of speed of stirring the sample solution
Agitation was used to facilitate mass transport between the bulk of the aqueous sample and the fiber, and thus shorten equilibration times [17]. In MAD/HS-SPME PAHs were first extracted from the XAD-2 into the bulk solution, which would be affected by agitation. From our experimental results agitation apparently increased the efficiency of extraction of PAHs for stirring speeds up to 2400 rpm. Precision then became worse because the stirring was not smooth.
Thermal desorption conditions
Because of the wide boiling-point range of the PAHs, the temperature of thermal desorption in the GC injection port was optimized to achieve sensitive quantification and prevent memory effects. The efficiency of desorption of the PAHs was optimum when the fiber was heated at 290 °C for 5 min. After desorption, no significant PAH signals appeared in the chromatogram after re-injection. Thus, no further regeneration of the fiber was required between runs. Although, according to the manufacturer the operating temperature of the PDMS–DVB fiber should not be >270 °C, the fiber was used more than 100 times in our MAD/HS-SPME system.
Validation of the method
To test the suitability of the method for quantitative determination of PAHs on XAD-2, XAD-2 samples spiked with standards were used for calibration after being subjected to the complete overall treatment procedure, i.e. MAD/HS-SPME, thermal desorption, and GC–MS analysis. A chromatogram obtained, under the conditions described in the section “GC–MS”, from PAH standards added to XAD-2 is shown in Fig. 3. Calibration plots for quantities of PAHs in the ranges listed in Table 1 were found to have good linearity with correlation coefficients (R 2) in the range 0.9953–0.9986. Calibration plot data are listed in Table 1. Detection limits were calculated on the basis of three times the average background noise divided by the detection sensitivity (slope of the calibration plot) and varied from 0.02 to 1 ng. Precision was estimated by performing seven extractions of XAD-2 samples spiked with all the PAHs at levels used for the calibration plots. Precisions ranged from 3 to 14% RSD.
Application to a real sample
To examine the suitability of the method for determination of PAHs in real samples a sample was collected as described in the section “Real sample collection” and analyzed by use of the proposed method. The chromatogram obtained from the PAHs in the sample is shown in Fig. 4. Although many unknown species were observed, four PAHs (NaP, AcPy, Flu, and PhA) were identified in the XAD-2 sample. Recovery was tested to investigate the effect of the real sample matrix by spiking an XAD-2 adsorbent tube with 2 ng Nap, AcPy, Acp and Flu, 10 ng PhA and AnT, and 40 ng FluA and Pyr and subjecting the tube to the proposed MAD/HS-SPME extraction/thermal desorption/GC–MS analysis. Recoveries of the PAHs varied from 80 to 108%, as listed in Table 2. This accuracy is acceptable in environmental analysis.
Comparison of the proposed MAD/HS-SPME method with other methods
To compare the efficiency of extraction of the proposed MAD/HS-SPME method with conventional pretreatment methods for desorption from XAD-2, three organic solvents, acetone, carbon disulfide, and dichloromethane (2 mL of each) were used separately to extract PAHs from spiked XAD-2 (275 mg) in 5-mL vials with ultrasonication. The extract (1 μL) was analyzed by GC–MS after extraction for 40 min. Analytical results for spiked XAD-2 samples pretreated by the MAD/HS-SPME method and by conventional ultrasound-assisted liquid–liquid extraction are listed in Table 3. The efficiencies of extraction of PAHs obtained by use of the proposed method were apparently much higher than those obtained by conventional ultrasonication extraction with organic solvents.
Conclusion
MAD/HS-SPME is proposed as an alternative pretreatment method for PAHs collected on XAD-2 adsorbent in air-quality monitoring. The optimum conditions have been established. Results from suitability tests indicate the proposed method is a simple, rapid, convenient, sensitive, and toxic-organic-solvent-free procedure for isolation of PAHs from XAD-2 before GC–MS determination.
References
Lee MI, Novotny MV, Bartle KD (1981) Analytical chemistry of polycyclic aromatic compounds. Academic Press, New York
Costantino J, Redmond C, Bearden A (1995) J Occup Environ Med 37:597–604
Andjelkovich D, Shy C, Brown M, Janszen D, Levine R (1990) J Occup Med 36:391–401
Chaloupka K, Harper N, Krishnan V, Santostefano M, Rodriguez LV, Safe S (1993) Chem Biol Interact 89:141–158
Kuusimaki L, Peltonen K, Mutanen P, Savela K (2003) Ann Occup Hyg 47:389–398
Chung JC, Kuhlman MR (1990) Environ Sci Technol 24:661–665
Oldaker GB III, Conrad JC Jr (1987) Environ Sci Technol 21:994–999
Williams RW, Watts RR, Stevens RK, Stone CL, Lewtas J (1999) J Expos Anal Environ Epidemiol 9:158–166
Oamh NT, Reutergardh LB, Dung NT (1999) Environ Sci Technol 33:2703–2709
Nolte CG, Schawer JJ, Cass GR (2001) Environ Sci Technol 35:1912–1919
Santos CYM, Azevedo DA, Neto FRA (2002) Atmos Environ 36:3009–3019
Li CT, Zhuang HK, Hsien LT, Lee WJ, Tsao MC (2001) Environ Int 27:61–67
Guillen MD, Manzanos MJ, Ibargoitia ML (2001) J Agric Food Chem 49:2395–2403
Lei L, Suidan MT, Khodadoust AP, Tabak HH (2004) Environ Sci Technol 38:1786–1793
Ré-Poppi N, Santiago-Silva M (2005) Atmos Environ 39:2839–2850
Chen J, Pawliszyn J (1995) J Anal Chem 67:2530–2533
Pawliszyn J (1997) Solid phase microextraction theory and practice. Wiley–VCH, New York
Lord HL, Pawliszyn J (2000) J Chromatogr A 885:153–193
Alpendura MF (2000) J Chromatogr A 889:3–14
Lee MR, Yeh YC, Hsiang WS, Chen CC (1998) J Chromatogr B 707:91–97
Koziel J, Jia M, Khaled A, Noah J, Pawliszyn J (1999) Anal Chim Acta 400:153–162
Hawthorne SB, Grabanski CB, Miller DJ, Kreitinger JP (2005) Environ Sci Technol 39:2795–2803
Diaz-Vazquez LM, Garcia O, Velazquez Z, Marrero I, Rosario O (2005) J Chromatogr B 825:11–20
Boyd-Boland AA, Pawliszyn J (1995) J Chromatogr A 704:163–172
King AJ, Readman JW, Zhou JL (2003) Environ Geochem Health 25:69–75
Chen HW (2004) Anal Sci 20:1383–1388
Chai M, Pawliszyn J (1995) Environ Sci Technol 29:693–701
Guan F, Watanabe K, Ishii A, Seno H, Kumazawa T, Hattori H, Suzuki O (1998) J Chromatogr B 714:205–213
Czerwinski J, Zygmunt B, Namiesnik J (1996) Fresenius J Anal Chem 356:80–83
Llompart M, Li K, Fingas M (1998) Anal Chem 70:2510–2515
Djozan DJ, Assadi Y (1999) Microchem J 63:276–284
Doong RA, Chang SM, Sun YC (2000) J Chromatogr Sci 38:528–534
Doong RA, Chang SM, Sun YC (2000) J Chromatogr A 879:177–188
Waidyanatha S, Zheng Y, Rappaport SM (2003) Chem Biol Interact 145:165–174
Wei MC, Jen JF (2002) Chromatographia 55:701–706
Jen JF, Su YS, Chen YI (2002) J Chromatogr A 976:349–355
Wei MC, Jen JF (2003) J Chromatogr A 1012:111–120
Jen JF, Li HP, Li GC (2003) J Chromatogr A 1012:129–137
Yan CT, Jen JF (2004) Chromatographia 59:517–520
Yan CT, Shih TS, Jen JF (2004) Talanta 64:650–654
Guillen MD, Sopelana P (2005) J Dairy Sci 88:13–20
Acknowledgments
The authors would like to thank the National Science Council of Taiwan for financial support of this work under Contract No. NSC 93-2113-M-166-004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, MC., Chang, WT. & Jen, JF. Monitoring of PAHs in air by collection on XAD-2 adsorbent then microwave-assisted thermal desorption coupled with headspace solid-phase microextraction and gas chromatography with mass spectrometric detection. Anal Bioanal Chem 387, 999–1005 (2007). https://doi.org/10.1007/s00216-006-0962-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0962-8