Abstract
This mini-review covers chelating sorbents anchored to silica gel and their analytical applications for the preconcentration, separation and determination of trace metal ions, focussing mainly on the last 20 years. The article summarizes also the experience gathered by our research group in the synthesis and characterization of new modified silica gels “via silanization”, and their affinity toward selective extraction and separation of trace elements. The introduction of 1,5-bis(di-2-pyridyl)methylene thiocarbohydrazide silica gel (DPTH-gel) and methylthiosalicylate silica gel (TS-gel) chelating sorbents in trace and ultratrace analysis provide vital breakthroughs in preconcentration methods. These home-made materials allow certain analytes to be selectively extracted from complex matrices without matrix interference and good detection limits. The advantages of these new chelating sorbents in comparison with 8-hydroxyquinoline chelating sorbent immobilized on silica gel are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few years solid-phase extraction (SPE) has become the most often used preconcentration technique for trace analysis. The basic principle of SPE is the transfer of analytes from the aqueous phase to the active sites of the adjacent solid phase. The transfer is stimulated by the selection of appropriate optimal conditions in the system of three major components: water (liquid phase), analyte and sorbent [1]. The analyte after sorption on the solid phase is either desorbed with a suitable eluate or the analyte along with the sorbent is dissolved in a suitable solvent and further analyzed. Another mode used is by analyzing the sorbent directly without eluting the analytes [2].
Hitherto, liquid–liquid extraction (LLE) was most often used among the various preconcentration or separation techniques in view of its simplicity, rapidity, ready adaptability to scale-up and easier recovery of analyte and extractant. SPE is replacing LLE because of several advantages that it offers [1, 3, 4], including: (1) flexibility, (2) low cost because of lesser consumption of reagents; (3) absence of emulsion; (4) speed and simplicity; (5) sampling in the field; (6) safety with respect to hazardous samples and (7) ease of automation.
New types of SPE sorbents, such as naphthalene, silica and silica gel, glass beads, cellulose, polyrethane foam, molecular imprinted polymers (MIPs) and other supports, are therefore currently being developed to allow more effective extractions. In the SPE with most commercial sorbents, many components of complex samples (e.g., biological, pharmaceutical and environmental samples) are co-extracted, so additional clean-up is usually needed before the chromatographic analysis is made. However, specific SPE materials avoid this problem by providing a selective extraction.
Silica gel can be used as a very successful adsorbing agent, as it does not swell or strain, has good mechanical strength and can undergo heat treatment. In addition, chelating agents can be easily loaded on silica gel with high stability, or be bound chemically to the support, affording a higher stability. The surface of silica gel is characterized by the presence of silanol groups, which are known to be weak ion-exchangers, causing low interaction, binding and extraction of ionic species [5]. In particular, silica gel presents high sorption capacity for metal ions, such as Cu, Ni, Co, Zn or Fe [6]. Retention is highly dependent on sample pH with quantitative retention requiring pH values over 7.5–8, as under acidic conditions silanol groups are neutral and the ion-exchange capacity of the silica gel is greatly reduced or even reduced to zero at low pHs. In addition, this sorbent has a very low selectivity, and is prone to hydrolysis at basic pH. Consequently, modification of the silica gel surface has been performed to obtain solid sorbents with greater selectivity. In most of the methods for preparation of immobilized silica gel, a two-step procedure has been used for loading the surface with specific organic compounds, physical adsorption and chemical immobilization. In the first case, the organic compound is directly adsorbed on the silanol group of silica gel surface (impregnated or loaded sorbent), either by passing the reagent solution through a column packed with the adsorbent, or by shaking the adsorbent in the reagent solution. In the second approach, a covalent bond is formed between the silica gel surface groups and those of the organic compound (functionalized sorbent). Chemisorption of chelating molecules on silica surface provides immobility, mechanical stability and water insolubility, thereby increasing the efficiency, sensitivity and selectivity of the analytical applications [7]. Chemical modification of silica surface by organic chelating group acts as ion-exchanger, which provides greater selectivity for the analyte than that offered by traditional ion-exchanger. The most convenient way to develop a chemically modified surface is by simple immobilization (or fixing) of the group on the surface by adsorption or electrostatic interaction or hydrogen bond formation of other type of interaction [8, 9]. Simple impregnation of the solution of modifiers [10] or covalent binding, the so called covalent grafting [11] of the chelating molecule to silica matrix via silanization, is the common practice of developing a functionalized silica surface. In 1998, our research group, mainly interested in sorbents based on silica with organic ligands chemically bonded to the surface, has synthesized and studied two new chelating sorbents pertaining to a group of thiocarbonohydrazide, 1,5-bis(di-2-pyridyl)methylene thiocarbohydrazide silica gel (DPTH-gel) [12] and methylthiosalicylate silica gel (TS-gel) [13], to determine the applicability of immobilized chelating groups as preconcentration aids for trace heavy metals. The DPTH-gel consists of chemically immobilizing on a silica gel support the reagent 1,5-bis(di-2-pyridyl) thiocarbohydrazide, which was synthesized for the first time in 1983 by the same group [14]. To be effective, these aids had to be inexpensive, readily available or easily synthesized and provide good stability, high sorption capacity for metal ions and good flexibility in working conditions. The two methodologies are frequently adopted for such designing. The first involves sorption of chelating ligands onto a matrix. The other is based on covalent coupling of a ligand with polymer backbone through a space arm, generally: –N=,=N–NH–, S=, –NH–, O= or –SH.
In analytical chemistry, these home-made chelating sorbents can be approached from two different points of view. The first sees DPTH-gel and TS-gel as analytes, which involves their determination in various samples, such as biological and environmental materials. The second sees DPTH-gel and TS-gel as analytical tools, including their use as chromatographic stationary phases, as different electrochemical sensors based on their activity and life times in continuous flow systems.
This paper will not try to review all publications on the determination and SPE of metal ions using chelating sorbents in the past 25 years, as this has already been done by others [15, 16]. Instead, it will focus on the important phenomena involved in chelating sorbents anchored to silica gel and try to illustrate these phenomena using representative contributions from the most current literature and contributions of our research group in the synthesis and characterization of new modified silica gels, and their affinity toward selective extraction and separation of trace elements.
Preparation of chelating sorbents based on silica gel
Most of the polymeric chelating sorbents known in the literature used for the preconcentration, separation and determination of trace metal ions, such as poly(acrylamidoxime)-divinylbenzene [17, 18], polyacrylonitrile-divinylbenzene [19, 20], epithiopropylmethacrylate-divinylbenzene [21], polyphenylethylene [22], Amberlite XAD and related polymeric supports [23–26], cellulose [27, 28], carbon fiber [29], activated alumina [30], fullerene [31] and silica gel [32, 33], have been prepared by a two-step synthesis:
-
1.
The first step is the insertion of an appropriate functional group on the surface of polymeric support or activation of the polymeric support or the preparation of the polymer.
-
2.
The second step is the immobilization of ligand of particular suitability by virtue of a condensation reaction or coupling reaction.
Immobilized silica gel is the most widely used chelating solid phase for preconcentration/separation and determination of trace metal ions [34–36]. In most of the methods for preparation of immobilized silica gel, a two-step procedure has been used; e.g., DPTH-modified silica gel [12] or methylthiosalicylate-modified silica [13] have been prepared by a two-step synthesis (Figs. 1 and 2). In the first step, activated silica gel was suspended in 3-aminopropyltrimethoxysilane in dry toluene and the mixture was refluxed for 10 h in a nitrogen atmosphere with constant stirring to obtain aminopropyl silica gel (APSG) while in the second step, APSG was reacted either with DPTH or methylthiosalicylate to get modified silica gel. In this paper, the preparation of these immobilized silica gel has been detailed. Another method reported for immobilization of crown ether esters on silica gel [37] involves refluxing crown ether esters with pendent vinyl groups, dimethoxy(methyl)silane and chloroplatinic acid for 20 h in dry benzene.
Preparation of DPTH-gel
The silica gel was refluxed with 6 M HCl for 3 h to remove metals, washed with deionized water and dried in an oven at 180°C for 24 h. Activated silica gel (10 g) was suspended in 100 ml of 10% v/v 3-aminopropyltrimethoxysilane in dry toluene. The mixture was refluxed through a calcium chloride tower for 24 h. The resulting product, aminopropyl silica gel (AP-gel), was filtered off and washed repeatedly with toluene and ethanol. This product was mixed with 100 ml of 3% v/n diglutaric aldehyde in deionized water and the reaction mixture was refluxed for 4 h. The solid obtained (GlutAP-gel) was filtered off, washed with deionized water and mixed with 1.5 g of thiocarbohydrazide previously dissolved in 100 ml of deionized water; ten drops of glacial acetic acid were added. After boiling and refluxing for 24 h the corresponding derivative (ThcGlutAP-gel) was obtained and suspended in 180 ml of 2% m/v di-2-pyridil ketone in ethanol. After refluxing for 24 h, the resulting product (DPTH-gel) was filtered off, washed with ethanol and dried in an oven at 50°C. The reaction for DPTH-gel formation is shown in Fig. 1.
The contents of carbon, hydrogen and nitrogen in the DPTH-gel measured with the CHN elemental analyzer were 10.39, 2.12 and 2.62%, respectively. The surface coverage, determined on the basis of per cent nitrogen by elemental analysis, was found to be 267 μmol g−1. The experiments showed that the DPTH-gel chelating sorbent was not soluble in common organic solvents.
Because an elevated temperature may be useful to dry the surface-modified silicas, to drive off volatile impurities, or to perform reactions under more vigorous conditions, it was desirable to have some idea of the thermal stability of this material. Thermogravimetry was therefore applied. Thermogravimetric profiles were recorded for silica gel and DPTH-gel. The results for silica gel conform to what is known about this material [37], most of physically adsorbed water is lost by 150°C and an essentially stable situation exists up to 250–300°C, when neighboring –SiOH groups begin to release water to form siloxane bridges (–Si–O–Si–). Up to 250°C, DPTH-gel displays virtually the same temperature profile as silica gel. The TGA data showed that the DPTH-gel exhibits a considerably larger mass loss (15%) between 250 and 600°C. Above 600°C, no further mass loss occurs. In the differential thermal analysis of this compound, two peaks appeared at 345 and 530°C. The important feature of the present result is that this silica-bound material seems to be stable up to about 250°C.
XPS provides a sensitive means of characterizing the chemical composition of the surface layer by following the photoelectron bands of carbon, silicon, oxygen, nitrogen and sulfur (in at.%) of the layer to be calculated. The results obtained are presented in Table 1. Apart from the presence of sulfur (the finding of sulfur confirmed that the synthesis had been effective), it is interesting that the nitrogen-to-sulfur ratio is less than that predicted by theory; this may be largely explained by the fact that the yields of the different stages of the synthesis were limited and it is highly probable that intermediate structures existed between each stage.
The infrared spectrum (KBr pellet) is complicated because the large mass of silica and the aromatic portion of the molecule produce numerous bands, the overlap of which makes detailed assignments difficult. The band at 1,280 cm−1 was attributed to C=S stretching and the bands at 1,590 and 2,990 cm−1 were assigned to C=N and N–H stretching, respectively. These bands were assigned on comparison with the spectrum of pure DPTH as reference material [14]. The spectrum exhibits several other bands, which are weak and difficult to assign, but those discussed above appeared to be the most useful. The 13C NMR spectrum shows peaks at 202, 181, 143 and 125 ppm, which were assigned, respectively, to the C=S, N=C, Py–C and –CHaromatic groups.
Preparation of TS-gel
The procedure is shown schematically in Fig. 2. The silica gel was refluxed with 6 M HCl for 3 h to remove metals, washed with deionized water and dried in an oven at 180°C for 24 h. Activated silica gel (3 g) was suspended in 100 ml of 10% v/v 3-aminopropyltrimethoxysilane in dry toluene. The mixture was refluxed through a calcium chloride tower for 3 h without stirring. The resulting product, AP-gel, was filtered off and washed repeatedly with methanol. This product was mixed with 5 g of methylthiosalicylate dissolved in 60 ml of ethanol. The mixture was refluxed for 8 h without stirring. The product obtained (TS-gel) was filtered off, washed with small portions of methanol and dried in an oven at 50°C. No stirring is required to obtain a chelating sorbent with a suitable pore size to pack the column; if stirring is performed, a small pore size is obtained, which hinders the flow of sample.
The contents of carbon, hydrogen and nitrogen in the TS-gel measured with the CHN elemental analyzer were 3.06, 1.01 and 0.89%, respectively. The surface coverage, determined on the basis of per cent nitrogen by elemental analysis, was found to be 636 μmol g−1. The experiments showed that the TS-gel chelating sorbent was not soluble in common organic solvents.
In order to ascertain the thermal stability of the material, thermogravimetry was applied. Thermogravimetric profiles were recorded for silica gel and TS-gel. The results for silica gel conform to what is known about this material [8], most of physically adsorbed water is lost by 150°C and an essentially stable situation exists up to 250–300°C, when neighboring –SiOH groups begin to release water to form siloxane bridges (–Si–O–Si–). The TGA data showed that the TS-gel exhibits a considerably large mass loss (19%) between 100 and 700°C. On the basis of the surface coverage value of 636 μmol g−1 obtained by CHN elemental analysis for the TS-gel and supposing that all the ligand is bonded to silica in the way depicted in Fig. 2, there is good concordance between the mass loss observed and that calculated theoretically for loss of organic group and water. Between 100 and 300°C, a mass loss of 8% occurs, corresponding to water; on incresing the temperature, a large mass loss (11%) is observed, corresponding to loss of the thiosalicylate group. Above 650°C, no further mass loss occurs. In the differential thermal analysis (DTA) of this material, two peaks appeared at 318 and 639°C. The important feature of these results is that this silica-bound material seems to be stable up to about 300°C.
XPS provides a sensitive means of characterizing the chemical composition of the surface layer by following the photoelectron bands of carbon, silicon, oxygen, nitrogen and sulfur. Integration of these bands and correction of the data by the equipment-specific factors enables the contents of carbon, silicon, oxygen, nitrogen and sulfur (in at.%) of the layer to be calculated. The results obtained are presented in Table 1. The presence of sulfur confirmed that the synthesis had been effective.
The infrared spectrum (KBr pellet) is complicated because the large mass of silica and the aromatic portion of the molecule produce numerous bands, the overlap of which makes detailed assignments difficult. However, the absorption observed at 1,650 cm−1 can be attributed to O=C–NH– stretching and that between 1,450 and 1,577 cm−1 can be assigned to C–Caromatic groups.
Preconcentration and elution
Chelating agents immobilized on silica gel support have various types of functional groups. These chelating agents form complexes with metal ions only in certain pH ranges. So when a sample containing metal ion is passed over a chelating sorbent at certain pH values, metal ion forms chelates with the chelating agent on the silica gel support and thus are retained by the sorbent; thus, the impurities are not retained and these pass through. During elution, the eluent chosen is such that it helps in knocking out the metal ion from the chelate site on the sorbent. This is generally achieved by changing the pH, i.e., by making use of acids. This also helps in regeneration of the sorbent and thus chelating sorbent can be reused. Such types of separation avoid use of carcinogenic organic solvents and are ecofriendly.
Analytical applications of chelating sorbents based on silica gel
Extraction of metal ions using chelating sorbents has several advantages over the conventional methods:
-
1.
Selective determination of metal ions will be possible by using a chelating sorbent having a ligand processing high selectivity to the targeted metal ion.
-
2.
It is free from difficult phase separation, which is caused by the mutual solubility between water and organic solvent layers.
-
3.
The chelating sorbent method is an economical method since it uses only a small amount of ligand, is free from difficult phase separation and extraction solvent, and this also increases the sensitivity of the system.
-
4.
Trace metal ions at concentrations as low as parts per billion (ppb) can be determined because the targeted ion is enriched on the solid phase.
-
5.
The concentration of metal ion can be visibly estimated from the color intensity of the solid phase if the metal complex formed process adsorption in the visible wavelength region.
-
6.
Use of carcinogenic organic solvents is avoided and thus the technique is ecofriendly.
The interest in chelating sorbents based on silica gel for preconcentration/separation and determination of metal ions can be charted in surveys of publications using chelating resin and silica gel key terms. In this work, we used papers abstracted by the Analytical abstracts Database (Royal Society of Chemistry, UK) as the data source. The time period of this study is from January 1980 to July 2004. The representative contributions and most relevant aspects of each application are reported in Table 2.
The number of papers represented in Table 2 from each chelating sorbent immobilized on silica gel is 113. 8-Hydroxyquinoline [quinolin-8-ol] immobilized on silica gel is the leading chelating sorbent used for separation of trace metals, with 16 papers represented in Table 2. This chelating sorbent has been used by many workers for preconcentration of trace elements [77–92]. Sturgeon et al. [91] has used this chelating sorbent for preconcentration of Cd2+, Pb2+, Zn2+, Cu2+, Fe3+, Mn2+ and Ni2+ from seawater prior to their determination by graphite furnace atomic spectrometry. This sorbent was found to permit large enrichment factors of 500 while providing rapid processing of large volume samples, quantitative recovery of these elements, and a matrix-free concentrate suitable for instrumental analysis. Luerhmann et al. [88] also used this sorbent for sorption of Cu2+, Ni2+, Co2+, Fe3+, Mn2+, Cd2+, Pb2+, Cr3+, Zn2+and Hg2+. The metal uptake capacities were in the range from 0.2 to 0.7 mmol g−1 and the distribution ratios were in the range from 103 to more than 9×104. Mercapto-modified silica gel has been used by many workers for preconcentration of some trace metals [65–73]. Volkan et al. [69] used this sorbent for separation and preconcentration of Cu2+, Cd2+, Pb2+ and Zn2+ from aqueous solutions. All the metals were quantitatively retained on the matrix and can be eluted with 3 M nitric acid with a recovery >95%. This mercapto-modified silica gel was used for the preconcentration of these metals from seawater. They also used this sorbent for selective preconcentration of arsenite from natural waters [70]. This sorbent selectively removes arsenite from the samples that also contain arsenate, monoethylarsenate and dimethylarsenate. Arsenite was quantitatively retained over the pH range 1.5–8.5. Since a number of potentially suitable acidic and basic eluting agents were all ineffective in removing arsenic from the mercapto-modified silica, they oxidized arsenite to arsenate by eluting with 2 g l−1 potassium iodate solution in 0.5 M hydrochloric acid. This sorbent was also used by Akman et al. [71] as adsorbent for preconcentration of Cu2+ and Cd2+ in water. These metals were quantitatively retained on the adsorbent at acidic media with a capacity for Cu2+ being 0.022 nmol g−1 modified silica. They also used this sorbent for separation and preconcentration of Co2+ and Ni2+ prior to their determination by graphite furnace atomic spectrometry [72]. Köklü et al. [73] had also used this sorbent for adsorption of Cu2+, Ag+ and Au3+.
Our home-made chelating sorbents, DPTH-gel and TS-gel, have been used for separation and preconcentration of Hg, Zn, Cd, Co, Pb, Ni, Pt and Cr. The most relevant aspects of each application are summarized in Table 3.
Analytical applications of DPTH-gel and TS-gel
SPE has rapidly established itself as an alternative to sample preparation, as it provides many advantages, including high recovery of analytes, effective concentration, highly purified extracts, the ability to extract simultaneously analytes of widely variable polarities, easy automation and reduced organic solvent consumption. A survey of SPE from the literature can give the impression that innovation in such a widely explored field is virtually impossible. However, the use of new solid materials can open previously unexpected prospects. Such is the case with DPTH-gel and TS-gel, as they can be used as new sorbent materials for clean-up and preconcentration. The analytical potential of these sorbent materials for the preconcentration of various species has apparently been developed only by the authors’ group.
Minicolumns of DPTH-gel and TS-gel were made from glass tubing (3 mm i.d.) packed with 500–1,000 mg of sorbent, and sealed at both ends with polyethylene frits (Omnifit) to prevent material losses; the columns were initially flushed with 2 M nitric acid and the subsequent use of eluents in each operating cycle was sufficient to make the columns ready for re-use for at least 3 months. The column was located in the loop of an injection valve, and therefore only continuous flow systems (CFSs) for automated sample pretreatment have been developed. The CFS operation comprises two steps: sorption (sample introduction) and elution. The final extract was introduced to the instrument on-line.
On-line preconcentration coupled to inductively coupled plasma atomic emission
Figure 3 shows the manifold used for this purpose. In the clean-up and preconcentration step, the sample solutions were continuously passed through the column (located in the loop of the valve). The metal was adsorbed on the sorbent mini-column and the sample matrix sent to waste. During the preconcentration step, a flow of eluent was being aspirated from the containers by the pump P1 to establish the baseline of the readout and to stabilize the plasma. In a second step, the eluent carried out by the pump is led into the sorbent column, thereby effecting the rapid desorption of the metal. From this point, the analyte is: (1) directly transported to the nebulizer of an inductively coupled plasma atomic emission spectrometer (ICP-AES) or (2) mixed with reductant and speared via gas–liquid separator and swept into the ICP torch by a stream of argon.
The first applications of DPTH-gel and TS-gel as sorbent materials used a cold-vapor inductively coupled plasma atomic emission model system for the determination of traces of mercury by using a sodium tetrahydroborate (III) as hydride generation [12, 13]. The use of a column filled with TS-gel showed similar results to those obtained with a column of DPTH-gel. These materials exhibit the highest preconcentration factor and lowest detection limit. Nevertheless, the most interesting feature of the new sorbent materials is this selectivity. The tolerance level for Zn2+, Cr3+, Ni2+, Cu2+, Mn2+ and Co2+ (>50 mg l−1) is very high, compared with other values obtained with using other chelating sorbents in the preconcentration and determination of mercury (5 mg l−1) in the presence of same metal interferences [150–152].
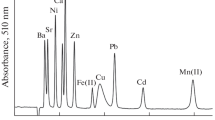
The effect of many common metals that can be chelated by the columns in the determination of cadmium by ICP-AES (see Fig. 3) is shown in Fig. 4. In this determination, the results obtained with DPTH-gel and TS-gel [39] were compared with those determined by Goswami et al. [77] using 8-hydroxyquinoline immobilized on silica gel. As can be seen, the selectivity was highest for DPTH-gel and TS-gel. The adsorptive potential of DPTH-gel for the preconcentration of trace zinc, manganese and cobalt from biological and water samples were also examined [40–42]. Best analytical results (sensitivity and selectivity) were obtained.
The CFS of Fig. 3 has been also applied for the determination of lead by preconcentration on TS-gel microcolumn and detection by ICP-AES [38]. The obtained detection limit was comparable with those obtained by other more sophisticated techniques and manifolds or expensive sorbent materials [153].
On-line preconcentration coupled to electrothermal atomic absorption spectrometry (ETAAS)
Figure 5 shows the manifold used for this purpose. It operated as follows: during the sample loading period, a flow of sample (standard or blank) is pumped (via P) through the microcolumn (located in the sampler arm); the metal ion is adsorbed on the sorbent microcolumn and the sample matrix is sent to waste; then, the switching valve (Vs) is actuated and the pumps of the AS-70 furnace autosampler, PAAS, are connected, permitting the operation of the autosampler in the normal mode; a wash step takes place with deionized water and, immediately after, the sample arm lowers the sample capillary into autosampler cup (filled with eluent) aspirating an eluent; then, the sampler arm swings over to the graphite furnace and the tip of the sampler capillary is inserted into the dosing hole of the graphite tube where the eluted metal was deposited as a drop; the sampler arm then returns to its initial position and the cycle of furnace operation commences; while the temperature program is running, the switching valve is again turned to start a new loading of the sample (standard or blank); thus, when the spectrometer gives the measurement, the microcolumn is ready for a new injection of eluent. The eluent injection system (normal operation of autosampler) provokes a flow though the column in both directions, up (when it is aspired) and down (when it is injected into the graphite tube), avoiding the continuous increase in column compactness. By using this approach, DPTH-gel has been used for on-line preconcentration and determination of nickel [43], cadmium [44] and platinum [45] in environmental samples. These systems have the advantage of simpler than other FI-ETAAS because the process is fully automated without complicated hardware and software; in fact modification of the software of the spectrometer was not necessary. High speed, ease of use and automation, selectivity and relative freedom from interference make these methods suitable for nickel, cadmium and platinum determination.
Another approach is the recently reported FI, on-line preconcentration and speciation of chromium in natural waters and human urine using a DPTH-gel chelating sorbent and Amberlite IRA-910 anionic ion exchange resin [46]. Diverse advantages are attained: (1) elimination of matrix, (2) increase of the sensitivity by the DPTH-gel preconcentration, and (3) automatization of different steps.
Conclusions and future directions
Probably the unavailability in the market of DPTH-gel and TS-gel has deterred these uses in analytical chemistry. They have a low cost compared with other chelating sorbents as Chelex-100 and Muromac A-1. The use of a column filled with DPTH-gel showed similar results to those obtained with a column of TS-gel. Although a better detection limit was obtained when DPTH-gel was used, the applicability of both the immobilized reagents was similar. The principal advantage of TS-gel is a single simple and fast reaction rather than the multi-step reaction reported in the synthesis of DPTH-gel and also with the other sorbents [154–156]. On the other hand, the presence of an excessive number of chelating centers may increase the complexing capacity of these resins for one or more metal ions with good selectivity. Using these newly synthesized chelating sorbents, better detection limits and enrichment factors were obtained when a DPTH-gel was used. Hg, Cd, Zn and Ni are the preference metals preconcentrated in the DPTH-gel sorbent with enrichment factors of 99, 86, 73 and 58, respectively, using 60 s preconcentration time. In the case of TS-gel the enrichment factors obtained for determination of Hg (t=60 s), Cd (t=120 s) and Pb (t=120 s) are 79, 62 and 41, respectively. The short time (60 s) required for loading metal ions onto the DPTH-gel sorbent is advantageous and better than required for loading metals onto TS-gel sorbent (120 s).
One of the foreseeable trends is the synthesis and use of their chelating sorbent derivatives that exhibit better properties than the original DPTH-gel and TS-gel, on the basis of the least number of chelating centers and to direct those centers to bind only the desired metal ion.
Because the DPTH-gel and TS-gel are insoluble in ordinary organic solvents and have a great capacity for formation of complexes with metals such as Hg, Zn, Cd, Mn, Co, Pt, Cr and Ni, they can be investigated as stationary phases, for liquid chromatography (LC) and for gas chromatography (GC).
References
Liska J (1993) J Chromatogr A 655:163
Carvahlo MS, Domingues MLF, Montovano JL, Filho EQS (1998) Spectrochim Acta B 53:1945
Martinez D, Cugat MJ, Borrull F, Callul M (2000) J Chromatgr A 902:65
Bruzzoniti MC, Sarzanini C, Mentássi EJ (2000) J Chromatogr A 902:289
Kvitek RJ, Evans JF, Carr PW (1982) Anal Chim Acta 144:93
Sarkar AR, Datta PK, Sarkar M (1996) Talanta 43:1857
Jal PK, Dutta RK, Sudershan M, Saha A, Bhattacharya SN, Chintalapudi SN, Mishra BK (2001) Talanta 55:233
Savvin SB, Mikhailova AV (1996) Zh Anal Khim 51:49
Sukhan VV, Zaporozhets OA, Lipkovskaya NA, Pogasi LB, Chuiko AA (1994) Zh Anal Khim 49:700
Ostrovskaya VM (1977) Zh Anal Khim 32:1820
Tertykh VA, Belyakova LA (1991) Noukova Dumka, Kiev
Cañada P, Garcia A, Cano JM, Rodríguez E (1998) J Anal Atom Spectrom 13:243
Cañada P, Cano JM, Sánchez F, Garcia A (1998) J Anal Atom Spectrom 13:1167
Bonilla JR, Garcia A, Cano JM (1983) Mikrochem J 28:132
Garg BS, Sharma RK, Bhojak N, Mittal S (1999) Microchem J 61:94
Camel V (2003) Spectrochim Acta B 58:1177
Colella MB, Siggia S, Barnes RM (1980) Anal Chem 52:2347
Colella MB, Siggia S, Barnes RM (1980) Anal Chem 52:967
Liu CY, Sun PJ (1981) Anal Chim Acta 132:187
Liu CY, Sun PJ (1984) Talanta 31:353
Maeda H, Egawa H (1984) Anal Chim Acta 162:339
Di P, Davey DE (1995) Talanta 42:685
Brajter K, Zlotorzynska ED (1988) Analyst 113:1571
Masi AN, Olsina RA (1993) Talanta 40:175
Hemmes M, Parrish JR (1977) Anal Chim Acta 94:307
Lim JH, Seol KM, An HS, Chung KC, Lee CH, Lee W (1996) Anal Sci Technol 9:364
Sutton RMC, Hill SJ, Jones P (1996) J Chromatogr A 739:81
Naghmush AM, Pyrzynska K, Trojanowicz M (1995) Talanta 42:851
Lin S, Zheng C, Yung G (1995) Talanta 42:921
Posta J, Berndt H, Luo SK, Schaldach G (1993) Anal Chem 65:2590
Gallego M, de Peña YP, Valcárcel M (1994) Anal Chem 66:4074
Leyden DE, Luttrell GH (1975) Anal Chem 47:1612
Leyden DE, Monidez WK, Carr PW (1975) Anal Chem 47:1449
Leyden DE, Luttrell GH, Monidez WK, Werho DB (1976) Anal Chem 48:67
Leyden DE,. Luttrell GH, Solan AE, De Anagelis NJ (1976) Anal Chim Acta 84:97
Unger KK (1979) Porous silica. Elsevier, New York
Hankins MG, Hayashita T, Kasprzyk SP, Bartsch RA (1996) Anal Chem 68:2811
Zougagh M, García A, Vereda E, Cano JM (2004) Talanta 62:503
Zougagh M, García A, Cano JM (2002) Talanta 56:753
Zougagh M, Cañada P, García A, Cano JM (2000) J Anal Atom Spectrom 15:1589
Zougagh M, García A, Cano JM (2003) Anal Lett 36:1115
Zougagh M, Cañada P, García A, Cano JM (2004) Anal Bioanal Chem 378:423
Siles MT, Vereda E, Cañada P, García A, Cano JM (1999) J Anal Atom Spectrom 14:1033
Vereda E, Palomo L, Siles MT, García A, Cano JM (2001) J Anal Atom Spectrom 16:293
Bosch C, Sánchez F, Cano JM, García A (2003) Anal Chim Acta 494:97
Siles MT, Vereda E, García A, Cano JM (2004) J Anal Atom Spectrom 19:398
Goswami A, Singh AK (2002) Anal Bioanal Chem 374:554
Goswami A, Singh AK (2002) Talanta 58:669
Wu XZ, Liu P, Pu QS, Sun QY, Su ZX (2003) Anal Lett 36:2229
Ince H, Akman S, Köklü Ü (1992) Fresenius J Anal Chem 342:560
Jin JH, Chen JW, Xu Y, Wang ZH, Zhao ZY (1999) Lihua Jianyan Huaxue Fence 35:339
Kocjan R, Swieboda R (1998) Chem Anal 43:657
Mahmoud ME, Soliman EM (1997) Talanta 44:15
Osman MM, Kholeif SA, Al-Maaty NAA, Mahmoud ME (2003) Microchim Acta 143:25
Wu XZ, Liu P, Pu QS, Su ZX (2002) Chem Anal 47:713
Ma WX, Liu F, Li KA, Chen W, Tong SY (2001) Anal Chim Acta 416:191
Pu QS, Su ZX, Hu Z, Chang XJ, Yang M (1998) J Anal Atom Spectrom 13:249
Pu QS, Sun QY, Hu ZD, Su ZX (1998) Analyst 123:239
Terada K, Matsumoto K, Kimura H (1983) Anal Chim Acta 153:237
Soliman EM, Mahmoud ME, Ahmed SA (2001) Talanta 54:243
Bagheri H, Gholami A (2001) Talanta 55:1141
Bagheri H, Gholami A, Najafi A (2000) Anal Chim Acta 424:233
Moreira JC, Pavan LC, Gushikem Y (1990) Mikrochim Acta 111:107
Bruening ML, Mitchell DM, Bradshaw JS, Izatt RM, Bruening RL (1991) Anal Chem 63:21
Walcarius A, Etienne M, Delacote C (2004) Anal Chim Acta 508:87
Losev VN, Elsuf’ev EV, Alennikova YV, Trofimchuk AK (2003) Zh Anal Khim 58:269
Losev VN, Kudrina YV, Maznyak NV, Trofimchuk AK (2003) Zh Anal Khim 58:146
Sahin F, Volkan M, Howard AG, Ataman OY (2003) Talanta 60:1003
Volkan M, Ataman DY, Howard AG (1987) Analyst 112:1409
Howard AG, Volkan M, Ataman DY (1987) Analyst 112:159
Akman S, Ince H, Köklü Ü (1991) Anal Sci 7:799
Köklü Ü, Akman S, Göçer Ö, Döner G (1995) Anal Lett 28:357
Köklü Ü, Tascioglu S (1998) Chim Acta Turc 16:2983
Su PG, Huang SD (1998) Anal Chim Acta 376:305
Mahmoud ME, Soayed AA, Hafez OF (2003) Microchim Acta 143:65
Dias NL, Gushikem Y, Polito WL, Moreira JC, Ehirim EO (1995) Talanta 42:1625
Goswami A, Singh AK, Venkataramani B (2003) Talanta 60:1141
Abbasse G, Ouddane B, Fischer JC (2002) J Anal Atom Spectrom 17:1354
Weeks DA, Bruland KW (2002) Anal Chim Acta 453:21
Uibel RH, Harris JM (2000) Appl Spectrosc 54:1868
Muenter MM, Stokes KC, Obie RT, Jezorek JR (1999) J Chromatogr A 844:39
Kasahara I, Takayama N, Yamamoto H, Sakurai K, Taguchi S (1997) Bunseki Kagaku 46:211
Jezorek JR, Tang JW, Cook WL, Obie R, Rowe JM (1994) Anal Chim Acta 290:303
Kasahara I, Willie SN, Sturgeon RE, Berman SS, Taguchi S, Goto K (1993) Bunseki Kagaku 42:107
Daih BJ, Huang HJ (1992) Anal Chim Acta 258:245
Ryabushko OP, Zaitseva GN (1990) Ukr Khim Zh 56:267
Ueda K, Koshino Y, Yamamoto Y (1985) Anal Lett 18:2345
Luehrmann M, Stelter N, Kettrup A (1985) Fresenius Z Anal Chem 322:47
Nojiri Y, Kawai T, Otsuki A, Fuwa K (1985) Water Res 19:503
Srivastava SP, Bhushan R, Chauhan RS (1984) J Liq Chromatogr 7:1341
Sturgeon RE, Berman SS, Willie SN, Desauiniers JAH (1981) Anal Chem 53:2337
Lan CR, Yang MH (1994) Anal Chim Acta 287:101
Kocjan R (1999) Mikrochim Acta 131:153
Iamamoto MS, Gushikem Y (1989) Analyst 114:983
Szczepaniak W, Szymanski A (1996) Chem Anal 41:193
Howard AG, Volkan M, Ataman DY (1987) Analyst 112:159
Tokman N, Akman S, Ozcan M (2003) Talanta 59:201
Tokman N, Akman S, Ozcan M, Köklü Ü (2002) Anal Bioanal Chem 374:977
Ekinci C, Köklü Ü (2000) Spectrochim Acta B 55:1491
Akman S, Ince H, Köklü Ü (1992) J Anal Atom Spectrom 7:187
Azeredo LC, Azeredo MAA, Castro RN, Saldanha MFC, Perez DV (2002) Spectrochim Acta B 57:2181
Zaporozhets OA, Ivanko LS, Marchenko IV, Orlichenko EV, Sukhan VV (2001) Talanta 55:313
Sharma RK (2001) Pure Appl Chem 73:181
Zhang SM, Pu QS, Liu P, Sun QY, Su ZX (2002) Anal Chim Acta 452:223
Pyell U, Stork G (1992) Fresenius J Anal Chem 343:576
Wolfbeis OS, Offenbacher H (1984) Fresenius Z Anal Chem 319:282
Hoang TT, Chen YF, May SW, Browner RF (2004) Anal Chem 76:2062
Zaporozhets OA, Ivan’ko LS, Sukhan VV (2000) J Anal Chem 55:130
Hankins MG, Hayashita T, Kasprzyk SP, Bartsch RA (1996) Anal Chem 68:2811
Mahmoud ME (2002) Anal Lett 35:1251
Osman MM, Kholeif SA, Abou-Almaaty NA, Mahmoud ME (2004) Anal Sci 20:847
Wu XZ, Liu P, Pu QS, Sun QY, Su ZX (2004) Talanta 62:918
Belikov KN, Blank AB, Shevtsov NI, Nadzhafova O, Tananaiko MM (1999) Anal Chim Acta 383:277
Zaporozhets OA, Nadzhafova OY, Zubenko AI, Sukhan VV (1994) Talanta 41:2067
Rakhman’ko EM, Tsvirko GA, Gulevich AL (1991) Zh Anal Khim 46:1525
Zaporozhets OA, Ivanko LS, Marchenko IV, Orlichenko EV, Sukhan VV (2001) Talanta 55:313
Zaporozhets OA, Nadzhafova OY, Verba VV, Zinchenko NM, Sukhan VV (1999) Int J Environ Anal Chem 74:243
Im HJ, Barnes CE, Dai S, Xue ZL (2004) Talanta 63:259
Arakawa Y, Wada O, Manabe M (1983) Anal Chem 55:1901
D’yachenko NA, Trofimchuk AK, Sukhan VV (1995) Zh Anal Khim 50:842
Garg BS, Bist JS, Sharma RK, Bhojak N (1996) Talanta 43:2093
Yamini Y, Chaloosi M, Ebrahimzadeh H (2002) Talanta 56:797
Liu P, Pu QS, Su ZX (2000) Analyst 125:147
Yan XP, Sperling M, Welz B (1999) Anal Chem 71:4216
Yan XP, Sperling M, Welz B (1999) J Anal Atom Spectrom 14:1625
Sperling M, Yan XP, Welz B (1996) Spectrochim Acta B 51:1875
Izatt RM, Bruening RL, Bruening ML, Tarbet BJ, Krakowiak KE, Bradshaw JS, Christensen J (1988) J Anal Chem 60:1825
Terada K, Nakamura K (1981) Talanta 28:123
Zaporozhets OA, Ivan’ko LS, Marchenko IV, Sukhan VV (2000) J Anal Chem 55:540
Szczepaniak W, Szymanski A (1996) Chem Anal 41:193
Wolfbeis OS, Offenbacher H (1984) Fresenius J Anal Chem 319:282
D’yachenko NA, Ishchenko VB, Trofimchuk AK, Sakhno AG (2000) J Anal Chem 55:851
D’yachenko NA (1999) J Anal Chem 54:144
Barbette F, Rascalou F, Chollet H, Babouhot JL, Denat F, Guilard R (2004) Anal Chim Acta 502:179
Venkatesh G, Singh AK, Venkataramani B (2004) Microchim Acta 144:233
Dudler V, Lindoy LF, Sallin D, Schlaepfer CW (1987) Aust J Chem 40:1557
Garg BS, Sharma RK, Bist JS, Bhojak N, Mittal S (1999) Talanta 48:49
Mahmoud ME, Al-Saadi MSM (2001) Anal Chim Acta 450:239
Liu P, Zu ZX, Wu XZ, Pu QS (2002) J Anal Atom Spectrom 17:125
Hutchinson S, Kearney GA, Horne E, Lynch B, Glennon JD, McKervey MA, Harris S (1994) J Anal Chim Acta 291:269
Kocjan R, Garbacka M (1994) Talanta 41:131
Liu P, Pu QS, Sun QY, Su ZX (2000) Fresenius J Anal Chem 366:816
Mahmoud ME, Al-Saadi MSM (2001) Anal Chim Acta 450–239
Azeredo LC, Azeredo MAA, Castro RN, Saldanha MFC, Perez DV (2002) Spectrochim Acta B 57:2181
Goswami A, Singh AK (2002) Anal Chim Acta 454:229
Terada K, Matsumoto K, Kimura H (1983) Anal Chim Acta 153:237
Sarkar AR, Dutta PK, Sarkar M (1996) Talanta 43:1857
Mahmoud ME (1996) Anal Lett 29:1791
Mahmoud ME, Gohar GA (2000) Talanta 51:77
Mondal BC, Das AK (2003) Anal Chim Acta 477:73
Vassileva E; Furuta N (2003) Spectrochim Acta B 58:1541
Hernandez O, Castro V, Arias JJ (1991) Anal Sci 7:341
Vochkova L, Arpadjan S (1996) Talanta 43:479
Marshall MA, Mottola HA (1983) Anal Chem 55:2089
Fadeeva VI, Tikhomirova TI, Yuferova IB, Kudryavtsev GV (1989) Anal Chim Acta 219:2
Falter R, Scholer HF (1995) Fresenius J Anal Chem 353:34
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zougagh, M., Cano Pavón, J.M. & Garcia de Torres, A. Chelating sorbents based on silica gel and their application in atomic spectrometry. Anal Bioanal Chem 381, 1103–1113 (2005). https://doi.org/10.1007/s00216-004-3022-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-3022-2