Abstract
A multiresidue method using gas chromatography coupled to ion trap tandem mass spectrometry (GC–ITD–MS/MS) associated with solid phase microextraction (SPME) was developed for the analysis of 20 pesticides commonly used in the Alsace region in rainwater samples. Since the pesticides were expected to be present at very low concentrations and in complex matrices, the analytical method used was both highly selective and sensitive. Therefore, fibers coated with polyacrylate (PA), polydimethylsiloxane (PDMS) and polydimethylsiloxane-divinylbenzene (PDMS-DVB) were tested, and the parameters affecting the precision and accuracy of the SPME method were investigated and optimized. These parameters include the type of fiber, the adsorption time, the effect of salt, and the extraction temperature. The PDMS fiber was the most polyvalent for the extractions of the different pesticides studied. Detection limits of between 5 and 500 ng L−1, depending on the compounds under study (except for those which could not be analyzed: captan and mevinphos), were obtained with this analytical procedure. This method was applied to the analysis of rainwater samples collected simultaneously on a weekly basis at one rural and one urban site between March 2002 and July 2003. While some of the 20 pesticides analyzed were constantly detected (such as lindane and atrazine), a strong temporal variability was observed for some of the others (including alachlor, metolachlor, atrazine).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emission of pesticides into the atmosphere can arise from drift during application, post-application volatilization from treated crops or from the ground, and from wind erosion. The most important environmental factors influencing dispersion on a local scale are the temperature, which affects emission rates, lateral and vertical dispersion, wind speed and height of cloud base. Once emitted into the atmosphere, the pesticides can be distributed between the gas, particle and aqueous phases, and this partitioning depends on the physicochemical properties of the compounds, such as the equilibrium vapor pressures or Henry’s law coefficients.
Pesticides are generally removed from the atmosphere by dry and wet deposition, and the partitioning between these two processes depends partly on the Henry’s-Law coefficient H. Compounds with a high H value are more effectively washed out by rain and therefore participate in the contamination of precipitation [1].
Since the mid-1960s, studies have demonstrated the presence of pesticides in precipitation in a variety of countries [2–8]. Recently, de Rossi et al. [6] have observed that concentration levels of pesticides measured in Germany in the year 1999 turned out to be much higher than the levels detected in the following year. Some studies in other parts of Europe have shown that pesticides are only present in rainwater during application times [5, 7–9].
These studies tends to demonstrate that the behavior of the pesticides in current use in the atmosphere is strongly dependent on the location of sampling, the quantities applied and the frequency of application. Due to the small number of papers actually published on this subject, more studies are needed in order to confirm this tendency.
The purpose of this study was to estimate and compare the pesticide contamination of precipitations collected in Alsace (eastern France) collected simultaneously in urban (Strasbourg) and rural (Erstein) areas on a weekly basis for two years (2002 and 2003), in order to evaluate spatial and temporal variations in pesticides concentration.
This type of study involved the determination of very low pesticide concentrations in complex environmental matrices with a large number of interfering compounds. Thus, a simple and highly sensitive analytical technique is required to detect and quantify these pesticides in rainwater at trace levels.
Various techniques have been shown to efficiently extract pesticides from water matrices, including liquid–liquid or solid phase extraction [5, 7, 10–12]. These methods are generally time-consuming, and use large quantities of expensive pure organic solvents. On the other hand, solid phase microextraction (SPME) is an inexpensive, rapid and solvent-free extraction method for isolating organic compounds from an aqueous sample. The main advantage of SPME is that it integrates sampling, extraction and concentration into one step. This method is actually rarely used for extracting organic pollutants from atmospheric water, probably due to the low levels commonly found in precipitation.
In order to evaluate the spatial and temporal differences in pesticide concentrations in rainwater between urban and rural areas, a method for the analysis of 20 pesticides was developed that was based on solid phase microextraction and ion trap GC–MS/MS. SPME was chosen because it permits rapid extraction and accurate analysis of a great number of samples collected over two years at the two sites, and MS/MS was chosen because it enables trace levels of pesticides to be determined in the presence of interfering compounds without losing any identification capabilities, due to a drastic reduction in the background noise.
The pesticides analyzed were selected due to their intensive application in the Alsace region to different kinds of crops. The aim of this paper is to present the development and the validation of the extraction procedure for the 20 pesticides which were easy to analyze via gas chromatography (including amides, organophosphorus and triazines). A second method, used for seven other compounds, which is a combination of derivatization with pentafluorylbenzylbromide (PFBBr) and solid phase microextraction, will be presented later in an other paper.
The influences of the composition of the fiber coating, the duration of adsorption, the ionic strength, and the extraction temperature were investigated. Finally, the results from monitoring rainwater sampled simultaneously in Strasbourg and Erstein between March 2002 and July 2003 are also presented.
Materials and methods
Standards, solvents and materials
Guaranteed pure standard pesticides—alachlor (99.7%), atrazine (99.2%), azinphos-ethyl (99.4%), azinphos-methyl (98.6%), captan (99.7%), chlorfenvinphos (98.4%), dichlorvos (99.0%), diflufenican (98.4%), α- and β-endosulfan (99%), iprodione (99.0%), lindane (99.0%), metolachlor (98.0%), mevinphos (91.0%), parathion-methyl (99.6%), phosalone (99.3%), phosmet (99.9%), tebuconazole (98.0%), triadimefon (99.9%) and trifluralin (99.3%)—and the internal standard (deuterated naphthalene) were obtained from Promochem (Molsheim, France) and Aldrich (L’Isle D’Abeau, France). The solvent used was HPLC grade methanol (Prolabo, France).
Mixtures of pesticides were prepared in methanol containing 100 mg.L−1 of each individual pesticide. Ultrapure Milli-Q water (Millipore) was used to prepare the standard solutions. The final percentage of methanol in all standard solutions did not exceed 0.1% and so did not have an influence on the efficiency of the extraction. [13].
The SPME holder and fiber assemblies (PDMS, PA and PDMS-DVB) for manual sampling were provided by Supelco (Paris, France).
Instrument
A Varian (Palo Alto, CA) Star 3400 CX equipped with a split-splitless injector and coupled to a Saturn IV Varian ion trap tandem mass detector (MS/MS) was used. An analytical capillary column was used (30m×0.32 mm, film thickness: 0.25 μm; DB-5MS, Macherey-Nagel, France).
Helium was used as the carrier gas and the inlet pressure was 12 psi (corresponding to a flow rate of 2 ml min−1). The GC temperature program varied between 60 °C and 163 °C at 25 °C/min then 163 °C to 165 °C at 0.3 °C/min, then 167 °C to 210 °C at 30 °C/min and finally 210 °C to 250 °C (10 min) at 5 °C/min. Injection was made in the splitless mode (2 min). The injector and the transfer line temperatures were kept at 250 °C while the manifold temperature was 200 °C.
Experimental
The chemical analyses were performed using two different steps. The pesticides (Table 1) were first extracted using solid phase microextraction (SPME) and then analyzed using a very specific analytical method, gas chromatography coupled with ion trap tandem mass spectroscopy (GC–ITD–MS/MS), as previously described in detail elsewhere [14, 15].
Quantification of rainwater samples was performed by comparing the samples with standards.
Optimization of SPME–GC/MS/MS
The parameters that can affect the SPME process were optimized: the type of fiber, the exposure time of the fiber, the temperature of extraction, the pH and ionic strength. In this work, sampling from the headspace was not investigated due to the different polarities and volatilities of the pesticides under study and because other studies have shown the limitations of this technique with these compounds (see [16] and references therein).
Sampling locations
In order to compare rainwater contamination in different locations, rain samples were collected at two sampling stations.
The first station was chosen in order to represent an urban area, and so the rainwater collector was placed in the botanical gardens of Strasbourg University, situated near the historic center of Strasbourg (400,000 inhabitants). One particular feature of this city is that intensive agricultural activity (essentially maize farming) takes place just 15 km south of the city center.
The second sampling point was installed in a rural area, situated 25 km southeast of Strasbourg, 2 km from a small town, Erstein (9,000 inhabitants), and 300 m from an area treated with pesticides. The collector was placed on the soil, but far from the treated area, in order to avoid any possible direct transfer of pesticides into the sampler during treatment.
Sample collection
Samples were collected simultaneously at the two sites on a weekly basis between January 2002 and September 2003 using a wet-only rainwater sampler (Précis Mécanique, Bezons, France) recommended by METEO-France. After sampling, the samples were stored in darkness at –18 °C before analysis.
In order to eliminate any variation in concentration from fluctuations in precipitation levels over the week of sampling, each station was also equipped with a graduated open collector (Précis Mécanique). The normalized concentration is calculated according to.
where C i1,norm is the normalized concentration of species 1 in sample i, C i1 is the concentration of species 1 in sample i, H i the precipitation level of sample i and H m is the mean precipitation height.
Results and discussion
Study of experimental variables
The first step in the development of the method was to choose the type of fiber. The fiber depth in the injector was set to 3.4 cm and the thermal desorption time in the split-splitless injector was 5 min at 250 °C, as recommended by Supelco and derived in our laboratory in a previous study [17]; all other parameters were fixed. Placing the fiber deeper into the injector gave rise to carryover effects, and feeding less than 3.4 cm of fiber in caused a loss of response. The liner purge was closed during desorption of the analytes from the SPME fiber in the split-splitless injector (2 min delay time). A blank was tested with the same fiber in order to confirm that all of the compounds were desorbed within 5 min of thermal desorption. Extractions were performed by immersing the fiber in 3 ml of sample, with permanent stirring and temperature control at 40 °C, for 30 min.
The ionic strength of the aqueous solution containing 5 μg L−1 of 20 pesticides was adjusted using a 50% NaCl saturated solution. The pH was not controlled (it was ~7), since the analyzed molecules are neutral and consequently not influenced by variations in the pH value [18].
The adsorption process: comparison of fibers
The fiber used for the analysis of rainwater must be able to efficiently extract the 20 pesticides even though they have very different physicochemical properties.
The fibers tested were coated with either 100 μm polydimethylsiloxane (PDMS), commonly used for nonpolar molecules, 85 μm polyacrylate (PA), which is more appropriate for more polar pesticides, or 65 μm PDMS/divinylbenzenze (DVB), a mixed phase consisting of porous polymer particles, particles of poly(divinylbenzene) (DVB) suspended in a matrix of PDMS that has complimentary properties to the DVB. All fibers were conditioned in the injector at 250 °C according to instructions provided by Supelco.
The adsorption efficiencies of the three different SPME fibers were then determined for the 20 pesticides under study (Fig. 1).
According to the results shown in Fig. 1, PDMS fiber, mainly used for the extraction of nonpolar pesticides with very low solubilities in water, such as organochlorine pesticides and some nonpolar organophosphorus compounds [19], gives the best extraction efficiencies for most of the compounds studied.
PA fiber generally gives a lower extraction efficiency than PDMS fiber. This fiber is normally used for the extraction of polar and semivolatile compounds, and so better extraction efficiencies were obtained for these kind of molecules (such as lindane, metolachlor).
PDMS/DVB fiber showed a much higher affinity than PDMS for eight compounds (atrazine, alachlor, lindane, methyl parathion, metholachlor, triadimefon, chlorfenvinphos, iprodione), but five pesticides were not extracted under these conditions (azinphos-methyl, phosmet, tebuconazole, captan, phosalone). This may be due to the structure of the fiber, since the PDMS-DVB coating is not directly attached to the fused-silica fiber. Gonçalves et al. [20] have compared three PDMS-DVB fibers, including 65 μm PDMS-DVB, for the analysis of multiresidue pesticides in water. They observed that the 65 μm PDMS-DVB fiber tested in this study has the lowest extraction ability for organochlorine, pyrethroid, organophosphate and triazine pesticides. They explained this by noting that this fiber does not have a polymer at the core and it has the smallest coating volume and fiber surface area. In the present study, it is difficult to interpret the results obtained for the 65 μm PDMS-DVB fiber, and anyway it is not the aim of the study, which is devoted to the development of an accurate extraction method for the analysis of pesticides in rainwater. Nevertheless, the structure of the coating could certainly play a role in its performance.
Regarding the results obtained for the three tested fibers, PDMS seems to be more polyvalent than PDMS-DVB for the extraction of pesticides under study, it gives better extraction efficiencies than PA, and it shows good reproducibility and a better linearity than the two other fibers [21, 22].
Therefore, 100 μm PDMS was preferred for the rest of the study, since the use of this fiber allow the simultaneous extraction of 18 out of a total of 20 pesticides in a single SPME procedure and a single chromatographic run.
Adsorption time
Since the SPME technique depends on an equilibrium process that involves the adsorption of analytes from a liquid sample into the polymeric phase according to their partition coefficients, it is important to determine the time required to reach this equilibrium for each compound.
The equilibration rate is limited by the mass transfer rate of the analyte through a thin static aqueous layer at the fiber–solution interface, the distribution constant of the analyte and the thickness and type of fiber coating [23]. Moreover, analytes with high molecular masses are expected to need longer equilibrium times due to their lower diffusion coefficients, since the equilibrium time is inversely proportional to the diffusion coefficient [24].
Although the time taken for the analyte to be adsorbed from the liquid phase into the stationary phase is primarily dependent upon the type of fiber, only PDMS was tested since this one was chosen for the study.
Different authors [13, 24] have shown that the diffusion coefficient of an analyte is higher in 100μm PDMS (a liquid and viscous polymer) than in PA (a solid polymer). The time profile of adsorption was studied by monitoring the area of each peak as a function of exposure time, under the same conditions used for the fiber tests.
Figure 2 shows the adsorption time profiles obtained for the pesticides under study. It is apparent that the equilibrium is compound-dependent and can vary significantly between the different compounds. Alpha-endosulfan, beta-endosulfan, trifluralin and triadimefon practically reached equilibrium after 15 min despite the high molecular mass of endosulfan. These compounds have a better affinity with the PDMS fiber because they have lower polarities and better hydrophobicities. This has also been observed by other authors [16, 25], who state that the more hydrophobic compounds (less polar) are adsorbed more readily by the polymeric phase. Some compounds reached equilibrium after 45 minutes (alachlor, tebuconazole), but the others only reached it after 60 min or more, as previously observed by Gonçalves and Alpendurada [20].
The relative peak responses of some pesticides (azinphos, phosalone, diflufenican) decrease after a long extraction time.
Although the majority of the analytes need between 45 and 60 min to approach equilibrium with the PDMS fiber, a shorter extraction time can be chosen [18] as long as the immersion time remains strictly constant. In the present study, a time of 40 min was selected since it is suitable for achieving the detection limits required to detect pesticides in rainwater, and because a good reproducibility is obtained for this time.
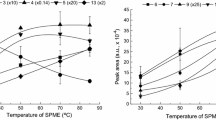
Effect of temperature
The effect of temperature was investigated between 30 and 60 °C with a constant extraction time of 40 minutes. The adsorption–temperature profiles obtained are shown in Fig. 3. The temperature controls the rate of diffusion of analytes into the coating, and so increasing the temperature improves the mobility of the pesticides. The best recoveries for some pesticides were obtained at <50 °C (chlorfenvinphos, tebuconazole, alpha- and beta-endosulfan, phosalone). The small decrease in extraction yield observed at higher temperatures could be due to the enhanced hydrolysis of organosphophorus at elevated temperatures [22].
The extraction efficiencies for another group of pesticides (atrazine, lindane, methyl parathion, triadimefon) are seen to decrease with increasing temperature. This could be due to a decrease in the distribution constant between the analytes and the fiber [26].
For most of the pesticides studied, the effect of temperature on the extraction is weak, and so this parameter was fixed to 45 °C, which seems to be a good compromise, in order to obtain reproducible results.
Effect of adding salt
The effect of salt addition was determined with NaCl standard solution for saturations of 0–100%, for initial concentrations of pesticides of 5 μg L−1. Pesticides that are more soluble in water have a lower affinity for the fiber coating.
In addition, a high salt content results in a solution with high ionic strength, which may cause the activity coefficients of some compounds to decrease significantly.
It was difficult to determine the quantity of salt that should be added, because the solubilities of the pesticides vary greatly. The different profiles obtained are shown in Fig. 4. The addition of salt generally improves the recovery, and this effect is particularly observed for the most polar compounds, such as dichlorvos and mevinphos. These compounds are hydrophilic and are very soluble. Extraction of these compounds is difficult without the addition of salt. [25].
Organophosphorus pesticides (phosmet, azinphos ethyl, chlorfenvinphos) show a different behavior when sodium chloride is added. The responses of most of these compounds decrease when the sodium chloride concentration is high.
The extraction efficiencies of nonpolar compounds such as trifluralin, endosulfan, phosalone and diflufenican (logK ow>4) decrease with the addition of NaCl. The solubilities of these non polar compounds tend to increase with the addition of NaCl.
It is important to note that a 100% saturated NaCl solution allowed the extraction of two compounds which were not extracted under normal conditions (phosmet and mevinphos).
Considering that high concentrations favor the extraction of several pesticides and make it possible to extract two more pesticides, a saturated salt solution was added to all samples.
Detection limits and precision
Finally, the best experimental conditions for pesticide extraction and analysis were selected using to Table 1. To check the linearity of the method, standard solutions with different concentrations of between 0.1 and 10 μg L−1 (0.1, 0.2, 1, 2.5, 5, 7.5 and 10 μg.L−1) were used. Each concentration was analyzed five times. Good linearity was observed for most of the pesticides in the range 0.1–5 μg L−1, with correlation coefficients ranging between 0.984 (azinphos methyl) and 0.999 (beta-endosulfan). The relative standard deviations, calculated using the mean of the peak areas of five consecutive SPME extractions from the same standard solution, varied from 10% (alachlor) to 34% (diflufenican) (Table 2).
The limits of quantification differed considerably for all of the compounds studied, ranging from 5 to 500 ng L−1. For eight compounds, the limit of quantification was 20 ng L−1 (Table 2). However, this limit was sensitive enough to determine the selected pesticides in rainwater samples.
Limits of detection were calculated by comparing the signal-to-noise ratio (S/N) of the lowest concentration to the limit S/N=3.
After optimization all of the parameters, only two compounds could not be quantified using this method: captan and mevinphos. Captan has no affinity with the fiber and is therefore not extracted, although diflufenican, which is from the same chemical family as captan, is extracted. Mevinphos is relatively unstable, and presents both a high limit of quantification (500 ng L−1) and a large standard deviation (119%) which make it difficult to analyze in rainwater. This is confirmed by other studies that showing the low affinity of this compound for the fiber [27].
Application to environmental samples
The effectiveness of the proposed method at determining the selected pesticides was tested by analyzing rainwater samples.
Different studies have shown that SPME is not influenced by nondissolved particles and other interfering compounds present in natural rainwater which can compete for adsorption sites at the fiber [13, 22].
The samples were quantified according to the procedure described previously. Rainwater samples were collected every week for more than a year, from March 2002 to July 2003 in both locations. A summary of the results is presented in Table 3.
For the 13 pesticides measured, the most frequently observed were alachlor, metolachlor, atrazine, α-endosulfan and γ-HCH (lindane). In addition, traces of other pesticides were occasionally found, which were associated with local spraying operations.
Atrazine, alachlor and metolachlor were found in precipitation between April and June, which is the period that they are normally applied to maize crops. This is in agreement with other studies that indicate that pesticides are usually only detected during spraying periods [28, 29].
The presence of atrazine and lindane in the rainwater during the period of collection will now be discussed in more detail.
Atrazine in rainwater
Atrazine is a herbicide that is widely used in both Europe and North America, where it is applied during spring and early summer. It was one of the herbicides most commonly applied to maize herbicides, although its use in now being restricted in several countries. In France, atrazine was taken off the market in 2002 and its use has been forbidden since September 2003. However, this pesticide was still detected during our rainwater sampling. Atrazine has a relatively short theoretical lifetime of 0.11 days (in relation to reactivity with OH radicals), but it appears that this degradation may be slower in the environment, possibly due to its adsorption on particles [30]. However, even given this scenario, its lifetime is relatively short and consequently atrazine was not detected in rainwater outside of the treatment period (Fig. 5). The highest concentrations, up to 1030 ng L−1 were found in Strasbourg in June 2002 and May 2003.
Lindane in rainwater
Lindane has been widely used as an insecticide in most parts of the world, but its use is now declining and it has been banned in many countries (in France since 1998). Results indicate that it can be found during all periods of the year. Lindane shows a pattern typical of continuous background contamination. The lindane concentrations found in rainwater were, for most of the samples, in the range 10–50 ng L−1 for both sites. Only two events exceeded 100 ng L−1. (Fig. 5).
According to the SAR method (structure reactivity relationship), lindane has a lifetime of about 12 days, which is much longer than that of atrazine. It is relatively stable in the atmosphere and can be transported over considerable distances. Therefore, no direct correlation with regional application is expected, but some contamination due to revolatilization from contaminated soils may occur [31].
Conclusion
This study clearly shows that the combination of SPME with GC-ITD (MS/MS) can be used to accurately determine 18 selected pesticides in rainwater, even though these compounds come from very different chemical classes. This method allowed us to make important improvements in selectivity and sensibility, and so it permits the identification and quantification of low traces of pesticides. This study showed that the SPME parameters, which affect the method’s sensitivity, need to be carefully evaluated and optimized in order to improve the detection limits obtained.
The PDMS coating was shown to be efficient at extracting 17 pesticides out of the 20 being studied. Nevertheless, for some pesticides the detection limits are relatively high, mainly due to a low affinity for the PDMS coating.
To solve this problem, analyses of selected pesticides should be performed in two separate runs with different fibers and extraction conditions. This procedure was not considered in this work in order to attempt to develop a technique based on one simple and rapid run. However, our technique did accurately quantify the vast majority of the 20 pesticides studied in rainwater samples, and it gave good detection limits. In addition, it gave good reproducibility and linearity, and the efficient combination of SPME with GC-ITD (MS/MS) was achieved.
With this method, pesticides were detected in rainwater, especially during the application periods of pesticides with short lifetimes (in agreement with the results from other studies). Pesticides with longer lifetimes can be transported over long distances and their concentrations in rainwater do not reflect local applications.
References
Sanusi A, Millet M, Mirabel Ph, Wortham H (1999) Atmos Environ 33:4941–4951
Abbot DC, Harrison RB, Tatton JOG, Thomson J (1965) Nature 208:1317–1318
Tarrant KR, Tatton JOG (1968) Nature 219:725–726
Millet M, Wortham H, Sanusi A, Mirabel Ph (1997) Environ Sci Pollut Res 4(3):172–180
Briand O, Seux R, Millet M, Clément M (2002) Rev Sci Eau 15 :767–787
de Rossi C, Bierl R, Riefstahl J (2003) Phys Chem Earth 28:307–314
Grynkiewicz M, Polkowska Z, Gorecki T, Namiesñik J (2003) Water Air Soil Pollut 149:3–16
Quaghebeur D, De Smet B, De Wulf E, Steurbaut W (2004) J Environ Monit 6:182–190
Chevreuil M, Garmouma M, Teil M-J, Chesterikoff A (1996) Sci Total Environ 182:25–37
Barcelo D (1993) J Chromatogr A 643:117–143
Albanis TA, Hela DG (1995) J Chromatogr A 707:283–292
Epple J, Maguhn J, Spitzauer P, Kettrup A (2002) Geoderma 105:327–349
Sampedro MC, Martin O, Lopez de Armentia C, Goicolea MA, Rodriguez E, Gomez de Balugera Z, Costa–Moreira J, Barrio RJ (2000) J Chromatogr A 893:347–358
Sauret N, Millet M, Herckes P, Mirabel Ph, Wortham H (2000) Environ Poll 110:243–252
Scheyer A, Morville S, Mirabel Ph, Millet M (2005) Anal Bioanal Chem 381:1226–1233
Aguilar C, Penalver S, Pocurull E, Borrull F, Marce RM (1998) J Chromatogr A 795:105–115
Sauret N (2002) PhD thesis. University of Strasbourg, pp 230
Beltran J, Lopez FJ, Hernandez F (2000) J Chromatogr A 885:389–404
Dugay J, Miege C, Hennion M-C (1998) J Chromatogr A 795:27–42
Gonçalves C, Alpendurada MF (2002) J Chromatogr A 963:19–26
Lambropoulou DA, Konstantinou IK, Albanis TA (2000) J Chromatogr A 893:143–156
Lambropoulou DA, Albanis TA (2001) J Chromatogr A 922:243–255
Lord H, Pawliszyn J (2000) J Chromatogr A 885:153–193
Louch D, Motlagu S, Pawliszyn J (1992) Anal Chem 64:1187–1199
Eisert R, Levsen K (1995) Am Soc Mass Spectrom 6:1119–1130
Hernandez F, Beltran J, Lopez FJ, Gaspar JV (2000) Anal Chem 72:2313–2322
Pi–Guey S, Huang S–D (1999) Talanta 49:393–402
Lode O, Eklo OM, Holen B, Svensen A, Johnsen AM (1995) Sci Total Environ 160/161:421–431
Huskes R, Levsen K (1997) Chemosphere 35:3013–3024
Unsworth JB, Wauchope RD, Klein AW, Dorn E, Zeeh B, Yeh SM, Akerblom, M, Racke KD, Rubin B (1999) Pure Appl Chem 71:1359–1383
Scheyer A, Morville S, Mirabel Ph, Millet M (2005) Chemosphere 58:1517–1524
Acknowledgements
The authors want to thanks the “Region Alsace”, the “DRIRE Alsace” and the French Ministry of Ecology and Sustainable Development through the Primequal-2 program for their financial support. Anne Scheyer particularly thanks ADEME and “Région Alsace” for their financial support of her PhD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheyer, A., Morville, S., Mirabel, P. et al. Analysis of trace levels of pesticides in rainwater using SPME and GC–tandem mass spectrometry. Anal Bioanal Chem 384, 475–487 (2006). https://doi.org/10.1007/s00216-005-0176-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0176-5