Abstract
A simple, sensitive, and reliable method based on a combination of multi-walled carbon nanotubes with incorporated β-cyclodextrin (β-CD-MWNTs) and a polyaniline (PANI) film-modified glassy-carbon (GC) electrode has been successfully developed for determination of dopamine (DA) in the presence of ascorbic acid (AA). The PANI film had good anti-interference properties and long-term stability, because of the permselective and protective properties of the conducting redox polymer film. The acid-treated MWNTs with carboxylic acid functional groups promoted the electron-transfer reaction of DA and inhibited the voltammetric response of AA. Sensitive detection of DA was further improved by the preconcentration effect of formation of a supramolecular complex between β-CD and DA. The analytical response of the β-CD-MWNTs/PANI film to the electrochemical behavior of DA was, therefore, better than that of a MWNTs/PANI film, a PANI film, or a bare glassy-carbon (GC) electrode. Under the conditions chosen a linear calibration plot was obtained in the range 1.0 × 10−7–1.0 × 10−3 mol L−1 and the detection limit was 1.2 × 10−8 mol L−1. Interference from AA was effectively eliminated and the sensitivity, selectivity, stability, and reproducibility of the electrodes was excellent for determination of DA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is an important neurotransmitter in mammalian central nervous systems [1]. Changes in DA levels have proved to be important in effective brain function, for example learning and memory formation, and in the physiological and pathological process of Parkinson’ s disease [2, 3]. Quantitative determination of DA is therefore important and has attracted much interest of neuroscientists and chemists. Electrochemical detection is a feasible method because DA is electrochemically active and because electrochemical methods have advantages such as simplicity, speed, and sensitivity. A major problem in the electrochemical detection of DA is the presence of high concentrations of ascorbic acid (AA), however, because AA is oxidized at a potential close to that of DA, which results in overlapping of the voltammetric response. The electrode surface can also be readily fouled by accumulation of products from oxidation of AA. Homogeneous catalytic oxidation of AA by oxidized DA is another major interference in the determination of DA. Selective measurement of DA in the presence of AA is therefore necessary and has become a major topic in electrochemical research.
Different approaches have been used to solve these problems. For example, Nafion ion-exchange membranes, widely used for in-vivo measurements, have been shown to be suppress interference from AA owing to the opposite polarities of DA and AA [4]. Electrodes modified with gold nanoparticle arrays [5], self-assembled monolayers [6, 7], and organic polymers [8, 9] have also proved very useful for improving selectivity against AA.
Carbon nanotubes (CNTs), a new kind of carbon material, have been widely recognized as the quintessential nanomaterial since their discovery in 1991 [10]. Two distinct types of structure are recognized—single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs). Because of their subtle electronic properties, CNTs promote electron-transfer reactions when used as electrode materials in electrochemical sensing. CNTs also have a strong electrocatalytic effect, rapid electron-transfer rate, high conductance, high tensile strength, high chemical stability, and are ultra-small in size.
Use of functional groups (e.g. carboxylic acid groups) on the open ends of short nanontubes may block the adsorption of species on the CNTs [11]. These intrinsic properties of CNTs are expected to shift the potential for AA oxidation more negatively than that for DA oxidation, which suggested an alternative means of selective determination of DA free from interference from AA. Many CNT-modified electrodes have therefore been used for the determination of DA in the presence of AA, with satisfactory results [12–15].
During recent years, polyaniline (PANI) has aroused much interest, because the conducting redox polymer film has good transducing properties and high stability and reproducibility. It has been widely used in the preparation of biosensors [16, 17]. Conducting PANI film is also known to be effective for eliminating the effect of electroactive compounds on the electrochemical response of the polymer-modified sensor [18]. It also has different electrocatalytic behavior toward DA and AA [19]. In addition, PANI films are positively charged, which would promote linking with negatively charged substances, for example negatively charged MWNTs with carboxylic acid functional groups.
Cyclodextrins (CD) are oligosaccharides composed of six, seven, or eight glucose units (α, β, or γ-CD, respectively) which are toroidal in shape with a hydrophobic inner cavity and a hydrophilic exterior [20]. This structure results in formation of inclusion complexes with a variety of guest molecules which fit inside the torus-shaped cavities, which serve as host sites. It has been reported that inclusion of DA in β-CD could be a result of hydrophobic and hydrogen-bonding interaction [21]. Electrodes modified with MWNTs in which β-CD have been incorporated have recently been successfully used to study and quantify many biological and other organic molecules, because of the promising properties of both materials [22–25]. As far as we are aware, however, detection of DA at a glassy-carbon electrode modified with a combination MWNTs incorporating β-CD and PANI film has not reported.
In the work discussed in this paper a simple, reliable method was developed for determination of DA in the presence of AA. It was based on use of multi-walled carbon nanotubes with incorporated β-cyclodextrin combined with polyaniline film-modified glassy carbon (GC) electrodes (β-CD-MWNTs/PANI). The electrochemical behavior of DA at the chemically modified electrodes was investigated in detail by cyclic voltammetry (CV). Interference from AA was efficiently eliminated and the electrodes had excellent sensitivity, selectivity, stability and reproducibility in the determination of DA.
Experimental
Chemicals
MWNTs of approximately 95% purity were obtained from Shenzhen Nanotech Port (Shenzhen, China) and purified by heating under reflux in 2.6 mol L−1 HNO3 for 10 h. This treatment produced oxygen-containing moieties (for example carboxylic acid groups) at the open ends of the nanotubes [15].
Dopamine and ascorbic acid were purchased from Sigma–Aldrich (USA). Aniline was from Shanghai Reagent Factory (Shanghai, China) and was redistilled before use. β-Cyclodextrin (β-CD) was provided by Sanland-Chem International. Phosphate buffer solutions (PBS, 0.1 mol L−1) of different pH were prepared by mixing stock standard solutions of Na2HPO4 and KH2PO4 and adjustment of the pH with HCl or NaOH.
All other reagents were analytical grade and were used without further purification. Double-distilled water was used in the experiments. Buffer and sample solutions were purged with high-purity nitrogen for at least 5 min before experiments. All experiments were conducted at room temperature.
Apparatus
Electrochemical measurements were performed with a CHI 660A electrochemical workstation (Shanghai Chenhua Apparatus), with CHI software, connected to a personal computer. All experiments were performed with a conventional three-electrode system comprising a modified glassy-carbon working electrode (GC, 3 mm in diameter), a platinum wire auxiliary electrode, and an Ag|AgCl|KCl (3 mol L−1) reference electrode (all from CH Instruments, USA). All potentials were relative to the reference electrode.
The surface morphology of the modified electrode was analyzed by field-emission scanning electron microscopy (SEM; Jeol JSM-6700 F).
Preparation of the β-CD-MWNTs/PANI-modified GC electrode
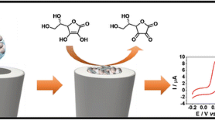
First, 2 mg MWNT was dispersed with the aid of ultrasonic agitation in 1 mL aqueous β-CD solution (2%) to give a 2 mg mL−1 black suspension. A GC electrode was carefully polished with 1.0 μm, then 0.3 μm, then, finally, 0.05 μm α-Al2O3 powder slurries on 1200 grit Carbimet disk, until a mirror-shiny surface was obtained, then sonicated sequentially in ethanol and double-distilled water for 3 min to remove traces of alumina and possible contaminants. The clean GC electrode was then immersed in a solution containing 0.025 mol L−1 aniline and 0.25 mol L−1 H2SO4 and the potential was swept between −0.2 and 0.8 V at a scan rate of 20 mV s−1. In this way a PANI film was formed on the electrode surface. Finally, 6 μL of the dispersion of 2 mg mL−1 MWNT in 2% β-CD solution was dropped on the PANI film-coated electrode and dried in air at room temperature.
Analytical procedure
The sensor was immersed in 0.1 mol L−1 PBS solution containing a known concentration of DA for 1 min accumulation at open circuit potential with stirring. A CV plot was then recorded from −0.1 to 0.4 V with a scan rate of 50 mV s−1. DPV was performed with an amplitude 50 mV, a pulse width of 50 ms, and a pulse period of 0.2s.
Results and discussion
SEM characterization
The SEM image of the surface of the β-CD-MWNTs/PANI film-modified GC electrode is shown in Fig. 1. Homogeneous MWNTs with a diameter of 20–40 nm were clearly observed at high magnification. The structure was also apparent; it consisted of a three-dimensional porous interspace formed by randomly tangled carbon nanotubes. This structure would therefore facilitate heterogeneous electron transfer and enhance the electrocatalytic reaction between electroactive DA species and the electrode surface, owing to the electronic properties of carbon nanotubes.
Electrochemical behavior of DA at the β-CD-MWNTs/PANI film-modified GC electrode
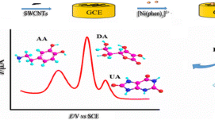
Figure 2 shows the CVs obtained, at a scan rate of 50 mV s−1, from 5 × 10−5 mol L−1 DA at the bare GC electrode (curve a), at the PANI film-coated GC electrode (curve b), at the MWNTs/PANI-modified GC electrode (curve c), and at the β-CD-MWNTs/PANI-modified GC electrode (curve d) in 0.1 mol L−1 PBS (pH 7.0). It is apparent from curve (a) that the electrochemical response of the bare GC electrode to DA was very poor; a broad, small anodic peak was obtained for DA. When the GC electrode surface was coated with a PANI film the response to DA became better and the peak current was slightly enhanced (curve b). This was attributed to the electrocatalytic effect and superior conductance of the redox PANI polymer. When the electrode was modified with the MWNTs/PANI film, an obvious couple of a well-defined redox peak of DA appeared and the peak current increased substantially (curve c). A large background current was observed, however, owing to the catalytically active surface of the MWNTs. The reason for the better performance may be the nanometer dimensions of the tubes, the electronic structure, and topological defects on the surface of the MWNTs. When curve (c) is compared with curve (d) it is apparent that the peak current for DA with the β-CD-MWNTs/PANI film-modified GC electrode was obviously greater than that with the MWNTs/PANI film-modified electrode. This signal enhancement could be because the concentration of DA at the β-CD-MWNTs/PANI film was greater than that at the MWNTs/PANI film, because of the formation of an inclusion complex between β-CD and DA. The β-CD-DA complex in the β-CD-MWNTs/PANI film would then dissociate and diffuse rapidly through the porous layer of MWNTs to the electrode surface, thus efficiently promoting electrochemical reaction of DA. These phenomena suggested that the β-CD-MWNTs/PANI-modified GC electrode not only benefited from use of MWNTs but also from the ability of β-CD to form inclusion complexes with organic molecules and the high conductivity and electrocatalytic effect of PANI. The β-CD-MWNTs/PANI-modified GC electrode thus gave the best analytical performance in the determination DA.
Effect of scan rate and pH
The effect of scan rate on the peak current of DA was investigated experimentally. Figure 3 shows that the CVs obtained from 5 × 10−5 mol L−1 DA in 0.1 mol L−1 PBS (pH 7.0) at the β-CD-MWNTs/PANI film-modified GC electrode depended on the scan rate. The anodic and cathodic peak currents were proportional to scan rate in the range from 25 to 300 mV s−1. As shown in Fig. 3, inset, the linearity of the plots was good—the correlation coefficients were 0.9990 and 0.9998, respectively. It was therefore assumed the electrode reaction of DA at the β-CD-MWNTs/PANI film-modified electrode was typical of an adsorption-controlled process. Redox peak currents that increased linearly with scan rate were also expected as a thin-layer electrochemical property [26, 27]. The surface concentration (г) of DA on the surface of the β-CD-MWNTs/PANI film could therefore be estimated by use of the equation:
Cyclic voltammograms obtained, at different scan rates, from the β-CD-MWNTs/PANI-modified GC electrode in 0.1 mol L−1 PBS (pH 7.0) containing 5 × 10−5 mol L−1 DA. Scan rate (mV s−1): (a) 25, (b) 50, (c) 75, (d) 100, (e) 150, (f) 200, (g) 250, and (h) 300. Inset: plots of anodic and cathodic peak currents against scan rate
In the experiment, under the conditions of saturated adsorption, the average surface concentration of DA was approximately 8.385 × 10−10 mol cm−2. This implied that the adsorbed DA was an approximate monolayer.
The pH of the solution also had a substantial effect on the electrochemical response to DA at the β-CD-MWNTs/PANI film-modified GC electrode, by altering both the peak current and the peak potential. Figure 4 shows the dependence of the anodic peak potentials of DA on pH. It was found that the anodic peak potential shifted negatively with increasing pH of the solution, suggesting that protons participated in the electrode reaction. The relationship between the anodic peak potential and pH could be expressed by the equation E pa = 0.7278–0.07341 pH (the E pa–V correlation coefficient, r, was 0.9989), which was also consistent with the transfer of two protons and two electrons during the oxidation of DA [28]. It was also observed that the anodic peak current increased with increasing solution pH value until it reached 7.0. When the pH value was greater than 7.0 the anodic peak current decreased. pH 7.0 was therefore chosen for the supporting electrolyte in the electrochemical detection of DA; this is also close physiological pH conditions.
Effect of accumulation time
The effect of accumulation time in 5 × 10−5 mol L−1 DA solution on anodic peak current at the β-CD-MWNTs/PANI film-modified GC electrode was investigated. It is clear from Fig. 5 that the anodic peak current of DA was almost constant after 1 min, indicating an accumulation time of 1 min was sufficient to achieve DA saturation of the electrode. An accumulation time of 1 min was therefore adopted in subsequent experiments.
Determination of DA
At the β-CD-MWNTs/PANI film-modified GC electrode in 0.1 mol L−1 PBS (pH 7.0), positively charged DA (pK a = 8.87) [29] would be attracted to the negatively charged MWNTs with carboxylic acid functional groups by electrostatic interaction. In addition, preconcentration was achieved, by formation of an inclusion complex between β-CD and DA, after 1 min accumulation at open circuit potential. The electrochemical reaction of DA was, therefore, efficiently promoted and sensitive detection of DA further improved.
Figure 6 shows a series of CVs obtained for DA at different concentrations at the β-CD-MWNTs/PANI-modified GC electrode in 0.1 mol L−1 PBS (pH 7.0). The results indicate that the logarithm of anodic peak current was proportional to the logarithm of DA concentration in the range 1.0 × 10−7–1.0 × 10−3 mol L−1. The linear regression equation was lg[Ipa]/μA = 3.2500 + 0.4326 lg[C]/mol L−1 with a correlation coefficient of r = 0.9986 (n = 3, RSD = 2.9%; Fig. 6, inset). The detection limit was 1.2 × 10−8 mol L−1 at a signal-to-noise ratio (S/N) of 3.
Cyclic voltammograms obtained from the β-CD-MWNTs/PANI-modified GC electrode for different concentrations of DA in 0.1 mol L−1 PBS (pH 7.0). DA concentration (μmol L−1): (a) 0.1, (b) 0.3, (c) 0.5, (d) 1.0, (e) 5.0, (f) 10.0, (g) 50, (h) 100, (i) 200, (j) 300, (k) 400, (l) 500, (m) 600, (n) 700, (o) 800, (p) 900, and (q) 1000 . Scan rate 50 mV s−1. Inset: concentration calibration plot of the anodic peak current for DA
The intra and the inter-assay reproducibility of the sensor were investigated. The maximum values of the intra and the inter-assay relative standard deviations were approximately 3.9% (n = 5) and 5.4% (n = 5), respectively. The reason for these results was the change of electrode positions and/or different surfaces from electrode to electrode. The results showed the reproducibility of detection of DA by the sensor was acceptable.
The stability of the modified electrode was also evaluated. After each measurement, the sensor was regenerated by immersing it in blank buffer, with stirring, to wash DA from the β-CD-MWNTs/PANI film-modified GC electrode. The sensor was then verified for the film purity, by recording a CV curve in a blank solution, and used repeatedly in a sample solution. It was found that the peak current was nearly unchanged after cyclic scanning for 100 cycles in PBS solution. When the modified electrode was stored in air for two weeks the shape of voltammetric curves for DA remained the same and only 7.5% decay of the initial current was observed.
Interference of ascorbic acid
Ascorbic acid (AA) is present with DA in mammalian brain, and its concentration is much higher than that of DA. It can be oxidized at a potential close to that of DA at traditional solid electrodes, resulting in overlapping voltammetric responses. Use of the electrode modified with the β-CD-MWNTs/PANI film effectively overcame the problem, however, enabling differentiation of the voltammetric response of AA and DA. The reasons for this were:
-
1.
the conducting polymer of PANI served as a permselective layer with excellent anti-interference properties. PANI also has different electrocatalytic effects on AA and DA, so AA and DA were oxidized at different potentials;
-
2.
electrostatic repulsion between the AA anion and the negatively charged carboxylate groups of MWNT in PBS (pH 7.0) blocked electron transfer, which altered the potential for AA toward a more negative value; and
-
3.
electrostatic attraction between the positively charged DA and the negatively charged MWNT facilitated movement of DA to the surface of the modified electrode.
Thus, the peak of DA was readily separated from that of AA and overlapping of the voltammetric responses of AA and DA was eliminated.
Differential pulse voltammetry (DPV) was performed to investigate the effect of AA on the electrochemical response to DA at the β-CD-MWNTs/PANI film-modified GC electrode. Figure 7 shows DPV recordings obtained from different concentrations of DA in the presence of AA. The electrochemical signals of DA and AA are clearly apparent. The oxidation peak potentials were 0.184 V for DA and −0.008 V for AA. The difference between DA and AA peak potentials was calculated to be 192 mV. It was also found the anodic peak current for DA increased linearly with increasing DA concentrations from 0.1 to 10 μmol L−1, with a correlation coefficient of 0.9954, whereas the anodic peak current of AA remained nearly constant (as shown in Fig. 7, inset). AA would, therefore, not interfere with detection of DA at the modified electrode and selective detection of DA was possible in the presence of AA.
Analytical applications
For preliminary evaluation of this electrochemical sensor, injection of dopamine hydrochloride was tested. In accordance with the methods proposed above, the amount of dopamine hydrochloride in the injection was determined by use of a calibration plot. Recovery was studied by adding a known volume of standard DA solution into the injection. The results, shown in Table 1, indicated that both recovery and RSD were acceptable and that the proposed method could be efficiently employed for determination of DA in injections.
Conclusion
A novel, simple, sensitive, and reliable method has been successfully developed for determination of dopamine, in the presence of ascorbic acid, based on incorporation of β-cyclodextrin on to multi-walled carbon nanotube and polyaniline film-modified glassy-carbon electrodes. The electrodes not only had high electrocatalytic activity toward DA oxidation but also high selectivity for determination of DA in the presence of AA. The reason for the excellent performance could be attributed to the combination of three kinds of functional material:
-
1.
PANI with superior transducing properties and good anti-interference characteristics;
-
2.
MWNTs with high electrocatalytic activity, rapid electron transfer rate, and different electrostatic interaction with AA and DA in PBS (pH 7.0); and
-
3.
β-CD for preconcentration by forming a supramolecular complex with DA.
The modified electrodes were highly stable and reproducible and were successfully used to detect DA in real samples. It is hoped the method will be suitable for application to further study of the electrochemistry of biological systems.
References
Wightman R, Amatorh C, Engstrom R, Hale P, Kristensen E, Kubr W, May L (1988) Neuroscience 25:513–523
Wightman R, May L, Michael A (1988) Anal Chem 60:769A–770A
Adams R (1976) Anal Chem 48:1128A–1137A
Kawagoe K, Wightman R (1994) Talanta 41:865–874
Raj C, Okajima T, Ohsaka T (2003) J Electroanal Chem 543:127–133
Raj C, Tokuda K, Ohsaka T (2001) Bioelectrochemistry 53:183–191
Raj C, Ohsaka T (2001) J Electroanal Chem 496:44–49
Ciszewski A, Milczarek G (1999) Anal Chem 71:1055–1061
Mo J, Ogorevc B (2001) Anal Chem 73:1196–1202
Iijima S (1991) Nature (London) 354:56–58
Kuznetsova A, Mawhinney D, Naumenko V, Yates Jr J, Liu J, Smalley R (2000) Chem Phys Lett 321:292–296
Wang Z, Liu J, Liang Q, Wang Y, Luo G (2002) Analyst 127:653–658
Zhang P, Wu F, Zhao G, Wei X (2005) Bioelectrochemistry 67:109-114
Ly S (2006) Bioelectrochemistry 68:227–231
Zhang M, Gong K, Zhang H, Mao L (2005) Biosensors Bioelectron 20:1270–1276
Hoa D, Suresh Kumar T, Punekar N, Srinivasa R, Lal R, Contractor A (1992) Anal Chem 64:2645–2646
Sangodkar H, Sukeerthi S, Srinivasa R, Lal R, Contractor A (1996) Anal Chem 68:779–783
Qu F, Yang M, Jiang J, Shen G, Yu R (2005) Anal Biochem 344:108–114
Li G, Fang H, Chen H (1994) Chemical Research and Application, China, 6:7–12
Rekharsky M, Inoue Y (1998) Chem Rev 98:1875–1917
Zhang Q, Wang N, Zhan W, Xie F, Chen X (2003) Chinese Journal of Spectroscopy Laboratory 20:749–752
Wang Z, Wang Y, Luo G (2002) Analyst 127:1353–1358
Wang G, Liu X, Yu B, Luo G (2004) J Electroanal Chem 576:227–231
Wang Z, Xiao S, Chen Y (2006) J Electroanal Chem 589:237–242
He J, Yang Y, Yang X, Liu Y, Liu Z, Shen G, Yu R (2006) Sensors Actuators B 114:94–100
Bard A, Faulkner L (1980) (eds) Electrochemical methods. Wiley, New York
Murray R (1984) (eds) Electroanalytical chemistry. Marcel Dekker, New York, 13:191–368
Adams R (1969) J Pharm Sci 58:1171–1184
Malem F, Mandler D (1993) Anal Chem 65:37–41
Acknowledgements
This work was supported by Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, T., Wei, W. & Zeng, J. Selective detection of dopamine in the presence of ascorbic acid by use of glassy-carbon electrodes modified with both polyaniline film and multi-walled carbon nanotubes with incorporated β-cyclodextrin. Anal Bioanal Chem 386, 2087–2094 (2006). https://doi.org/10.1007/s00216-006-0845-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0845-z