Abstract
The use of natural antioxidants is of increasing importance in the human diet, because they are recognised as compounds essential to health which minimize or delay the aging process. Despite apparent simplicity, however, it is very difficult to measure and quantify such properties, for which a robust analytical method is required. Because oxidation usually is caused by the presence of OH· radicals, a new method involving the in-situ, vapour-phase generation of these radicals and their quantification in the presence and absence of potential antioxidant extracts has been developed. The oxidant atmosphere generated from hydrogen peroxide is carried by an air stream through an empty quartz chamber in which UV radiation promotes the formation of radicals by a photochemical reaction. The products then pass through a cartridge containing the essential oil, finally bubbling into an impinger containing an aqueous solution of salicylic acid, at pH 4.5, which reacts with the OH· radicals forming 2,5-dihydroxybenzoic acid. This solution is quantified by RP-HPLC using UV and fluorescence detectors connected in series. Detection and quantification limits for OH· radicals were approximately 0.01 pg g−1 air. Description and optimization of the method are discussed, as also is the antioxidant performance of an extract of ginger (Zingiber officinale R.), which reduced the oxidation process by up to 92%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumption of natural products with antioxidant properties is increasingly used to delay or prevent the aging process and to protect humans against severe diseases, for example cancer. Among natural products, essential oils are becoming of great importance, because they have been used for decades to increase the shelf life of food, because of their antioxidant and antimicrobial properties [1, 2]. Essential oils are a group of volatile oils responsible for the characteristic odours or fragrances of plants. They contain a complex mixture of compounds from different chemical groups, including terpenoids, aldehydes, ketones, and phenols. Some of these compounds are known to be antioxidants but to establish their antioxidant capability is very difficult, because a multistep procedure involving the oxidation process and a final quantitative measurement are required. The methods more commonly used are based on spectrophotometric determinations such as the Trolox equivalent antioxidant capacity (TEAC) [3, 4], ferric-reducing antioxidant power (FRAP) [3], or thiobarbituric acid reactive substances (TBARS) [5], chemiluminiscence techniques with luminol [3, 4], and other, more specific, methods in which enzymes are involved [4].
It is known that the oxidation process is a radical reaction initiated by the presence of OH· and O· radicals. Because these radicals are the main oxidation agents responsible for most organic reactions [6], their direct determination is very interesting, because it would enable measurement of the antioxidant properties of compounds by quantitative determination of radicals. This is not an easy task, however. OH· exists in the atmosphere at very low concentration (0.04 pg g−1 air, approx. 106 molecules cm−3 air), with a very short half-life [7]. This means that to measure such low concentration of radicals a detection limit of 0.04 pg g−1 air would be necessary. Alternatively, longer sampling periods could be used to reach the quantification limits of existing analytical procedures for analysis of OH· radicals. This step involves sampling periods of more than 10 days for each sample, which is not useful for practical purposes. One solution is to generate an OH·-enriched atmosphere which can be placed in contact with antioxidant substances; quantitative measurement could then be performed. For this reason, to measure, in a reasonably short time, the antioxidant capacity of essential oils and simultaneously demonstrate the antioxidant mechanism, generation of a significant, known concentration of OH· in the gas phase is required.
Generation of an oxidant atmosphere with a given concentration of OH· radicals has previously been studied using aqueous media. Although very energetic (λ=185 nm) UV-photolytic OH· generation directly from water is possible [7]. Most previous studies have been performed with hydrogen peroxide, which decomposes under the action of UV radiation at 254 nm giving very reactive species, mainly OH· radicals [8]. Generation of OH· radicals in vapour phase, however, one of the main targets of this work, is difficult, because radical stability is very low and the radicals readily disappear as a result of chemical reaction.
Because of these difficulties, quantification of OH· radicals is usually performed by indirect methods which entail determination of hydroxylation products, for example 2-hydroxyterephthalic acid [9–11] or 8-hydroxy-2′-deoxyguanidine [12, 13], generated in aqueous media from radical scavengers. Among these, especially for biological systems, the most frequently used is an approach developed by Halliwell et al. [14], based on measurement of hydroxylated species from salicylic acid. This method has become the most common procedure for determination of OH· radicals in the aqueous phase, because of the ease of analysis of the resulting compounds by HPLC with fluorescence detection. Nevertheless, application of this method to the determination of OH· in gas phase is relatively new [7, 8]. After hydroxylation, dissolved oxygen abstracts hydrogen from the intermediate releasing HO2 and a range of oxidation products, the most important of which are 2,5-dihydroxybenzoic acid (2,5-DHB), 2,3-dihydroxybenzoic acid (2,3-DHB), and catechol. Because these are the main products, they are used as tracers to determine the OH· concentration [7]. In in-vivo studies only 2,3-DHB and 2,5-DHB are considered, because catechol formation is negligible [15] under the test conditions.
It is well known that, in addition to being produced by radical reactions, 2,5-DHB is also a reaction product of microbial metabolism of salicylic acid. Consequently, in biological research 2,3-DHB evaluation is preferred, because its origin is solely via radicals [14, 16–18]. The fluorescence intensity of 2,5-DHB is, however, much higher than that of 2,3-DHB. Because microbial metabolism can be reduced to a minimum by controlling the generated OH·, only 2,5-DHB will be considered as target compound for OH· evaluation.
This paper reports a new system developed to generate, in a controlled manner, a known concentration of OH· radicals in the gas-phase. They are then passed through a cartridge containing the antioxidant compound under test, in this work a natural essential oil of ginger (Zingiber officinale R.). Finally, OH· radicals are trapped in an aqueous solution of salicylic acid, then converted into 2,5-DHB. The final solution is measured by HPLC with highly sensitive fluorescence detection. Although assessment of the antioxidant capacity of different plant species is not a challenge analytically [3], the complexity of existing methods makes the study presented here a relatively simple, cheap, and portable option for monitoring hydroxyl radical (OH·). It is also important to confirm that these antioxidant extracts are efficient radical scavengers, by demonstrating protection against oxidation mechanisms. Optimization of the device used and results obtained in the presence of different concentrations of the essential oil are reported and discussed.
Experimental
Reagents
Sodium salicylate >99.5% (CAS 54-21-7), salicylic acid (SA) >99.0% (69-72-7), and 2,5-dihydroxybenzoic acid (2,5-DHB) >99.0% (490-79-9) were from Sigma–Aldrich (Madrid, Spain). Methanol, gradient HPLC grade (67-56-1) was from Scharlab (Barcelona, Spain). Ultrapure water was obtained from a Milli-Q system (Millipore. Billerica, MA, USA). Hydrogen peroxide >34.5% (7722-84-1) was from Scharlab. Silanized glass wool was from Supelco (Bellefonte, PA, USA). Ginger, Zingiber officinale R., extract (084696-15-1) was from Artibal (Sabiñánigo, Spain). Because SA and its oxidation products are light sensitive, special care was taken to prevent their degradation by covering the flasks containing the solutions with aluminium foil in both storage solutions and experimental setup, thus avoiding possible interferences.
Apparatus
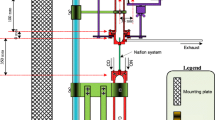
OH· radical generation in the gas phase was achieved by means of the set-up shown in Fig. 1. Compressed air was supplied by a Cecatto Bluair compressor (Brendola, Italy). Aerosol was generated by means of the complete pneumatic section (flow controller and nebulizer) of an old Perkin Elmer 370A atomic absorption spectrophotometer (Wellesley, MA, USA). To improve the accuracy of hydrogen peroxide feed flow, the capillary inlet tube was connected to a peristaltic pump from Bio-Rad (Hercules, CA, USA), set at 0.8 mL min−1. Total air flow was set at 940 mL min−1. The reaction chamber used to generate OH· from H2O2 (2.9×10−1 mol L−1) consisted of a 300 mm×30 mm cylindrical quartz tube with opposite inlet and outlet. UV radiation required for the reaction was supplied by four 140 mm×15 mm Philips fluorescent UV lamps TL 4W/08 F4T5/BLB Hg, (Eindhoven, Holland), placed axially around the quartz tube. Two home-made glass traps (two pieces each, with 29/32 male-female connectors) were used to contain the glass wool on which the essential oil was supported in one of the lines; the other contained only glass wool as blank reference. Salicylate solution (50 mL, 1.25×10−5 mol L−1 sodium salicylate in 250 μmol L−1 H3PO4 at pH 4.5) was contained in two glass Drechsler-type gas-washing bottles. To minimize interferences, all connections were made with PTFE tubing (6 mm i.d., 9 mm o.d.) of the shortest possible length to achieve maximum efficiency.
Chromatographic analysis of 2,5-DHB and remaining SA were performed with a Hewlett–Packard 1050 Series HPLC system (Palo Alto, CA, USA) coupled to a Waters 474 fluorescence detector (Waters, Milford, MA, USA) operating at the optimum wavelengths for both compounds (2,5-DHB and SA: λ exc=324 nm, λ ems=448 nm). Separation was achieved on a reversed-phase column (250 mm long, 4.6 mm i.d., 5 μm) Luna C18(2) 100 Å (Phenomenex, Torrance, CA, USA). The mobile phase (isocratic mode, 1.5 mL min−1) was aqueous acetate buffer (pH 5.9, 35 mmol L−1)–methanol, 90:10 (v/v); the injection volume was 20 μL. A UV detector (Hewlett–Packard 1050 Series HPLC System) coupled in series before the fluorescence detector was used to monitor the non-fluorescent compounds.
Results and discussion
Generation of OH· radicals
As mentioned in the Introduction, generation of OH· radicals in the gas phase is possible by photolysis of aqueous hydrogen peroxide solution using the experimental device depicted in Fig. 1 and sketched in Fig. 2. Because yield is critically affected by particle size, to obtain the smallest possible drops, in a controlled and reproducible manner, the described nebulizer was used. When nebulized solution is irradiated with UV light at 254 nm, the aerosol drops decompose, giving OH· radicals which can be carried by the air stream to another chamber. This was the basis of the developed device.
Flow-chart diagram of the experimental setup depicted in Fig. 1
Critical conditions for obtaining the maximum concentration of OH· radicals were:
-
1
the concentration of hydrogen peroxide;
-
2
the air flow;
-
3
the size of the photoreactor; and
-
4
the intensity of the UV radiation.
Maximum generation of radicals was achieved by using 2.9×10−1 mol L−1 H2O2 and 940 mL min−1 air flow. Greater flows carried the largest drops of the aerosol to the cartridge in which the antioxidant was supported on silanized glass wool (to eliminate possible adsorption by normal glass wool), producing larger signals for both 2,5-DHB and free H2O2 in the final impinger, which was not the purpose. To demonstrate that the essential oil can act as antioxidant without direct contact with the oxidant solution of OH· radicals, and consequently in vapour phase, it was necessary to guarantee that the OH· radicals were generated and transported in the vapour phase. The concentration of H2O2 should not, therefore, exceed 2.9×10−1 mol L−1 and the air flow was maintained at 470 mL min−1 for each impinger.
Three different sizes of cylindrical quartz photoreactor were tested. The first, 100 mm×30 mm, resulted in very low concentrations of OH· radicals and the pathway of drops was too short to guarantee that no drops of the H2O2 solution reached the cartridge. The second reactor tested was 500 mm×50 mm; this resulted in an even lower concentration of radicals, although the H2O2 drops did not reach the cartridge. The pathway was so long, however, that the radicals did not survive long enough in the system. Finally, a 300 mm×30 mm chamber gave the best results and was therefore selected for the study.
Oxidation reaction
As already mentioned above, SA was used to trap the OH· radicals. Figure 3 shows the mechanism of the reaction.
It is commonly accepted that reaction occurs via an intermediate hydroxycyclohexadienyl radical [19], because of ring activation, the positions ortho and para to the hydroxyl group are more easily oxidized than the meta position [20]. Different conditions affect the reaction. First, pH is expected to affect the reaction because of the acidity constants of SA. The pK a1 corresponding to dissociation of the carboxyl group is 2.97 and that for –OH· dissociation, pK a2, is 13.74 [21]. Because the reaction is supposed to be based on the presence of carboxylate species, the optimum pH should be between 2.97 and 13.74, which is a large range.
When the effect of pH was studied in depth it was found that at pH below 4.0 no signal was obtained from 2,5-DHB, because the response was below the detection limit. Maximum response was obtained at pH 4.5, which means the reactive species is the salicylate, as mentioned by other authors [19, 22, 23]. This can be explained on the basis of the inductive effect of the functional groups. In particular, the main difference is the presence of –COOH or –COO−. The first is electron-withdrawing (as also is –OH· which remains unchanged) whereas the second is electron-donating, which contributes more efficiently to stabilization of the ionized structure than does the carboxyl form. The concentration of SA also has a critical effect. The range of concentrations studied was between 4.00×10−6 and 1.30×10−5 mol L−1 and the optimum SA concentration was 1.25×10−5 mol L−1.
Assuming that the concentration of generated radicals is very low, long periods of photochemical reaction were required to produce enough OH· radicals to exceed the quantification limit of 2,5-DHB, the final fluorescent compound to be measured. For this reason, numerous experiments were required with different photoreaction times to discover the optimum conditions: 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 96, and 240 h were tested. Not detectable or very low concentrations of 2,5-DHB were observed when sampling times were less than 8 h. When samples were subjected to oxidation for more than 48 h, on the other hand, and despite adequate conversion of SA into 2,5-DHB, high RSD values (more than 50%) were observed. Taking both considerations into account, a reaction time of 24 h was finally selected for optimum sensitivity and reproducibility.
Analytical features
As mentioned above, the final product to be measured was 2,5-DHB, a fluorescent compound. Calculated analytical data for the quantitative determination were as shown in Table 1.
The detection limit for 2,5-DHB was calculated from the errors associated with the calibration plot [24]. The second isomer (2,3-DHB), a side product of oxidation, was not used because of its low fluorescence sensitivity.
The concentrations of OH·, expressed as molecules per cm3 air, were determined from the concentration of 2,5-DHB, expressed in mol L−1, by use of Eq. (1):
Where V (L) is the post-reaction volume, N A is Avogadro’s constant, F (L min−1) the air flow during the test, t (min) is the time of the test, and B the hydroxylation coefficient, which is the ratio of the amount of SA remaining to the amount of SA eliminated by the reaction [7], as shown by Eq. (2):
To determine this coefficient and the concentration of 2,5-DHB it is crucial to quantify the OH· radicals. During the test, substantial loss of water by evaporation was observed, between 1.7×10−2 and 3.3×10−2 mL min−1, because of the high air flow used (940 mL min−1). To avoid miscalculations during quantification, the volume was kept constant during the process by adding the required volume of water to the impinger when required.
Quantification of the antioxidant capability
When generation of OH· radicals and their measurement had been optimized using the system described, the behaviour of different concentrations of ginger essential oil, with known antioxidant properties [25], was studied. After screening experiments to select the most appropriate concentration range (more exactly mass), 0.1 to 0.5 g was tested in the cartridges. Use of the larger amounts led to the presence of the essential oil in the SA solutions, which was not acceptable, whereas masses below than 0.1 g gave results which were not statistically significantly different from those for the blank. Figure 4 shows the dependence on the concentration of ginger in the cartridge of the size of the chromatographic peak obtained from the reaction product 2,5-DHB.
It is apparent that the higher the concentration of ginger, the smaller the peak. Thus, the OH· radicals are trapped by the ginger, which acts as a scavenger, and, consequently, they do not reach the salicylate solution. Quantitative values were obtained for both 2,5-DHB (Fig. 5) and OH· radicals and are listed in Table 2, confirming the initial hypothesis of antioxidant properties of ginger.
To verify that the differences found in the study were a result of the absence or presence of different concentrations of ginger, a significance test was applied. Table 3 lists the values obtained.
From these data it can be concluded there are significant differences, because t was much higher than the critical t value for the 95% confidence level, and the same was true of the F-value. This means that the variance of the data are not of the same magnitude. So, there is no homogeneity of variances and, consequently, there is a statistically significant difference.
References
Tovar L, Salafranca J, Sánchez C, Nerín C (2005) J Agric Food Chem 53:5270–5275
López P, Sánchez C, Batlle R, Nerín C (2005) J Agric Food Chem 53:6939–6946
Kranl K, Schlesier K, Bitsch R, Hermann H, Rohe M, Bohm V (2005) Food Chem 93:171–175
Mantle D, Anderton JG, Falkous G, Barnes M, Jones P, Perry EK (1998) Comp Biochem Phys B 121:385–391
Rey AI, Hopia A, Kivikari R, Kahkonen M (2005) LWT Food Sci Technol 38:363–370
Tang B, Zhang L, Geng Y (2005) Talanta 65:769–775
Salmon RA, Schiller CL, Harris GW (2004) J Atmos Chem 48:81–104
Librando V, Tringali G (2005) J Environ Manage 75:275–282
Li LX, Abe Y, Nagasawa Y, Kudo R, Usui N, Imai K, Mashino T, Mochizuki M, Miyata N (2004) Biomed Chromatogr 18:470–474
Mason TJ, Lorimer JP, Bates DM, Zhao Y (1994) Ultrason Sonochem 1:S91–S95
Benito Y, Arrojo S, Nerín C (2004) Unpublished results
Nuñez MT, Tapia V, Toyokuni S, Okada S (2001) Free Radical Res 34:57–68
Golden MC, Hahm SJ, Elessar RE, Saksonov S, Steinberg JJ (1998) Mycoses 41:97–104
Halliwell B, Kaur H, Ingelman-Sundberg M (1991) Free Radical Bio Med 10:439–441
Halliwell B, Grootveld M (1988) Method Biochem Anal 33:59–90
Dupont I, Berthou F, Bodenez P, Bardou L, Guirriec C, Stephan N, Dreano Y, Lucas D (1999) Drug Metab Dispos 27:322–326
Ingelman-Sundberg M, Kaur H, Terelius Y, Persson JO, Halliwell B (1991) Biochem J 276:753–757
Strolin-Benedetti M, Brogin G, Bani M, Oesch F, Hengstler JG (1999) Xenobiotica 29:1171–1180
Lang K, Brodilova J, Lunak S (1996) Collect Czech Chem C 61:1729–1737
Buxton GV, Langan JR, Smith JRL (1986) J Phys Chem 90:6309–6313
Daniel CH (2001) Quantitative chemical analysis, 5th edn. Reverté, Barcelona
Jen JF, Leu MF, Yang TC (1998) J Chromatogr A 796:283–288
Lang K, Wagnerova DM, Brodilova J (1994) Collect Czech Chem C 59:2447–2453
García I, Ortiz MC, Sarabia L, Vilches C, Gredilla E (2003) J Chromatogr A 992:11–27
Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R (2005) Food Chem 91:621–632
Acknowledgements
This paper has been financed by the Project CAL03-080-from INIA – Ministerio de Ciencia y Tecnología, Spain. D. Pezo acknowledgements the grant obtained from SCH – University of Zaragoza.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pezo, D., Salafranca, J. & Nerín, C. Design of a method for generation of gas-phase hydroxyl radicals, and use of HPLC with fluorescence detection to assess the antioxidant capacity of natural essential oils. Anal Bioanal Chem 385, 1241–1246 (2006). https://doi.org/10.1007/s00216-006-0395-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0395-4