Abstract
There is an urgent need for natural water reference materials certified for nutrients. In 1996, NRC collected seawater for a proposed CRM at a depth of 200 m in the North Atlantic; this was immediately filtered through 0.05-μm cartridge filters into 50-L carboys. The water was later homogenized in the NRC laboratories in Ottawa and stabilized via gamma irradiation. Over six years of stability testing no significant deterioration was detected. In addition to the usual customary standard colorimetric procedures, alternative analytical methods were developed to enable the certification process. The production of a CRM called MOOS-1 will be discussed. Certified values, with uncertainty components addressing the homogeneity, stability, and characterization of the material, were calculated to be: orthophosphate=1.56±0.07 µmol L−1, silicate=26.0±1.0 µmol L−1, nitrite=3.06±0.15 µmol L−1, and nitrite and nitrate=23.7±0.9 µmol L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The measurement of silicon, phosphorus, and nitrogen macronutrients in seawater is fundamental to the marine sciences. However, there is a conspicuous lack of certified reference materials (CRMs) to support this activity. Recently, the National Research Council (US) Committee on Reference Materials for Ocean Science was tasked with identifying critical reference materials required by the marine sciences. Their final report recommended a CRM for nutrients as a priority, citing an “urgent need for a CRM for these nutrients” and “the success of future surveys as well as the development of instruments capable of remote-time series measurements will rest on the availability and use of good nutrient reference materials” [1].
The greatest difficulty to overcome in the preparation of a natural seawater CRM for nutrients is the stabilization of analytes in solution. Biological activity must be eliminated, as it can rapidly alter the concentration of the analytes. For this purpose the use of autoclaving was reported by Aminot and Kérouel [2, 3]. This approach was successfully used for a series of International Council for the Exploration of the Sea (ICES) and Quasimeme intercomparison exercises [4, 5]. Autoclaving had a negligible impact on the concentration of nitrite and nitrate. However, on prolonged storage, phosphate was shown to leach from the glass storage bottles and it was reported that a slight amount of ammonia permeated through their plastic caps. Nevertheless, this approach was used to prepare two reference materials for ammonia, orthophosphate, and nitrite+nitrate in seawater by VKI of Denmark [6].
Use of chemical preservatives such as mercuric chloride [7, 8] has also been proposed to stabilize nutrient solutions; however, this method was not pursued as minimal sample alteration is generally preferable for reference materials.
The National Research Council of Canada (NRC) has recently prepared a seawater CRM for micronutrients called MOOS-1 using gamma irradiation to sterilize the sample. The certification of this material for dissolved silicate, orthophosphate, nitrite, and nitrite+nitrate is discussed herein.
Experimental
Nutrient measurements were conducted at NRC in order to assess stability and homogeneity of MOOS-1. These measurements were performed using a Technicon AutoAnalyser II (Technicon Industrial Systems, W. Tarrytown, NY, USA) following manufacturer-supplied methods: industrial methods No. 155–71 W for orthophosphate, No. 186–72 for silicon, and No. 158–71 W for nitrite and nitrate.
For orthophosphate this involved reaction of the sample with ammonium molybdate and antimony potassium tartrate. The resulting complex is reduced with ascorbic acid and the absorbance is measured. Silicon is measured as molybdenum blue as produced from the formation of β-molydosilicic acid. Nitrite is determined by diazotizing with sulfanilamide and coupling with N-1-naphthylethylenediamine dihydrochloride to form a colored azo dye. Nitrate and nitrite are measured using the same procedure; however, the nitrate is first reduced to nitrite in a cadmium column. Standards were prepared fresh from KH2PO4, Na2SiF6, NaNO2, and NaNO3.
Additional results were contributed by NRC using ion-exclusion chromatography in combination with inductively coupled plasma mass spectrometry (IEC-ICP-MS). A Perkin–Elmer Elan 6000 ICP-MS (Concord ON, Canada) was interfaced with a Model AGP-1 advanced gradient pump (Dionex, Sunnyvale, CA) for the measurement of orthophosphate [9] and silicate [10].
An ion chromatography system consisting of a Waters 600 s Controller, 626 Dual Pump, and 486 UV Detector ( Waters Corporation, Milford MA) was used for nitrite and nitrate determinations. Both ions were analyzed using a Dionex AG10 guard column on a Dionex AS10 analytical column. Nitrate was eluted with 200 mM HCl and detected by UV at 225 nm. Nitrite was eluted with 80 mM NaCl and detected by UV at 225 nm. It was necessary to wash the column with 200 mM NaOH for 30 min before analyzing for NO2.
Sample collection
MOOS-1 was collected at lat. 47.06°N, long. 59.98°W, off the northern tip of Cape Breton Island, Nova Scotia, Canada from a depth of about 200 m using a rosette containing 22 Niskins each of about 10-L volume. The contents of each Niskin were transferred, using a peristaltic pump, through a 0.05-µm cartridge filter into precleaned 50-L carboys. The water was returned to the NRC laboratories in Ottawa, homogenized in a 400-L polypropylene tank, bottled in 50-mL aliquots in precleaned polyethylene bottles, sealed, and irradiated. The bottles were precleaned by soaking overnight in 10% HCl followed by a rinse with deionized water. Other than the processes described above, the water was not intentionally altered.
The water was collected on June 24, 1996, bottled on July 11 and 12, and irradiated to a minimum 25 kGy on July 16, 1996.
Due to bottle-to-bottle inhomogeneity, as noted below, the samples were subsequently reblended and bottled in a Class 10 clean room located in the NRC laboratories in April 2001.
Discussion
Effect of irradiation
The destruction of micro-organisms through the use of cobalt-60 gamma irradiation has found widespread acceptance as an effective and technically viable method for treating a large variety of samples, such as food and medical devices. NRC has successfully used this “cold pasteurization” method to stabilize CRMs with respect to trace metal analysis for its suite of biological tissues, sediments, and natural water CRMs. However, conversion of the chemical composition of certain analytes has been shown to occur, the extent of which is dependent upon the dose of ionizing radiation received by the sample [11, 12]. When treated, the product is exposed to a specified minimum dose of radiation, required to ensure proper microbiological reduction, and a maximum, to ensure the functional specifications of the product or packaging are not exceeded. These overall limits are managed by controlling the time spent in the irradiation chamber. However, the dose received by individual bottles varies slightly, depending upon on their placement with respect to the irradiation source. This effect was demonstrated by preparing twenty 50-mL samples from a batch of seawater; ten bottles were subjected to irradiation and the remaining bottles were untreated. Following irradiation, all twenty samples were randomly analyzed using colorimetric methods. In addition to an increase in the concentrations for nitrite and nitrate+nitrite, a significant decrease in the interbottle precision is evident in the irradiated samples, as shown in Table 1. The determination of silicate and orthophosphate in these samples was not made.
To overcome this problem, the sample was irradiated prior to blending and bottling. This requires that the samples are not contaminated with bacteria during final processing. Although this preservation method is not satisfactory for field sampling, for which accurate concentrations are required, it is quite suitable for a reference material wherein a slight change in concentration is not detrimental to the usefulness of the material and sample stability is of utmost importance.
Certified value
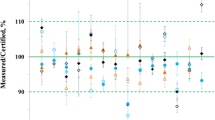
MOOS-1 was analyzed by NRC and a number of expert laboratories coinciding with an annual intercomparison exercise for nutrients sponsored by the National Oceanic and Atmospheric Administration (NOAA)[13]. Laboratories were requested to provide duplicate results using analytical methods of their choice. Data from a select sub-group of participants were used for the certification of MOOS-1. These laboratories were selected based on their satisfactory performance history in a previous intercomparison. The analytical methods employed utilized instrumentation from a variety of manufacturers. The procedures employed were based on the colorimetric procedures of Strickland and Parsons [14] and Morris and Riley [15], some with slight variations to the NRC procedure as briefly described earlier. For calibration, laboratories generally used nutrient-free seawater to prepare the standards and a few laboratories used NaCl solutions or the method of standard additions.
Colorimetric methods are universally used by oceanographers for these determinations; however, alternative analytical methods were developed at NRC to provide independent results for the certification process. An ion chromatographic procedure with UV absorbance detection was developed for NO3 − and NO2 − determinations. Methods for the direct determination of orthophosphate [9] and dissolved silica [10] in seawater by ICP-MS in combination with ion-exclusion chromatography (IEC) were also developed. IEC can be used to provide a temporal separation of the dissolved analytes from the major seawater ions to permit their direct determination despite the high dissolved solids content of the sample.
The certified values for the analytes were calculated from the unweighted means of the results. Data were first examined for outliers using the Grubb’s Test.
Uncertainty
Guidelines for CRM producers suggest all sources relevant to the user of the material contribute to the overall uncertainty of the certified value [16, 17]. The expanded uncertainty (U CRM) in the certified value is equal to ku c,where u c is the combined standard uncertainty calculated according to the ISO Guide [18] and k is the coverage factor. The value of u c is determined from the combined uncertainties of the various methods used to generate the characterization data (u char) as well as uncertainties related to possible between-bottle variation (u hom) and instability derived from effects relating to long-term storage and short-term transport (u stab).
When expressed as standard uncertainties these components can be combined as:
It is intended that U CRM encompass every aspect that reasonably contributes to the uncertainty of the measurand [16]. A coverage factor of 2 was applied for all analytes. Table 2 presents the certified values for MOOS-1.
Characterization
The characterization uncertainties (u char) were calculated in accordance with Eq. 2, where s is the standard deviation of the laboratory means and p is the number of submitted mean results included in the calculation [19].
Table 3 summarizes the data from the participating laboratories and NRC that was used for the certification of MOOS-1.
Homogeneity
The uncertainty components for homogeneity were derived according to the recommendation of an international study group [20]. The material was tested for homogeneity at NRC using standard colorimetric procedures. Results from triplicate sub-samples from ten bottles were evaluated using ANOVA.
For all four analytes, the inhomogeneity contribution to uncertainty, u hom, was set equal to the experimentally determined between-unit standard deviation (s between). These results are the best estimate of the uncertainty due to homogeneity and are reported in Table 3.
Stability
Uncertainty components for long- and short-term stability were also evaluated. To determine possible uncertainty associated with these components, samples of MOOS-1 were stored at −25°C, 20°C, and 40°C for one month. These samples were analyzed at the same time as several bottles stored under recommended conditions at +4°C. No between-bottle differences were detected. Consequently, the component for short-term stability was set to zero. It should be noted that it has been reported that the recovery of silicate from frozen samples may vary depending upon the thawing conditions [21].
The uncertainty components related to the long-term stability of this CRM were also calculated according to the recommendations of an international study group [20]. MOOS-1 has been continuously monitored since 1996 and found to be stable with respect to nutrient concentration over this period. The slope and uncertainty in the regression fit of the slope of these stability data were used to calculate the uncertainty components in Table 3, based on a projected 60-month lifetime.
The stability of this CRM will continue to be monitored and users will be notified if any significant irregularity occurs prior to the expiry date.
Based on sample stability, the certified values for MOOS-1 listed in Table 3 are considered valid until December 2007, provided the CRM is stored at 4°C in accordance with instructions in the certificate [22].
Conclusions
Although it has been shown in this paper and reported that gamma irradiation slightly alters the nutrient concentrations [10], our experience with MOOS-1 over six years has shown exceptional stability. This material does not possess ideal nutrient concentrations for the study of some oceanographic processes as outlined in the NAS report [1], however, it is hoped that MOOS-1 will partially fill a void and become a useful quality assurance tool for nutrient measurements. It is available from the NRC in units comprising of two 50-mL bottles.
References
Chemical reference materials (2002) National Research Council, The National Academies Press, Washington DC
Aminot A, Kérouel R (1995) Mar Chem 49:221–232
Aminot A, Kerouel R (1991) Anal Chim Acta 248:277–283
Aminot A, Kirkwood DS (1994) Mar Poll Bull29:159–165
Fifth ICES intercomparison for nutrients in seawater (1994) International Council of the Exploration of the Sea, Copenhagen, Denmark
Merry J (1995) Fresenius J Anal Chem 352:148–151
Kattner G (1999) Marine Chem 67:61–66
Kirkwood DS (1992) Marine Chem 38:151–164
Yang L, Lam JWH, Sturgeon RE (2001) J Anal At Spectrom 16:1302–1306
Hioki A, Lam JWH, McLaren JW (1997) Anal Chem 69:21–24
Yang L, Bancon-Montigny C, Mester Z, Sturgeon RE, Willie SN, Boyko VJ (2003) Anal Bioanal Chem 376:85–91
Benoliel MJ, Quevauviller Ph (1998) Analyst 123:977–979
Willie SN, Clancy VP (2002) Second intercomparison for nutrients in seawater. NOAA Technical Memo 158. Available from the web site of the National Oceanic and Atmospheric Administration, Center for Coastal Monitoring and Assessment. http://ccmaserver.nos.noaa.gov/. Cited 10 July 2003
Strickland JD, Parsons TR (1972) Bull Fish Res Board Can 167:310p
Morris AW, Riley JP (1963) Anal Chim Acta 29:272–279
Pauwels J, van der Veen A, Lamberty A, Schimmel H (2000) Accred Qual Assur 5:95–99
Pauwels J, Lamberty A, Schimmel H (1998) Accred Qual Assur 3:180–184
Guide to the expression of uncertainty in measurement (1993) ISBN 92–67–10188–9, 1st edn. ISO, Geneva, Switzerland
van der Veen AMH, Linsinger TPJ, Schimmel H, Lamberty A Pauwels J (2001) Accred Qual Assur 6:290–294
Ellison SLR, Burke S, Walker RF, Heydorn K, Månsson M, Pauwels J, Wegscheider W, te Nijenhuis B (2001) Accred Qual Assur 6:274–277
Zhang J-Z, Ortner PB (1998) Wat Res 32:2553–2555
National Research Council of Canada (2003) Certificate for MOOS-1. Institute for National Measurement Standards, Ottawa
Acknowledgements
The authors thank Dr Peter Strain of the Bedford Institute of Oceanography, Fisheries and Oceans Canada, Dartmouth Nova Scotia for advice concerning sample collection and analytical methods, L. Yang of NRC for provided the IEC-ICP-MS results, and A. Hioki for developing the ion chromatography methods.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clancy, V., Willie, S. Preparation and certification of a reference material for the determination of nutrients in seawater. Anal Bioanal Chem 378, 1239–1242 (2004). https://doi.org/10.1007/s00216-003-2473-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2473-1