Abstract
A semiautomatic method based on application of ultrasounds has been developed to leach and hydrolyse phenolic compounds, such as naringin, rutin, naringenin, ellagic acid, quercetin and kaempferol, from strawberries. Two grams of lyophilized sample was placed into a sample cell and 5 mL of acetone containing hydrochloric acid was added. The cell was immersed in a water bath and sonicated for 30 s (duty cycle 0.8 s, output amplitude 50% of the nominal amplitude of the converter, applied power 100 W and with the probe placed 2 cm from the top surface of the extraction cell) for three times: each time 5 mL extractant displaced the previous extract. When the extraction was completed, the combined extracts were evaporated for 10 min, diluted to 10 mL with water adjusted to pH 8, and transferred to a cleanup–preconcentration manifold; here the analytes were retained in two in-series minicolumns packed with HR-P sorbent and then eluted with 4 mL methanol, and injected for individual separation–quantitation into a chromatograph–photodiode array detector assembly. Optimisation of the extraction was carried out using samples spiked with 4 mg kg−1 of each analyte. Calibration curves using the standard addition in red strawberries typically gave linear dynamic ranges of 4–40 mg L−1 for all analytes, except for ellagic acid (40–400 mg L−1). The r 2 values exceeded 0.98 in all cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds occur as secondary metabolites in all plants. Flavonols constitute one of the major subclasses of flavonoids endowed with a C-ring structure with a double bond at the 2,3 position. Flavonols in plants are usually bound to sugars as O-glycosides. This is a general feature of flavonoids, with one notable exception: catechins. Flavonoids free from their attached sugars are called aglycones. Flavonol aglycones are not present in fresh plants, but may appear as a result of food processing.
These compounds are considered generally to be non-nutritive agents; however, interest in these substances arises from their possible effects on human health. Several studies on the mutagenicity of some major flavonoids (e.g. quercetin, kaempferol and myricetin) have been published [1–4]. The antimutagenic and anticarcinogenic properties of these phenolic compounds have been demonstrated by experimental studies on animals [5–7]. Furthermore, these compounds can act as signalling molecules (host-recognition substances) in the interaction between the plant and nitrogen-fixing bacteria in certain leguminous plants [8]. An important function of flavonoids and phenolic acids is their action in plant defence mechanisms [9, 10]. Stress conditions such as excessive UV light, wounding or infection induce the biosynthesis of these compounds. Thus, environmental factors may have a significant contribution to the content of flavonoids and phenolic acids in plants such as strawberries.
Knowledge of the levels of these compounds in plants is of interest but problems for their analysis arose from the structural diversity of phenolics and their effect on physicochemical behaviour such as solubility. For these reasons, the efficient extraction of these compounds constitutes a challenging analytical problem. Different methods for identification and quantification of phenolic compounds in food samples have been published. The most widely used are based on high-performance liquid chromatography (HPLC) with photodiode array and/or mass spectrometry detection [11–13]. The latter, liquid chromatography-mass spectrometry (LC-MS), has become the best alternative for separation, identification and quantification of these compounds in plants (because of the analysis complexity and different distribution of antioxidants in the sample) [14]. When dealing with liquid samples, HPLC with direct injection is the analytical technique of choice most times [15], and filtration of the samples is the only pre-treatment needed. Solid foods require an appropriate prior extraction step [16, 17], for which extractants such as ethanol, acetone or methanol are used [18]; the extractant is a water/methanol mixture containing both hydrochloric acid and an antioxidant to protect the analytes from oxidation [13]. The extraction step of phenolic compounds requires special care because these compounds are easily oxidized and rapidly degraded by light. Different techniques such as supercritical fluid extraction (SFE), which uses either pure or modified CO2 [19], and microwave-assisted extraction [20] have been used. The methods based on these techniques offer better control of the extraction conditions compared with conventional alternatives, thus allowing the extraction to be carried out within relatively short times and in a selective way.
Ultrasound radiation is a powerful aid for acceleration of various steps of the analytical process. This energy provided is of great help in the pre-treatment of solid samples as it facilitates and accelerates operations such as the extraction of organic and inorganic compounds [21, 22], homogenization [23] and various others [24]. Ultrasound-assisted extraction processes are often used for the isolation of target compounds from plant material by liquid solvents. This extraction process is faster and more complete compared with traditional methods such as maceration/stirring because the surface contact area between the solid and liquid phases is increased by particle disruption [24].
This paper presents research devoted to the development of a semiautomatic method for the analysis of phenolic compounds from strawberries (naringin, rutin, naringenin, ellagic acid, quercetin and kaempferol), based on ultrasound-assisted extraction, cleanup, preconcentration, chromatographic separation, and photodiode array detection. The inclusion of two in-series minicolumns packed with HR-P sorbent in a flow injection (FI) system coupled on-line to a chromatograph allows the cleanup and preconcentration of the extracted analytes before individual separation and photodiode array detection.
Experimental
Reagents
Catechin, naringin, naringenin, rutin, quercetin, kaempferol and ellagic acid were purchased from Sigma–Aldrich (Madrid, Spain). An N,N-dimethylformamide (DMF) tock standard solution of 1,000 mg L−1 each analyte was prepared and stored at 4°C in the dark. Solutions were prepared daily by dilution of this stock solution with methanol. Ultrapure water from Mili-Q system (Millipore, Bedford, Mass., USA) was used throughout. Acetone, DMF and methanol (Panreac, Barcelona, Spain) were of HPLC grade. Formic and hydrochloric acids, sodium hydroxide, and tert-butylhydroquinone (TBHQ) (Panreac) were of analytical grade.
Instruments and apparatus
Ultrasound irradiation was applied by means of a Branson 450 sonifier (20 kHz, 100 W) equipped with a cylindrical titanium alloy probe (2.54-cm diameter) which was immersed in a water bath in which the sample cell was placed. The extraction chamber used consisted of a stainless steel cylinder (10-cm long and 10-mm i.d.) closed with screws at either end, which permitted the circulation of the leaching fluid through it. The screws caps also contained stainless steel filter plates, 2 μm in thickness and 0.25-in. i.d., to ensure that the sample remained in the extraction chamber.
A four-channel Gilson Minipuls-3 low-pressure peristaltic pump, a Rheodyne low-pressure injection valve, three Rheodyne low-pressure selection valves, two laboratory-made minicolumns (4 and 6 cm×3-mm i.d.) packed with HR-P sorbent and Teflon tubing of 0.8-mm i.d. were used to construct the overall arrangement.
A rotary evaporator (R-200, Büchi, Switzerland) was used for partial evaporation of the solvent after extraction.
The HPLC system used for individual separation, identification and quantification was an HP1100 liquid chromatograph (Agilent Technologies, Avondale, Pa., USA) consisting of a G1311A high-pressure quaternary pump, a G1322A vacuum degasser, a Rheodyne 7725 high-pressure manual injector valve (10-μL injection loop) and a G1315A diode array UV-VIS detector (DAD). A Hypersil ODS column (250 mm×4.6-mm i.d., 5 μm) protected with a Hypersil ODS guard column (10 mm×4.6-mm i.d., 5 μm) was also used.
Sample preparation
Red strawberries were purchased in a local market of Córdoba, Spain, lyophilized, crushed and stored at −4°C until use. All the strawberries used in this study were from the same prior homogenized batch.
Spiked red strawberries were used to carry out the optimization study, as no CRMs of this type are available. Approximately 2 g of lyophilized sample was spiked with the necessary amount of phenolic compounds (0.032 mL of a stock multistandard solution of 2,500 mg L−1 for ellagic acid and 250 mg L−1 for all other analytes) to obtain a final total concentration in the food of 4 mg kg−1 in each analyte, except for ellagic acid (40 mg kg−1). Then, the samples were stored for 2 h at 4°C before extraction to simulate the normal interaction between strawberries and the phenolic compounds. To minimize errors in the spiking step, sampling was done according to the protocol established by legislation [25].
Procedure
The method was developed using the approach shown in Fig. 1, which allows the automated leaching and—after partial extractant release in a rotary evaporator—preconcentration, individual chromatographic separation and photodiode array detection of the analytes. Lyophilized strawberry (2 g) was weighed and placed into the extraction chamber (EC). After assembling, this unit was placed into the water bath, and 5 mL of an acetone solution containing 0.2 M hydrochloric acid and 2 g L−1 TBHQ was pumped into the chamber cell by via of the channels of the peristaltic pump PP1 for simultaneous extraction and hydrolysis. Then, the ultrasound extraction program, which consisted of three cycles of irradiation of 30 s (duty cycle 0.8 s, output amplitude 50% of the nominal amplitude of the converter, applied power 100 W and with the probe placed 2 cm from the top surface of the extraction cell), was developed. After each cycle, fresh solvent displaced the extract from the cell, which was collected in an extract reservoir, and a new extraction cycle was performed. After the last cycle, an air stream purged the system for collecting the extract (from the final cycle) in the reservoir.
Experimental set-up used. Top Extraction step: EX extractant, PP peristaltic pump, IV injection valve, EC extraction chamber, ER extract reservoir. Bottom left Cleanup-preconcentration step: M methanol, A air, SV selection valve, CE conditioned extract, EL elution loop, F filter, W waste, MC minicolumn. Bottom right Individual separation-detection step: SR solvent reservoirs, HPP high-pressure pump, HPIV high-pressure injection valve, AC analytical column, DAD diode array detector
After complete extraction, the extract was evaporated close to dryness by using a rotary evaporator for 10 min to remove the acetone from the extract. The extract was not dried completely as further dissolution was otherwise more difficult and degradation occurred. The concentrated sample was diluted up to 10 mL with water adjusted to pH 8.
In order to obtain a cleaner and more concentrated extract, a cleanup preconcentration step was then developed. The filtration–preconcentration manifold required the use of three injection valves. The extract was firstly freed from particles by passage through a filter (F), which was located in the loop of injection valve IV1; the extract was then transferred to two in-series sorption columns where the analytes were retained. The columns were also located in the loop of an injection valve (IV3), thus enabling elution in the direction opposite to retention with a volume of methanol selected using the third injection valve (IV2). The eluate from the sorption columns was transferred to the loop of the high-pressure injection valve of the chromatograph using an air stream as carrier. Between samples and during the chromatographic separation, water was circulated through the filter in the opposite direction to filtration at high flow rate to clean the filter, and the sorbent in the minicolumns was conditioned by circulating methanol and water through it.
The time program for valves and pump channels was as follows: after extraction and extract conditioning, PP2 and PP3 remained in stop and go positions, respectively; meanwhile, IV1 and IV2 were in the load position. In this way, the conditioned extract passed through the filter for retention of in-suspension particles, and—after passing through the two sorbent minicolumns for cleanup preconcentration—it was sent to waste. After passage of the extract through the minicolumns, PP2 was changed to its go position. The loop of IV2 was filled with methanol adjusted to pH 2.5 with formic acid; meanwhile air passed through the loop of IV3, now in its inject position. After a preset interval for filling the loop, valve IV2 was switched to the inject position and a given volume (4 mL) of methanol, pH 2.5, eluted the retained analytes from the minicolumns and transferred them to the high-pressure injection valve (HPIV) which was in its load position. After a delay time from injection of the eluent by valve IV2, HPIV was switched to the inject position and a portion of the eluate was injected onto the chromatographic column. During the individual separation and detection, the preconcentration minicolumns were conditioned by passage of methanol and water (valve IV3 in the inject and load position, respectively). After conditioning, a new extraction step started, overlapped with the chromatographic separation and detection of the analytes from the previous sample.
The HPLC separation of the phenolic compounds was performed using a methanol/water gradient. The mobile phase consisted of methanol/water (50:50 v/v) adjusted to pH 2.5 with formic acid (A) and methanol (B). The gradient program was as follows: 0–8 min, 90% A and 10% B, flow rate 1 mL min−1; 8–10 min, 90–85% A and 10–15% B, flow rate 1 mL min−1; 10–15 min, 85–50% A and 15–50% B, flow rate 1 mL min−1; 15–20 min, 50% A and 50% B, flow rate 1 mL min.1; 20–30 min, 50–0% A and 50–100% B, flow rate 1 mL min−1; 40–41 min, 0–90% A and 100–10% B, flow rate 1 mL min−1; 41–45 min, 90% A and 10% B, flow rate 1 mL min−1. The chromatograms were acquired at 254, 275, 285, 355 and 370 nm. HPLC-DAD quantitative analyses were expressed in area units for all analytes (see Fig. 2).
Chromatograms of the phenolic compounds after ultrasound-assisted extraction, under the optimal working conditions. Analyte peaks: 1 catechin (275 nm), 2 naringin (285 nm), 3 rutin (254 nm), 4 ellagic acid (254 nm), 5 naringenin (285 nm), 6 quercetin (254 nm), 7 kaempferol (355 nm). a Strawberry sample. b Standard
Results and discussion
Optimisation of the individual chromatographic separation–detection step
Phenolic compounds absorb in the UV region and the most commonly used detector for HPLC is a multiwavelength UV or UV-VIS detector. No single wavelength is appropriate for monitoring all phenolics, since they display absorbance maxima at different wavelengths. Thus, five wavelengths were selected as a compromise solution. Rutin, ellagic acid and quercetin show their maximum absorbance close to 254 nm, catechin at 275 nm, naringenin and kaempferol at 285 and 355 nm, respectively. These wavelengths were studied using standard solutions of the analytes. The retention time and the profile of the absorption spectra of the compounds were used for identification of the analytes.
The experimental variables optimised to obtain an appropriate separation of the analytes were the type of the column, separation program, composition, pH and flow rate of the mobile phase.
Two columns, a Hypersil ODS column and a Superspher 100 RP-18 were compared to provide maximum retention of the phenolic compounds. The best results were obtained using the former.
Different water/methanol mixtures (90:10, 75:25 and 50:50) as A and methanol and acetonitrile as B were tested. The pH of A was studied in the range 2–5. The best separation was achieved using the composition, the pH of the mobile phase and gradient program given in the “Procedure” section. The influence of the flow rate of the mobile phase was studied in the range 0.5–1.2 mL min−1. The flow rate given in the “Procedure” section was selected as the value providing the best separation in a shorter time.
Two loop volumes (10 and 20 μL) were tested for injection. As the number of phenolic compounds present in strawberries is high, coelution of some of the compounds (e.g. naringenin and quercetin) was observed; thus, an injection loop of 10 μL was selected to minimize peak overlapping.
Optimisation of the preconcentration step
Prior to optimisation of the preconcentration step, the temperature and the time required to evaporate the acetone from the extract were studied. The temperature range studied was 25–90°C. Degradation of the analytes was observed in increasing temperature; thus, 25°C was selected as the best value. The time necessary to remove the acetone from the extract was 8–9 min; 10 min was the time selected for ensuring complete removal of the organic solvent.
The study of the preconcentration step was performed using the flow injection manifold in Fig. 1. In all cases, 10 mL of a standard solution containing 80 μg of each analyte, except ellagic acid (800 μg) was used to reproduce the real conditions—in terms of volume of extract and concentration of the analytes—obtained when the overall process (including the extraction step) was performed with quantitative extraction of the target analytes.
Three types of sorbent were tested; C18, C18 Hydra and HR-P, and the best results were obtained with the last of these. An HR-P minicolumn provided partial and preferential retention of the target analytes. Enlarging the column length caused high overpressure in the system. Two in-series minicolumns provided quantitative retention of the target analytes with pressure values usual in FI systems.
The pH for optimum retention of the analytes in the sorbent was studied between 2.5 and 9.0. The chromatograms obtained showed the highest peak at pH 8.0.
The eluents assayed were a methanol/DMF mixture and methanol adjusted to different pHs. Methanol adjusted to pH 2.5 was the best for quantitative elution of the analytes.
The minimum volume of methanol for total elution of the analytes—and thus for obtaining the highest preconcentration factor—was 4 mL. The eluent carrier was air, which drove out the eluent from the minicolumn, thus avoiding dilution of the analytes.
The flow rates for retention and elution were studied between 0.8–1.5 and 0.3–0.7 mL min−1, respectively; 1.2 and 0.5 mL min−1 were the optimum.
To determine the breakthrough of the HR-P minicolumns, the volume of sample containing 80–800 μg of the analyte passed through them was studied in the range 5–20 mL. The signal remained constant up to 20 mL; hence, in subsequent experiments, volumes lower than 20 mL were used in order to not exceed the breakthrough volume of the minicolumn. Under these conditions, quantitative retention and elution of the analytes was achieved.
Table 1 summarizes the tested ranges and the optimal values for all variables under study.
Optimisation of the extraction–hydrolysis step
Preliminary studies were aimed at selecting the extractant producing the lowest decomposition of the target analytes when subject to ultrasound. Standard solutions of the analytes were subject to ultrasonic energy using five of the solvent more frequently used as extractants of phenolic compounds: a 50:50 water/methanol mixture, water, acetonitrile, DMF and acetone, with two concentrations of hydrochloric acid 0.6 and 1.2 M. Acetone was considered to be the best as, under the same working conditions, the minimum analytes decomposition was observed when this solvent was used.
A multivariate optimisation approach was used for the extraction–hydrolysis step due to the interrelationship between the variables influencing it. The variables optimised were the probe position (distance between the tip horn of the ultrasound probe and the top surface of the extraction cell), radiation amplitude, percentage of duty cycle of ultrasound exposure, sonication time, extractant volume, concentration of hydrochloric acid in the extractant, extractant flow rate, time interval for changing the flow direction of the leachant, and number of cycles (Table 1).
A Plackett–Burman design allowing three degrees of freedom and involving 12 randomized runs plus three centre points was built for a screening study of the behaviour of the nine variables affecting the extraction step. The conclusions of this screening were that all factors were not significant for catechin, naringin, naringenin and rutin; meanwhile, the ultrasound radiation amplitude, probe position, time interval for changing the flow direction of the leachant and sonication time were not significant factors in the ranges under study for ellagic acid; all factors but the concentration of hydrochloric acid in the extractant were not significant factors for quercetin; and all factors but concentration of hydrochloric acid in the extractant and number of cycles were not significant factors for kaempferol. However, the results showed better recoveries with the highest value of probe height (2 cm), intermediate value of ultrasound radiation amplitude (50%), and both the shortest time interval for changing the flow direction of the extractant (no change) and sonication time (30 s). Lower concentrations of hydrochloric acid in the extractant—and higher concentration of acetone, as a result—could be selected for subsequent experiments due to their negative effect, but it is not advisable because deterioration of the tubes was observed. The lower value of the extractant flow rate was selected due to their negative effect (0 mL min−1). Higher value of the extractant volume could be tested due to their positive effect, but 5 mL was selected because this was the maximum volume of the extraction system.
Thus, higher values for the percent of duty cycle of ultrasound exposure and number of cycles were tested in subsequent experiments due to their positive effects. A two-level full-factor design involving four randomized runs plus three centre points was used. In this case, the duty cycle and the number of the cycles were significant factors in the range studied. The lower values tested (0.8 s and three cycles, respectively) were selected due to their positive effects. The optimum values of all variables are shown in Table 1.
Characterisation of the overall method
Calibration with standards
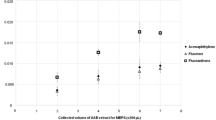
Calibration curves were obtained by plotting the peak area as a function of standard concentrations (Fig. 3). The linear dynamic ranges (4–40 mg L−1) for all analytes but ellagic acid (40–400 mg L−1) revealed good linearity, with r 2 values exceeding 0.98 in all cases.
Application of the method to ultrasound-assisted extracts from real-life samples
Quantification was based on the standard addition method using samples spiked with the necessary amount of phenolic compounds (0.032, 0.056, 0.08, 0.16 and 0.32 mL of a stock multistandard solution of 2,500 mg L−1 for ellagic acid and 250 mg L−1 for the rest of the analytes) to obtain different total concentrations (0, 4, 7, 10, 20 and 40 mg L−1 for each analyte and 0, 40, 70, 100, 200 and 400 mg L−1 for ellagic acid). The peak area was used for quantification of the analytes.
It is worth to emphasising that, as shown in Fig. 2, the retention times of the analytes in the chromatograms from standards and extracts do not coincide. For this reason, identification of the analytes in the latter was based on their corresponding spectra, which were then validated by standard addition of each individual analyte, thus corroborating and quantifying the retention time displacement of each analyte owing to differences between standards and extracts composition.
The limits of detection and quantification, respectively, of the analytes calculated for real-life samples are: 0.40±0.30 mg kg−1, 1.0±0.4 mg kg−1 for catechin, 2.01±1.44 mg kg−1, 4.80±2.01 mg kg−1 for naringin, 0.42±0.31 mg kg−1, 1.40±0.31 mg kg−1 for rutin, 4.12±3.40 mg kg−1, 11.30±4.10 mg kg−1 for ellagic acid, 0.34±0.21 mg kg−1, 1.13±0.21 mg kg−1 for naringenin, 0.33±0.15 mg kg−1, 0.51±0.33 mg g−1 for quercetin and 3.28±1.44 mg g−1, 4.80±3.28 mg g−1 for kaempferol in red strawberries. The limits of detection and quantification of the analytes are defined as the concentration producing a peak height three times and ten times, respectively, the standard deviation of the baseline noise.
The precision of the method was evaluated using the optimal conditions to develop a reproducibility study using the extracts and involving replicates on different days and two replicates on the same day, morning and afternoon, for 7 days. The range of within-day relative standard deviations (RSDs) for all target analytes was 1.3–7.5%; meanwhile, the between-day study yielded RSDs within 1.2–7.5%. The recoveries of the analytes added to the sample were in the range 80.3–101.4%.
Significant amounts of catechin (12.34 mg kg−1), naringin (38.72 mg kg−1), rutin (2.17 mg kg−1), ellagic acid (50.03 mg kg−1), quercetin (0.87 mg kg−1) and kaempferol (16.1 mg kg−1) were found in the red strawberries.
Conclusions
A semiautomatic approach which enables ultrasound-assisted extraction and partial evaporation of the extractant followed by solid-phase concentration prior to individual chromatographic separation of the target analytes and quantification by a photodiode array detector is proposed for the analysis of phenolic compounds in red strawberries.
Ultrasound-assisted extraction is faster and more efficient than traditional methods such as maceration/stirring: for example, three cycles of 30 s are required compared with 2 or 20 h (flavonols or ellagic acid, respectively) at 85–90°C [12, 13, 26, 27], because the surface contact area between the solid and liquid phases is increased by particle disruption.
Acid hydrolysis, especially under drastic conditions may result in losses of polyphenols through degradation and derivatisation.
The number of cycles of the extraction process increases the efficiency.
The pH (concentration of hydrochloric acid in the extractant) contributes to efficient extraction as its value determines the solubility of the analytes in the extractant.
Temperatures higher than 25°C are inadvisable and were not used to avoid degradation of the polyphenolic compounds.
References
Bjeldanes LF, Chang GW (1997) Mutagenic activity of quercetin and related compounds. Science, Washinton, DC 197:577
Stavric B (1984) Mutagenic food flavonoids. Fed Proc 43:2454
Van der Hoeven JCM, Bruggeman IM, Debets FMH (1984) Genotoxicity of quercetin in cultured mammalian cells. Mutat Res 136:9
MacGregor JT (1984) Genetic and carcinogenic affects of plant flavonoids: an overview. Adv Exp Med Biol 177:497
Huang M-T, Wood AW, Newmark HL, Sayer JM, Yagi H, Jerina DM, Conney AH (1983) Inhibition of the mutagenicity of bay-region diol-epoxides of polycyclic aromatic hydrocarbons by phenolic plant flavonoids. Carcinogenesis 4:1631
Francis AR, Shetty TK, Bhattacharga RK (1989) Modifying role of dietary factors on the mutagenicity of aflatoxin B1: in vivo effect of plants flavonoids. Mutat Res 222:393
Deschner EE , Ruperto J, Wong G, Newmark HL (1991) Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis 7:1193
Strack D (1997) Phenolic metabolism In: Dey PM, Harborne JB (eds) Plant biochemistry. Academic, London, p 387
Bennet RC, Wallsgrove RM (1994) Secondary metabolites in plant defence mechanisms. Tansley review, 72 New Phytol 127:617
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085
Bomser J, Madhavi DL, Singletary K, Smith MAL (1996) Planta Med 62:212
Häkkinen S, Auriola S (1998) J Chromatogr A 829:91
Häkkinen S, Törrönen AK (2000) Food Res Int 33:517
Dugo P, Modello L, Errante G, Zappia G, Dugo G (2000) J Agric Food Chem 49:3987
Revilla E, Ryan JM (2000) J Chromatogr A 881:461
Estribano-Bailón T, Gutiérrez-Fernández Y, Rivas-Gonzalo J, Santos-Buelga C (1992) J Agric Food Chem 40:1794
Antolovich M, Prensler P, Robards K, Ryan D (2000) Analyst 125:989
Häkkinen S, Karenlampi S, Heinoner I, Mykkänen H, Törrönen A (1998) J Sci Food Agric 77:543
Palma M, Piñeiro Z, Barroso CG (2002) J Chromatogr A 968:1
Llompert MP, Lorenzo RA, Cela R, Li K, Bálanger JMR, Paré JRJ (1997) J Chromatogr A 744:243
Mierzura J, Sun YC, Yang MH (1997) Anal Chim Acta 355:277
Ashley K, Andrews RN, Cavazos L, Demenge M (2001) J Anal At Spectrom 16:1147
Vinodgopal K, Peller J, Makogon O, Hieffje GM (1998) Appl Spectrosc 52:515
Luque de Castro MD, Luque-García JL (2002) Acceleration and automation of solid sample treatment, Elsevier, Amsterdam
Commision Directive of 24 July 1979 establishing community methods of sampling of the official control of pesticide residues in and on fruit and vegetables 79/700/EEC. Official Journal of European Community, European Community, Brussels
Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR (1999) J Agric Food Chem 47:2274
Justense U, Knuthsen P, Leth T (1998) J Chromatogr A 799:101
Acknowledgements
Spain’s Comisión Interministerial de Ciencia y Tecnología (CICyT) is gratefully acknowledged for financial support (project BQU-2002-01333).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera, M.C., Luque de Castro, M.D. Ultrasound-assisted extraction for the analysis of phenolic compounds in strawberries. Anal Bioanal Chem 379, 1106–1112 (2004). https://doi.org/10.1007/s00216-004-2684-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2684-0