Abstract
The aim of the present study was to develop a chromatographic method for the analysis of atorvastatin, o- and p-hydroxyatorvastatin (acid and lactone forms) in human plasma after administration of atorvastatin at the lowest registered dose (10 mg) in clinical studies. Sample preparation was performed by solid-phase extraction and was followed by separation of the analytes on an HPLC system with a linear gradient and a mobile phase consisting of acetonitrile, water and formic acid. Detection was achieved by tandem mass spectrometry operated in the electrospray positive ion mode. Validation of the method for the compounds for which reference compounds were available (acid forms of atorvastatin, o- and p-hydroxyatorvastatin) showed linearity within the concentration range (0.2–30 ng/ml for atorvastatin acid and p-hydroxyatorvastatin acid, and 0.5–30 ng/ml for o-hydroxyatorvastatin acid) (r2≥0.99, n=5 for all analytes). Accuracy and precision (evaluated at 0.5, 3 and 30 ng/ml for atorvastatin, p-hydroxyatorvastatin and 1, 3 and 30 ng/ml for o-hydroxyatorvastatin) were both satisfactory. The detection limit was 0.06 ng/ml for atorvastatin and p-hydroxyatorvastatin, and 0.15 ng/ml for o-hydroxyatorvastatin. The method has been successfully applied in a clinical study where atorvastatin, o- and p-hydroxyatorvastatin (both acid and lactone forms) could be detected in a 24-h sampling interval after administration of the lowest registered dose of atorvastatin (10 mg) for one week.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
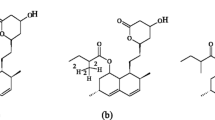
Atorvastatin is a HMG-CoA reductase inhibitor, which has a widespread use in the prevention of cardiovascular events. Atorvastatin is administered as the calcium salt of its active acid form, and undergoes interconversion to its inactive lactone form in vivo [1, 2] (Fig. 1). It is metabolised by CYP3A4 to two hydroxylated metabolites, o-hydroxyatorvastatin and p-hydroxyatorvastatin, which are also present in both acid and lactone forms in vivo [3] (Fig. 1). About 70% of the total plasma HMG-CoA reductase inhibitory activity is accounted for by active metabolites [4].
In several clinical studies regarding statins in general, including atorvastatin, determination of the statins has been performed using alternative methods, such as enzyme inhibition assays, other than determination of the actual plasma concentrations of the compounds [5–8]. Such indirect measurements are relevant when determining the HMG-CoA reductase inhibitory activity of the statin in plasma, but they do not give any further information on metabolites. Information about the actual plasma concentration of both parent compound and metabolites is of interest in pharmacokinetic studies, especially with regard to possible side effects and toxicity of metabolites, and also in investigations of the mechanisms of drug–drug interactions. Although the lactone forms of the statins are inactive with regard to lipid-lowering effects, it is not clear whether they contribute to the side effects of statins. Specific chromatographic methods are therefore required in order to achieve specific determinations of the parent compound and both active and inactive metabolites.
Two high-performance liquid chromatographic (HPLC) methods for the determination of atorvastatin in human plasma and serum have previously been published [9, 10]. Both methods use liquid–liquid extraction (LLE) with ether for sample preparation. Both of these methods have been applied (with minor modifications) in clinical studies, with atorvastatin doses ranging between 40 and 80 mg [3, 11]. For our purpose, a method for specifically detecting both acid and lactone forms of atorvastatin and its hydroxyacid metabolites after the administration of a low dose of atorvastatin (just 10 mg) was needed. Sample preparation by solid-phase extraction (SPE) was desirable in order to reduce the amount of organic solvent used compared to the LLE methods [9, 10]. Thus, the aim of this study was to develop a chromatographic method for determining the acid and lactone forms of atorvastatin, o- and p-hydroxyatorvastatin, in human plasma following administration of atorvastatin in a low dose (10 mg), using SPE for sample preparation.
Experimental
Chemicals and reagents
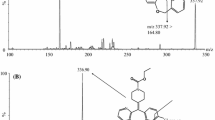
Atorvastatin, o-hydroxyatorvastatin and p-hydroxyatorvastatin were provided by Parke-Davis (Ann Arbor, MI, USA). Unfortunately, we were not able to obtain reference substances of atorvastatin lactone, o- and p-hydroxyatorvastatin lactone for method validation. Lactone forms of atorvastatin, o- and p-hydroxyatorvastatin were obtained—for the purpose of optimizing the helium collision energy during tandem mass spectrometric (MS) detection and estimation of in-source interconversion—by adding 1 ml chloroform and 50 μl 0.1 M sulfuric acid to 100 μg of each of the available substances, atorvastatin, o- and p-hydroxyatorvastatin, and heating to 55 °C for 1 h, as acidic conditions favor the interconversion from the acid to the lactone form [1, 12]. Formation of atorvastatin lactone, o- and p- hydroxyatorvastatin lactone by this procedure was confirmed by comparing their fragmentation pattern with a previous publication after LC–tandem MS analysis [10] (Fig. 2). A complete interconversion from acid to lactone could not be confirmed, so the lactones obtained by this procedure could only be used for qualitative and not quantitative purposes. Deuterated analogs of atorvastatin were not available, and methaqualone (Sigma-Aldrich, Norway) was therefore used as internal standard. Methaqualone is not a registered drug in Norway, and it was therefore unlikely to be used by patients, in subsequent pharmacokinetic studies applying this method. All chemicals used for chromatographic purposes were of analytical grade.
MS/MS spectra of the reference compounds of atorvastatin acid, o- and p-hydroxyatorvastatin acid, and of the interconversion products atorvastatin lactone, o- and p-hydroxyatorvastatin lactone, after chemical interconversion from acid forms (acid forms treated with 1 ml chloroform, 50 μl 0.1 M sulfuric acid at 55 °C for 1 h). MS/MS conditions are described in the “Experimental” section. Precursor ion masses, product ion masses and relative collision energies are shown in Table 1
Preparation of standard samples
Stock solutions of atorvastatin acid, o- and p-hydroxyatorvastatin acid were prepared in acetonitrile–water (90:10, v/v), whereas stock solutions of the synthesized lactone forms of atorvastatin, o-and p-hydroxyatorvastatin were prepared in acetonitrile. Stock solution of the internal standard, methaqualone, was prepared in methanol. All stock solutions were stored at −20 °C. Heparin drug-free pooled plasma was obtained from five healthy volunteers. Plasma samples for method validation were spiked with aliquots of the stock solutions.
Biological samples
Biological samples were obtained from healthy volunteers serving as controls in a clinical study investigating the pharmacokinetic interaction between atorvastatin and cyclosporine in renal transplanted patients [13]. Briefly, healthy volunteers were given 10 mg atorvastatin daily for seven days and plasma samples were drawn on fixed times from 0 to 24 h post-dose on day 7. Plasma samples were frozen immediately after sample withdrawal to minimize acid–lactone interconversion and stored at −80 °C. Individuals participating in this study signed an informed consent, and the study was recommended by the Regional Ethics Committee of Health Region South and by the Norwegian Medicines Control Authority, Oslo, Norway.
Instrumentation
The HPLC equipment consisted of a Spectra System P4000 quarternary pump and a Spectra System AS 3000 autosampler. For detection, a Finnigan LCQDUO ion trap MS detector equipped with an electrospray interface operated in the positive mode was used. Xcalibur version 1.2 software was used for system operation and data handling (all LC-MS equipment was from Instrument Teknikk AS, Ø sterås, Norway). A Universal 32R centrifuge (Hettich Zentrifugen, Tuttlingen, Germany) was used for both centrifugation steps in the sample preparation procedure.
Sample preparation
Preparation of samples from healthy volunteers and plasma samples spiked with aliquots of the analytes was performed by SPE, and the samples were kept on ice at all times during sample preparation. The plasma samples (1 ml) were spiked with 10 ng of the internal standard methaqualone, and 1 ml of sodiumformat (pH 3; 1 M) was added after which they were vortexed and centrifuged at 1,600g and 4 °C for 5 min. The supernatant was subsequently transferred to 3 ml C18 (100 mg) SPE cartridges (Varian, Harbor City, CA, USA) pre-conditioned with 1 ml acetonitrile followed by 2 ml water. The cartridges were washed with 1 ml water followed by 1 ml methanol–water (30:70, v/v), and the analytes were eluted with 750 μl acetonitrile–ammonium acetate (0.1 M; 95:5, v/v) and evaporated to dryness under a stream of N2. The residues were reconstituted in 120 μl mobile phase A and centrifuged at 35,000g and 4 °C, prior to transferral to HPLC vials and injection of 100 μl in the HPLC system.
Chromatographic conditions
An Omnisphere C18 (3 μm, 30×2 mm) analytical column with a Chromguard (5 μm, 10×2 mm) guard column (both Varian, Harbor City, CA, USA) was used for the chromatographic separation of the compounds. The analytes were eluted by a gradient mobile phase system consisting of mobile phase A (acetonitrile–formic acid (1 mM; 30:70, v/v) and mobile phase B (acetonitrile–formic acid (1 mM; 60:40, v/v). During the first 5 min after injection a linear change from 100% mobile phase A to 60% A and 40% B was performed. This combination was held for 11 min, and then reversed back to 100% A within 1 min before re-equilibrating for 4 min with mobile phase A. The flow rate was 0.2 ml/min during the first 17 min of the run, and 0.4 ml/min during the 4 min of re-equilibration. The autosampler tray temperature was set at 4 °C.
Mass spectrometric conditions
The mass spectrometer was operated in positive ion mode and connected to the chromatographic system using an API electrospray interface. Tuning the MS detector for both optimal operating conditions and the optimal collision energy for dissociation was achieved through infusion of atorvastatin, o- and p-hydroxyatorvastatin (acid and lactone forms) through a T-connector. Helium was used as collision gas. Sheet gas (N2) flow was set at 40 AU (arbitrary units), auxiliary gas (N2) at 10 AU, source voltage at 5 kV and capillary temperature at 200 °C. In order to obtain maximum sensitivity, seven segments were used (Table 1). The MS detector was operated in the single reaction monitoring (SRM) mode within each of the seven segments. All transitions and relative collision energies are listed in Table 1. Isolation width was set at m/z=2.
Method validation
The concentrations of the compounds in the plasma samples were determined from their peak heights, using methaqualone as an internal standard. Linearity was tested in the range of 0.2–30 ng/ml for both atorvastatin and p-hydroxyatorvastatin and at 0.5–30 ng/ml for o-hydroxyatorvastatin. Separate batches of drug-free pooled plasma was spiked with atorvastatin, p-hydroxyatorvastatin (0.5, 3 and 30 ng/ml) and o-hydroxyatorvastatin (1, 3 and 30 ng/ml) (n=5) to determine the accuracy and precision of within-day and between-day runs. Recovery was measured at 1 and 30 ng/ml by comparing the response of extracted samples spiked before extraction with the response of extracted blank samples spiked immediately before injection (n=3 at each concentration). The recovery of methaqualone was determined using atorvastatin as internal standard (n=3). Limit of detection (LOD) was determined at a signal-to-noise ratio of 3/1. Effect of the matrix on ion suppression or signal enhancement was determined by comparing the peak heights of blank plasma samples spiked post-extraction with the analytes at a concentration of 10 ng/ml with direct injection of the analytes at the same concentration. Long-term, bench-top and freeze–thaw stability was not tested, as they have been thoroughly investigated previously by Jemal and Xia [10]. Autosampler stability of atorvastatin, o-hydroxyatorvastatin and p-hydroxyatorvastatin at 4 °C was tested on extracted plasma samples in the injection matrix (mobile phase A).
Results and discussion
Sample preparation, chromatography and MS detection
In the present study, a SPE extraction procedure for the preparation of human plasma samples prior to LC–tandem MS analysis was developed. Plasma samples were mixed with sodiumformat-buffer (pH 3; 1 M) in order to ensure that the analytes were in the unionized form prior to transferral to the SPE cartridges. Wash steps with solutions containing amounts of organic solvent greater than 20% acetonitrile or 30% methanol in water resulted in a dramatic decrease in the extraction recoveries of the metabolites. A wash step with 30% methanol in water resulted in cleaner samples and less variable recovery than wash solutions containing 20% acetonitrile, and was therefore preferred. Acetonitrile–ammoniumacetate (0.1 M; 95:5, v/v) was used for elution of the analytes. A volume of 750 μl was needed in order to achieve maximum recovery for this eluent.
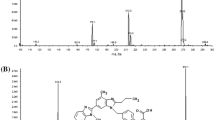
A representative chromatogram from the analysis of a biological sample taken after daily administration of 10 mg atorvastatin for one week is presented in Fig. 3. Separation of the compounds needed to be good in order to enable the use of SRM, which resulted in lower limits of detection than those obtained by full scan. The total time of analysis in the chromatographic system was therefore 21 min, including the time for re-equilibration. The retention times of atorvastatin and metabolites were between 6.2 and 15.6 min, and the retention time for the internal standard was 3.9 min (Fig. 3). Identification of atorvastatin, o- and p-hydroxyatorvastatin (acid and lactone forms) was achieved by injecting reference substances obtained as described in the “Experimental” section.
Representative chromatograms of an extracted plasma sample taken 30 min post-dose after administration of 10 mg atorvastatin to a healthy volunteer for one week (steady state). Plasma concentrations of compounds for which reference compounds were available were 12.5 ng/ml (atorvastatin acid), 0.5 ng/ml (o-hydroxyatorvastatin acid) and 0.4 ng/ml (p-hydroxyatorvastatin acid). IS* internal standard (methaqualone)
Validation of the method
Validation of the method showed linearity within the concentration range 0.2–30 ng/ml for atorvastatin acid and p-hydroxyatorvastatin acid, and 0.5–30 for o-hydroxyatorvastatin acid (r2≥0.99, n=5 for all analytes). Relative standard deviations (RSDs) of the estimated slopes were less than 15% over the whole concentration range. The intercepts of the calibration curves were shown to be not statistically different from zero for any of the analytes. LOD was 0.06 ng/ml for atorvastatin and p-hydroxyatorvastatin, and 0.15 ng/ml for o-hydroxyatorvastatin. Recoveries for all analytes ranged between 53 and 78% on average. This was highly reproducible at 30 ng/ml (RSD<8%); however, at 1 ng/ml, the recovery was within the same range (56–73%), but RSD values were higher (19–32%). Recovery of the internal standard was 85±12%. Within-day and between-day RSD (evaluated at 0.5, 3 and 30 ng/ml for atorvastatin and p-hydroxyatorvastatin and at 1, 3 and 30 ng/ml for o-hydroxyatorvastatin) were within acceptable limits for the lowest and highest concentrations (Tables 2 and 3) [14]. Some of the RSD values at the middle concentration (3 ng/ml) showed variations slightly above the acceptance criteria for bioanalytical method validation (Tables 2 and 3) [14]. However, taking into account the labile nature of these compounds, these variations were considered acceptable. The specificity of the method was tested using extracted drug-free plasma from five individuals. No interfering peaks were found. Also, no interfering ion suppression or matrix effect on signal enhancement was observed. No significant concentration changes in any of the analytes were observed upon autosampler storage at 4 °C for a period of up to 24 h.
Interconversion
Atorvastatin and its metabolites undergo interconversion between the acid and lactone forms not only in vivo, but also after sample withdrawal (during storage, sample preparation, analysis and detection (in-source interconversion)). Upon validation of analytical methods regarding compounds susceptible to interconversion, when both forms are present in vivo this can be taken into account by selecting relative concentrations of the analytes in the quality control samples to mimic the anticipated concentrations in the biological samples [12]. However, as was pointed out by Jemal and Xia, this is a challenging task, as these concentrations are unknown prior to analysis and may vary from sample to sample [12]. This was clearly demonstrated in our study with administration of 10 mg atorvastatin to 18 healthy volunteers, where the ratio between atorvastatin acid and atorvastatin lactone showed a 175-fold difference between patients 24 h post-dose (Table 4). The change in ratio between samples from the same patient during a 24-h sampling interval was up to sevenfold for atorvastatin and around 20-fold for o- and p-hydroxyatorvastatin (Table 4).
Lack of reference compounds with known concentrations of the lactones prevented addition of both acid and lactone forms of the compounds in order to mimic the in vivo concentrations. Due to the substantial intra- and inter-patient variability in the ratio between the acid and lactone forms it was therefore of crucial importance to minimize post-sampling interconversion (Table 4). The present study showed that keeping the samples on ice or at a maximum of 4 °C at all times during sample handling is a useful approach when reference compounds are lacking for one or more of the interconverting compounds. This is in agreement with previous observations for pravastatin [12].
However, despite the low temperatures used at all times during sample handling, in-source interconversion remains a problem, demonstrating the need for chromatographic separation of interconverting substances to enable identification of interconversion occurring prior to or during MS detection. In-source interconversion was tested in the single MS full-scan mode (m/z from 540 to 578) with identical operational conditions as the tandem MS method, described in the “Experimental” section. No in-source interconversion could be detected from acid to lactone form. A small but stable amount of lactone was interconverted in-source to the acid form (RSD<4%, n=5).
Application of the method
This method was developed to detect atorvastatin and its metabolites in human plasma in pharmacokinetic studies after administration of the lowest registered dose of atorvastatin (10 mg). The present method has been successfully applied in our laboratory in a study investigating the pharmacokinetic interaction between atorvastatin and cyclosporine in renal transplant patients [13]. In order to serve as a control group in this study, 18 healthy volunteers were administered 10 mg atorvastatin daily for one week. Atorvastatin, o- and p-hydroxyatorvastatin in both acid and lactone forms were detected in plasma samples from all 18 healthy volunteers. Figure 4A shows examples of plasma concentration vs time curves of atorvastatin acid, o- and p-hydroxyatorvastatin acid for a healthy volunteer in the control group following administration of 10 mg atorvastatin daily for one week (steady state).
Plasma level vs time curves of atorvastatin (filled circles), o- hydroxyatorvastatin (filled triangles) and p-hydroxyatorvastatin (filled squares) in (A) acid forms and (B) lactone forms after administration of 10 mg atorvastatin to a healthy volunteer for one week (steady state). * Plasma levels of atorvastatin lactone, o- and p-hydroxyatorvastatin lactone are presented in arbitrary units (MS/MS signal in relation to MS/MS signal of the internal standard per milliliter plasma)
In addition to the validated acid forms of the compounds, the lactone forms of the compounds were also determined in all patient samples. Their presence was confirmed by comparing retention times and MS/MS fragmentation patterns with those of the synthesized lactones. Due to lack of reference compounds, plasma level vs time curves for atorvastatin lactone, o- and p-hydroxyatorvastatin lactone were obtained on the basis of arbitrary units (Fig. 4B) (in other words, MS/MS signal of the lactone in relation to MS/MS signal of internal standard per milliliter plasma). These arbitrary plasma measurements can be used to estimate pharmacokinetic parameters as terminal half-life and time to maximum plasma concentration, and will provide pseudo-values for maximum and minimum plasma concentration as well as area under the plasma concentration vs time curve. Such data are useful for relative comparisons, but it must be noted that pharmacokinetic parameters derived from such arbitrary units are subject to uncertainty, as linearity, precision and accuracy have not been evaluated. This approach has previously been successfully applied to other compounds for which there is a lack of reference compounds [15].
Conclusion
In the present work, sample preparation of human plasma samples was performed by SPE, in contrast to previous studies using LLE. Sample preparation was followed by HPLC with tandem MS detection. Validation results for atorvastatin acid, o- and p-hydroxyatorvastatin acid were satisfactory. However, due to lack of reference compounds, validation could not be carried out for the lactone forms of atorvastatin, o- and p-hydroxyatorvastatin. The method has been successfully applied in a clinical study, where atorvastatin, o- and p-hydroxyatorvastatin (both acid and lactone forms) could be detected in a 24-h sampling interval on steady state after the administration of the lowest registered dose (10 mg) of atorvastatin to healthy volunteers.
References
Kearney A, Crawford L, Mehta S, Radebaugh G (1993) Pharm Res 10:1461–1465
Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, Pearson PG, Baillie TA (2002) Drug Metab Dispos 30:505–512
Kantola T, Kivisto K, Neuvonen P (1998) Clin Pharmacol Ther 64:58–65
Parke-Davis (2004) Product information: Lipitor (Atorvastatin Calcium). Parke-Davis (Division of Pfizer Inc.), Ann Arbor, MI
Asberg A, Hartmann A, Fjeldså E, Bergan S, Holdaas H (2001) Am J Transplant 1:382–386
Amsden GW, Kuye O, Wei GC (2002) J Clin Pharmacol 42:444–449
Hsyu PH, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM (2001) Antimicrob Agents Chemother 45:3445–3450
Siedlik PH, Olson SC, Yang BB, Stern RH (1999) J Clin Pharmacol 39:501–504
Bullen W, Miller R, Hayes R (1999) J Am Soc Mass Spectrom 10:55–66
Jemal M, Ouyang Z, Chen BC, Teitz D (1999) Rapid Commun Mass Sp 13:1003–1015
Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, Lameire NH (2003) Nephrol Dial Transplant 18:967–976
Jemal M, Xia YQ (2000) J Pharm Biomed Anal 22:813–827
Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL (2004) Clin Pharmacol Ther 76:388–391
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Pharm Res 17:1551–1557
Molden E, Helen Boe G, Christensen H, Reubsaet L (2003) J Pharm Biomed Anal 33:275–285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermann, M., Christensen, H. & Reubsaet, J.L.E. Determination of atorvastatin and metabolites in human plasma with solid-phase extraction followed by LC–tandem MS. Anal Bioanal Chem 382, 1242–1249 (2005). https://doi.org/10.1007/s00216-005-3266-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3266-5