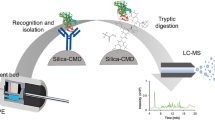
Abstract
Silane-immobilized magnetic iron oxide particles were used as the assisting material in surface-assisted laser desorption/ionization (SALDI) mass spectrometric analysis. This approach can be used to analyze small proteins and peptides. The upper detectable mass range is approximately 16 kDa. The detection limit for peptides is about 20 fmol. Silanized iron oxide particles with negatively charged functionalities can also be used as the affinity probes to selectively trap oppositely charged species from sample solutions by adjusting the pH of the solution. A tryptic digest product of cytochrome C at a concentration as low as 10 nM can be enriched by the particles and directly analyzed by iron oxide SALDI MS without the need for elution steps. Affinity-based mass spectrometry using the bifunctional silanized magnetic iron oxide particles as the SALDI matrix and concentrating probe is demonstrated in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A mixture of inorganic particles and glycerol is a typical matrix used for assisting desorption/ionization of analytes in surface-assisted laser desorption/ionization mass spectrometry (SALDI MS) [1]. Inorganic particles play the role of energy absorber and energy transfer medium, while glycerol provides protons during ionization in SALDI MS analysis. The advantage of using this inorganic/liquid hybrid system is the lack of so-called sweet spots that are commonly observed when organic matrices are used in matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) analysis [2, 3]. However, the presence of liquid in the SALDI samples may affect the vacuum in the mass spectrometer, limiting its capability for high-throughput analysis. Citrate salts and phosphoric acid can replace the use of liquid glycerol. The concern about the use of liquid in the sample preparation can be neglected. The use of inorganic particles in the development of methods of laser desorption/ionization mass spectrometry [1, 4–13] has attracted attention with the rapid growth of materials science research. Tanaka et al. reported the first study using 30-nm cobalt nanoparticles mixed with glycerol as a two phase-matrix to assist laser desorption/ionization mass spectrometric analysis for proteins in the 1980s [4]. Sunner et al. [1] and Dole et al. [5] later demonstrated that micro-sized graphite powder mixed with glycerol could be used as an effective matrix. Sunner et al. termed the method surface-assisted laser desorption/ionization mass spectrometry (SALDI MS) [1] to distinguish it from the method using organic chemicals as the matrix, which was discovered by Karas and Hillenkamp [2, 3]. Hillenkamp and co-workers investigated a series of micro- and nano-sized inorganic particles mixed with glycerol as the SALDI matrix [9]. They found that particles such as titanium nitride, tantalum, and nano-soot mixed with glycerol could be used as the assisting material for SALDI MS analysis of peptides and proteins. They also demonstrated that iron oxide particles (300 nm) mixed with glycerol as the assisting material are effective only for analytes with molecular weight <5 kDa. Recently we have demonstrated that titania-coated magnetic particles can be used as the SALDI matrix and highlighted the absorption capacity of titania toward laser irradiation [10]. No further studies using iron oxide particles alone as the SALDI matrix have been reported.
Herein, we investigate the feasibility of using silanized magnetic iron oxide particles as the SALDI matrix in SALDI MS analysis. This follows our previous demonstration that the role of glycerol as the proton source can be replaced by citric acid or ammonium citrate [10–13]. We used organic acids such as citric salts and phosphoric acid to replace the use of glycerol as the proton source for iron oxide (Fe3O4) SALDI MS analysis. Elimination of a liquid such as glycerol would simplify the preparation of SALDI samples and suit the requirement of high vacuum in the mass spectrometer. Additionally, one of the potential problems using iron oxide particles directly as the SALDI matrix is the retention of impurities such as iron ions on the surfaces of particles, causing serious interference during analysis. Although most iron ions can be removed by rinsing the particles with acid, our experience is that it is impossible to quantitatively eliminate all interferences. We attempted to solve the problem by coating a thin layer of silane onto the surface of the iron oxide particles to prevent undesirable release of iron ions.
Magnetic particles can be easily isolated from sample solutions using an external magnetic field and they have therefore been widely used as concentrating probes for the separation and purification of specific analytes from sample solutions. Previously, we have demonstrated that magnetic particles coated with gold nanoparticles are effective affinity probes for the concentration of trace peptide residues from protein enzymatic digest solutions [14]. The trapped peptide residues are then eluted by using MALDI matrix, followed by MALDI MS analysis. If the magnetic particles could be used directly as the assisting material in SALDI MS analysis, it would not be necessary to elute the trapped species and so the loss of sample during elution could be avoided. We explored the possibility of using iron oxide magnetic particles coated with functional silane to enrich peptide residues from enzymatic digest products of proteins.
Experimental
Reagents
Trifluoroacetic acid (TFA) and hydrochloric acid were obtained from Merck (Seelze, Germany). Iron(III) chloride hexahydrate, ammonium hydrogen carbonate, sodium sulfite, and diammonium hydrogen citrate (DHC) were obtained from Riedel-de Haën (Seelze, Germany). Ammonium hydroxide and tetraethoxysilane (TEOS) were purchased from Fluka (Buchs, Switzerland); ethanol was obtained from Showa (Tokyo, Japan). Insulin, bradykinin, melittin, ubiquitin, cytochrome C, myoglobin, citric acid, 3-aminopropyl triethoxysilane (APTES), and trypsin (from bovine pancreas) were obtained from Sigma (St. Louis, MO). Dimethyl formamide (DMF) was purchased from J. T. Baker (Phillipsburg, NJ);α-cyano-4-hydroxycinnamic acid (CHCA) was obtained from Aldrich (Milwaukee, WI).
Preparation of silanized magnetic iron oxide particles
Magnetic iron oxide nanoparticles were prepared based on the procedures reported by Cao et al. [15]. Ferric chloride (2 M) in hydrochloride solution (2 M, 12 mL) was diluted to 100 mL with air-free distilled water under nitrogen. Freshly prepared aqueous sodium sulfite (0.08 M, 50 mL) was slowly added to the diluted ferric chloride solution. A mixture of ammonia solution (28 %, 8 mL) and water (40 mL) was added slowly to the solution with vigorous stirring under nitrogen. The mixture was then held at 70 °C in a water bath for 15–30 min before cooling to below 45 °C. The magnetite nanoparticles which had formed were aggregated by placing an external magnet on the edge of the vial and the solution was removed. The particles were rinsed several times with distilled water and water/ethanol (2:1) mixture and re-suspended in ethanol (50 mL). Ethanol (7 mL), water (8 mL), TEOS (2 mL), and ammonia solution (10 %, v/v) (2 mL) were added in sequence to 25 mL of the particle suspension (40 mg mL−1) with stirring at 40 °C and stirring was continued for 12 h. The particles were rinsed with methanol several times to remove unreacted TEOS and re-suspended in methanol (40 mL). APTES (98 %, 8 mL) was added to this solution with sonication for 10 min followed by stirring for a further 12 h in the water bath at 60 °C. The particles were rinsed with methanol and re-suspended in 40 mL DMF. A solution of succinic anhydride (4 g) dissolved in DMF (10 mL) was added to the particle suspension and stirred under nitrogen for 8 h. The particles were then rinsed with DMF and water and then dispersed in water (25 ml).
Use of magnetic iron oxide particles as SALDI matrix
The particles prepared above were immersed in an HCl (4 N) solution for 10 min to dissolve unsilanized Fe3O4 magnetic particles. The rinsed particles were re-suspended in a solution containing citric acid (75 mM), DHC (75 mM), and triammonium phosphate (100 mM) to prepare a particle solution at a concentration of 50 mg mL−1. A solution of particle suspension (0.2 μL) was deposited onto a sample plate followed by the analyte solution (0.15 μL). After evaporation of the solution, the sample was ready for SALDI MS analysis.
Use of silanized magnetic iron oxide particles as affinity probes to enrich target species
The samples used for enrichment included insulin and the tryptic digest product of cytochrome C. Both cytochrome C and trypsin were prepared in aqueous ammonium bicarbonate solutions (25 mM, pH 8.0). Cytochrome C and trypsin, at a weight ratio of 41:1, were incubated at 37 °C for 24 h. The digest product was then diluted in a given volume and adjusted to a specific pH before performing the enrichment. A silanized iron oxide particle suspension (0.3 μL, 30 mg mL−1) was added into the sample solution (50 μL) to carry out the enrichment. After incubation for 1 h, the particles conjugated with their target species were isolated by applying an external magnetic field, and rinsed with the same solution as used in the enrichment step. The rinsed particles were then mixed with citrate solution (0.2 μL) containing citric acid (150 mM) and DHC (150 mM). The mixture was then applied to the sample target. After evaporation of the solution, the sample was ready to load into the mass spectrometer for SALDI MS analysis.
Instrumentation
All mass spectra were obtained using a Biflex III (Bruker Daltonics, Germany) time-of-flight mass spectrometer equipped with a 337-nm nitrogen laser, a 1.25-m flight tube, and a sample target having the capacity to load 384 samples simultaneously. The accelerating voltage was set at 19 kV. The TEM image was obtained using a JEOL 2000FX (Japan) transmission electron microscopy (TEM).
Results and discussion
Figure 1 displays the Fe3O4 SALDI mass spectrum of bradykinin using bare Fe3O4 magnetic particles mixed with citrate salts (citric acid (150 mm) and DHC (150 mM)) as the SALDI matrix. The mass spectrum contains the protonated pseudomolecular ion of bradykinin at m/z 1,060.6. The result indicates that this approach can be used for peptide analysis. However, when we employed bare magnetic iron oxide particles mixed with citrate salts as the SALDI matrix for SALDI MS analysis of small proteins such as ubiquitin, the quality of the SALDI mass spectra was undesirable due to the presence of iron ions on the surfaces of the particles. A series of iron–analyte adducts appear in the mass spectrum. Figure 2a displays the SALDI mass spectrum of ubiquitin using bare magnetic iron oxide particles as the SALDI matrix. Two broad peaks representing for the doubly and singly charged ions of ubiquitin appear in the mass spectrum. A series of iron adduct ions of ubiquitin are adjacent to the pseudomolecular ions of ubiquitin ([M+H]+, [M+2H] 2+). With higher molecular weights of analyte molecules, the series of iron–analyte adduct ions becomes an unresolved and broad peak (results not shown). We found that if the molecular weights of analytes are larger than 5 kDa, the iron–analyte adduct ions become the dominant peaks. However, iron–analyte adduct ions with molecular weight <3 kDa are not observed in the Fe3O4 SALDI mass spectra. We believe that this effect probably arises from the chelating ability of Fe(III) for functional groups such as carboxylic and amino groups on the protein molecules. When small proteins such as insulin and ubiquitin were used as the samples, several functional groups may coordinate with iron ions and result in the formation of iron–analyte adducts. Because smaller analytes such as peptides contain a limited number of amino acids, there are insufficient functional groups to chelate with iron ions. Therefore, no iron adducts appear in the Fe3O4 SALDI mass spectra when using peptide samples. To improve the mass spectral quality, we added triammonium phosphate to reduce the formation of iron adducts of ubiquitin (Fig. 2b) because iron ions form complexes with phosphate with higher affinity than with ubiquitin. The results demonstrate that the addition of triammonium phosphate can significantly reduce the iron ion adducts.
The magnetic particles were coated with a thin layer of silane and subsequently chemically modified to improve the Fe3O4 SALDI MS analysis by reducing interference due to iron ions and impurities. Scheme 1 presents the proposed structure of this modification. The particle surfaces have negatively charged groups after the modification, which was designed for studies as affinity probes.
Figure 3 presents the TEM image of the silanized magnetic iron oxide particles. The average size of the nanoparticles is approximately 25 nm. After silanization, several nanoparticles were linked together to form nano-composite particles. Thus, the surfaces of the magnetic particles are rough and have high specific surface areas.
To investigate the feasibility of using the silanized magnetic iron oxide particles as the SALDI matrix, several peptides and small proteins were analyzed using Fe3O4 SALDI MS. 4 presents the Fe3O4 SALDI mass spectra of bradykinin (141 fmol), melittin (53 fmol), insulin (26 fmol), and ubiquitin (174 fmol). The use of silanized particles effectively eliminated undesirable interferences such as those due to iron ions. Figure 5 presents the Fe3O4 SALDI mass spectra of cytochrome C (1.2 pmol) and myoglobin (890 fmol). Myoglobin is the largest molecule detected by Fe3O4 SALDI MS so far. The detection limit is approximately 20 fmol for peptides but several hundred fmol for small proteins.
We investigated the feasibility of using silanized magnetic nanoparticles with negatively charged surfaces as selective affinity probes toward positively charged species. The isoelectric point (pI) of insulin is 5.3, giving it a net positive charge at pH 5. Our negatively charged magnetic particles were used as affinity probes to trap target species from a sample solution containing insulin (10 nM, 50 μL) at pH 5. This was followed by direct Fe3O4 SALDI MS analysis of the magnetic particles conjugated with target species.
After trapping insulin in this way, the protonated pseudomolecular ion of insulin at m/z 5,734.5 appears in the Fe3O4 SALDI mass spectrum (Fig. 6a). However, after adjusting the pH of the same sample solution to pH 8, no peaks appear in the mass spectrum (Fig. 6b) after trapping treatment by the affinity probes. This result indicates that these negatively charged affinity probes can trap trace positively charged species from sample solutions.
We applied this approach to enrich trace peptides from enzymatic digest products. To demonstrate enrichment by our affinity probes, we used a low concentration of tryptic digest of cytochrome C (10−8 M) as the sample. No peptide ions were detected in either direct MALDI mass spectrum (Fig. 7a) or direct Fe3O4 SALDI mass spectrum (Fig. 7b) before enrichment. However, after the digest product was enriched by our affinity probes, several peaks with quite good intensities appeared in the Fe3O4 SALDI mass spectrum (Fig. 7c). The peptide sequences and theoretical pI values for each peptide ion appearing at m/z 11,68.9, 1,296.7, 1,350.9, 1,433.5, 1,478.7, 1,607.1, and 1,735.3 are listed in Table 1. Besides a weak peptide ion peak at m/z 1,350.9, the pI values of the other peaks are larger than 5. The results indicate that those peptides having pI >5 have net positive charges at pH 5 and can be trapped and enriched by the negatively charged magnetic particles. The appearance of the peak at m/z 1,350.9 may be owing to the presence of two acidic amino acids (E) in the peptide sequence.
a Direct MALDI mass spectrum [CHCA (25 mg/mL, 0.2 μL) dissolved in the solution of acetonitrile/1 % TFA (2/1, v/v) was used as the matrix] and b direct Fe3O4 SALDI MS mass spectrum of the tryptic digest product of cytochrome C analysis sample (10 nM, 0.15 μL). c Fe3O4 SALDI MS mass spectrum obtained after using the silanized magnetic iron oxide particles to selectively concentrate target species from the tryptic digest product of cytochrome C (10 nM, 0.2 mL) at pH 5
Conclusions
We have demonstrated that bifunctional magnetic particles can be used as SALDI matrix and affinity probes. Solutions containing traces of enzymatic digests can be enriched using bifunctional magnetic iron oxide particles and directly analyzed using Fe3O4 SALDI MS. We demonstrated that negatively charged surfaces of magnetic nanoparticles are capable of concentrating traces of positively charged species from aqueous solutions. However, the iron oxide magnetic particles could be further modified to develop selective affinity probes for specific analytes. Affinity-based mass spectrometry using silanized magnetic particles both as the SALDI matrix and affinity probe could be further developed for analysis of trace target species from complex samples.
Rererences
Sunner J, Dratz E, Chen Y-C (1995) Anal Chem 67:4335–4342
Karas M, Bachmann D, Hillenkamp F (1985) Anal Chem 57:2935–2939
Karas M, Hillenkamp F (1988) Anal Chem 60:2299–2301
Tanaka K, Waki H, Ido Y, Akita S, Yoshida Y, Yoshida T (1988) Rapid Commun Mass Spectrom 2:151–153
Dale MJ, Knochenmuss R, Zenobi R (1996) Anal Chem 68:3321–3329
McLean JA, Stumpo KA, Russell DHJ (2005) Am Chem Soc 127:5304–5305
Hoang TT, Chen YF, May SW, Browner RF (2004) Anal Chem 76:2062–2070
Finkel NH, Prevo BG, Velev OD, He L (2005) Anal Chem 77:1088–1095
Schürenberg M, Dreisewerd K, Hillenkamp F (1999) Anal Chem 71:221–229
Chen C-T, Chen Y-C (2005) Anal Chem 77:5912–5919
Chen C-T, Chen Y-C (2004) Anal Chem 76:1453–1457
Chen C-T, Chen Y-C (2004) Rapid Commun Mass Spectrom 18:1956–1964
Chen W-Y, Wang L-S, Chiu H-T, Chen Y-C, Lee C-YJ (2004) Am Soc Mass Spectrom 15:1629–1635
Teng C-H, Ho K-C, Lin Y-S, Chen Y-C (2004) Anal Chem 76:4337–4342
Cao J, Wang Y, Yu J, Xia J, Zhang C, Yin D, Häfeli UO (2004) J Magn Magn Mater 277:165–174
Acknowledgements
The authors would like to thank the National Science Council of Taiwan for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, WY., Chen, YC. Affinity-based mass spectrometry using magnetic iron oxide particles as the matrix and concentrating probes for SALDI MS analysis of peptides and proteins. Anal Bioanal Chem 386, 699–704 (2006). https://doi.org/10.1007/s00216-006-0427-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0427-0