Abstract
A new device is described which allows the recovery of compounds from thin layer chromatograms in short times, within a small volume, and without contamination. This apparatus can be coupled online to an electrospray mass spectrometer, but can also be used with other detectors or for micropreparations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thin layer chromatography (TLC), invented in 1938 by Ismailov and Schraiber [1], is an effective, simple, and inexpensive method for the separation of a wide range of organic compounds. Despite the introduction of high-performance liquid chromatography (HPLC) in the 1970s, TLC still plays an important role in academia and industry. Among its many advantages is the possibility of performing many experiments in parallel and its tolerance against “dirty” samples.
TLC is a development chromatography and hence the compounds remain on the adsorbent layer after their separation; however, it is often necessary to recover the compounds for further investigations. The standard method to accomplish this is to scratch the adsorbent at the spot site from the plate and extract the adsorbent externally. This task is time consuming, produces dilute solutions, and often recontaminates the compound.
To overcome these difficulties many procedures have been tried especially in combination with mass spectrometric methods. There are approaches in which the sample is desorbed and ionized from the TLC plate with an IR laser [2] within the ion source, or introduced into a secondary ion mass spectrometer (SIMS) [3] sometimes with addition of high-boiling auxiliary liquid (LSIMS, FAB) [4, 5]. Matrix-assisted laser desorption ionization (MALDI) from standard TLC plates has been reported [6, 7, 8], or separations have been done on a hybrid plate with subsequent introduction to (MALDI)-MS [9].
In an early approach, Esteban [10] published a device called the “Eluchrom” for the quantitative extraction of spots. With this technique a circle of the adsorbent layer is removed to allow a tight seal of an extraction head to the supporting material. An alternative extractor was described by Anderson and Busch [11]. With this device the spot on the TLC plate is desorbed in a solvent and then recovered from the plate by capillary forces of a wick. To prevent the solvent from spreading across the plate the spot is optionally isolated from the surrounding by a ring of wax.
A recent survey of TLC coupling to MS is given by Wilson [12]. Van Berkel published an interesting continuous probe for TLC chromatograms for use with a special reversed-phase adsorbent [13]. Another approach uses small-scale TLC plates to couple the chromatography with an electrospray mass spectrometer [14].
In this paper we describe a new device for the transfer of TLC-separated compounds into a solvent stream. The apparatus works with aluminium and polyester-backed plates. It was successfully tested with silica, reversed phases, cellulose, and polyamide adsorbents. In this publication the apparatus is demonstrated online with electrospray mass spectrometry but there are many more applications for this extractor, like couplings to other detectors that allow the introduction of liquids (UV, electrochemical detectors, etc.). The independence of separation and spectrometry makes it possible to make many chromatograms in parallel and to concentrate on the identification of only the interesting spots. In this way the load on the spectrometer is minimized. The extractor is also an effective micropreparation device in conjunction with MALDI, GC, GC-MS, and biological tests, and serves as a precleaning step for sensitive HPLC separations etc.
Materials and methods
The solvent flow is provided by HPLC-pumps HP1100 (Agilent, Palo Alto, USA) or LKB2150 (LKB, Bromma, Sweden). The extractor was linked to a Rheodyne 7000 column switch valve (Rheodyne, Rhonert Park, CA, USA).
A Z-spray electrospray mass spectrometer Quattro LCZ (Micromass, Manchester, UK) was used. Its capillary voltage was set to 3 kV, and the cone voltage was adjusted for maximum signal intensity. MS-MS measurements of alkaloids: capillary 3 kV, cone 52.2 V, collision energy 26 V, cycle time 0.53 s, gas cell pressure 1.4×10−3 mbar.
UV detection was performed with an DAD HP1100 (Agilent, Palo Alto, USA).
All solvents and chemicals were reagent grade. TLC plates from different sources were used (Merck, Macherey&Nagel, Germany; Sigma Chemicals, USA).
Yohimbine and ajmalicine were obtained from Acros and Fluka, Oligosaccharide samples were produced in-house. Human brain ganglioside reference (Supelco) was a donation from the Institute of Medical Physics Münster, University of Münster.
Different TLC spot localization methods were used: a) fluorescence attenuation; b) charring with naphthoresorcinol (200 mg) in sulfuric acid (2 N, 100 mL) and ethanol (100 mL) by dipping and subsequent heating [15]; c) dipping in a primulin 0.2% in acetone/H2O solution and observing fluorescent spots [16].
The extractor prototype was built in the workshop of the Institute of Organic Chemistry (Münster, Germany). The PTFA frits were punched out from 5-μm filter frits (Bohlender, Grünsfeld, Germany). The extractor is commercially available under the name ChromeXtrakt (ChromAn, Leipzig, Germany) [17].
Construction and mode of action
The main part of the apparatus is a stainless steel plunger (Fig. 1) with a solvent inlet and an outlet capillary. It has a ring-shaped cutting edge on the face. The edge is 0.1 mm higher than the adsorbent layer. Two models are used in this investigation with edge diameters of 2 and 4 mm. The plunger is pressed on the TLC plate by means of a frame press as is shown in Fig. 2. The edge of the plunger penetrates through the adsorbent layer and forms a tight seal with the carrier foil. Glass-backed plates cannot be used. When the plunger is in its end position the enclosed adsorbent is somewhat pressurized. By this action a flat tablet of compressed adsorbent material containing the separated compound is formed. Solvent can enter the disk through a small hole at the periphery, pass through it, dissolve the compound, and leave via the outlet capillary. To prevent the exit from clogging it is protected by a 5-μm PTFA frit. The complete setup is shown in Fig. 3.
The complete setup. Solvent flow is supplied by an HPLC pump (a), in standby it is fed through the bypass of the switching valve (b) or during extraction through the plunger (c) across the selected spot on the TCL plate (d). The connections between extractor, switching valve, and spectrometer (e) should be made as short and narrow as possible (in our setup 50-cm-length and 0.13-mm-ID PEEK capillary was used)
In standby mode the liquid flow provided by the pump is bypassed to the mass spectrometer through a loop. The spectrometer is set to scan mode with the appropriate polarity and mass range. When the target molecules are known, it is alternatively possible to select only their characteristic masses or apply MS-MS experiments to enhance the sensitivity. Now the interesting spot on the TLC is positioned beneath the plunger and pressurized with the screw. The valve is rotated so that the solvent is directed through the compressed adsorbent within the ring edge. The compound is flushed to the spectrometer and detected. After complete extraction the valve is switched back, the screw is turned down, and the plunger can be repositioned to a new spot. If the extracted tablet of compressed adsorbent should remain in the plunger it can be easily blown out with pressurized air.
Identification of oligosaccharide derivatives
Pivaloylysis of cellulose acetate [18] produces a mixture of oligomers with pivaloyl groups at both ends. The progress of the reaction and the chromatographic purification of the reaction mixture is typically controlled by TLC. For this purpose approximately 200 μg of the reaction mixture was applied to the starting line as a 50-mm-long band. After predevelopment with methanol just above the startline, the chromatogram was finally developed with ethyl acetate/cyclohexane (1:1) up to 50 mm. By charring a side cutoff of the plate with naphthoresorcinol reagent the position of the oligomers on the chromatogram was determined (Fig. 4).
The corresponding regions are marked with a soft pencil on the untreated part of the plate. These bands are eluted with the extractor (2-mm diameter) and sent directly to the ES-MS by a 0.1 mL min−1 stream of CHCl3/MeOH (1:1). The ion signal appears approximately 20 s after the plunger is placed on a spot and the valve has been activated. The carbohydrate esters form stable sodium adducts which can be detected easily. The extraction time for each spot was approximately 1.5 min, so that all 8 compounds are identified within 13 min.
Figure 5 shows the mass chromatograms of the sum of characteristic peaks [M+Na]+, [2M+Na]+, [M+2Na]2+ within a mass range of m/z 400 to m/z 2,000. The mass spectrometer was in scan mode, so that full spectra are registered. The spectrum of the pentamer is shown as an example in Fig. 6.
Elution profile of characteristic ions during the extraction of the eight bands. The spectrometer was set to full scan mode so that all masses are registered. Peak number (masses [m/z], intensity [counts]): 1 (497.3, 3.1×105), 2 (785.4+1,547.8, 2.4×105), 3 (1,073.5, 7.1×104), 4 (692.8+1,361.7, 6.2×104), 5 (836.7+1,649.9, 5.5×104), 6 (980.7+1,939.0, 2.3×104), 7 (1,124.8, 1×104), 8 (1,268.9, 3.1×103)
The electrospray mass spectrum from spot (5) corresponding to the pentameric carbohydrate. The molecular weight (calculated 1,626.5 Da) can be clearly identified by means of the [M+Na]+ peak at m/z 1,649.9 and the [M+2Na]2+ peak at m/z 836.7. The peak m/z 413 is due to the plasticizer contaminant from the non-precleaned TLC plate
Complex lipid isolation
Thin layer chromatography is widely used because of its good separation quality and robustness against impurities in complex lipid research [8, 19, 20] and is therefore often preferred over HPLC. For example, a reference mixture of human brain gangliosides (HBG) was separated on a silica TLC plate with chloroform/methanol/water with addition of CaCl2 [21]. The spot localization can be done destructively with naphthoresorcin reagent or better with primulin (Fig. 7). When the latter is used, it is possible to identify the lipids by mass spectrometry in presence of the fluorescent dye, because there are no interfering peaks in the lipid mass region.
Upper left side TLC separation of a mixture of human brain gangliosides with chloroform/ methanol/water (120:85:20) containing 2 mM CaCl2. Spot detection by fluorescence of the primulin-treated TLC. The chromatogram was illuminated with a UV lamp; documentation was done with a digital camera. Upper right side The negative electrospray spectrum shows the [M−H]− peaks at m/z 1,544.1 and m/z 1,573.1. The spectrum represents about 1 μg compound and identifies it as GM1 (bottom)
The bands are fast and easily identified as GM1, GD1a, GD1b, and GT1; the negative electrospray mass spectrum of GM1 (structure shown) is reproduced in Fig. 7.
Speed of operation
From the mass traces in Fig. 5 the elution time for a peak under standard conditions of 0.1 mL min−1 solvent flow and a 4-mm diameter plunger is estimated to be approximately 1 min. To find out if it is possible to shorten this time, small spots of 200 ng ajmalicine were re-extracted from a silica plate (Fig. 8).
As expected, the peak width decreased with increasing flow and faster cycle times are possible, but in conjunction with the flow rate, the backpressure increases linearly. Because of the risk of leakage one should avoid pressures beyond 30 bar which corresponds to a flow rate 0.5 mL min−1.
A second effect of increasing flow rates is the decreasing concentration (=increasing peak volume) of the compound in the effluent stream which gives lower sensitivity with ES-MS (and other concentration detectors like UV monitors).
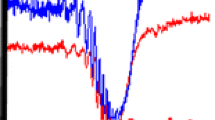
When speed is the most important parameter, then spot elution times of less than 15 s can be achieved by using a smaller plunger with a 2-mm diameter edge in conjunction with a high flow rate. In Fig. 9, three elutions of equal amounts of dye (approximately 0.1 μg) from a silica TLC with increasing solvent flow are shown. The detection was made with a diode array detector. The decrease of signal area and height is obvious.
Quantitative aspects and detection limits
Although the device was developed mainly for qualitative work, it is worth looking at its quantitative abilities because this indicates the quality of the extraction process. An equimolar mixture of ajmalicine and yohimbine was spotted on a TLC plate; the spot diameters were less than 3 mm. The deposited amounts ranged from 5 to 1,000 ng. Then the compounds were extracted with methanol with a flow rate of 0.1 mL min−1. To guarantee a complete coverage of the spots, the 4-mm-diameter plunger was used. The detection was made with the ESI mass spectrometer detecting characteristic collision-induced decays 353→144 (ajmalicine) and 355→144 (yohimbine) (Fig. 10).
The response is linear (R=0.9945) over 3 decades between 0.1 ng and 100 ng. The observed good linearity is proof of the completeness of the extraction process. With low amounts, the measurement is limited by chemical noise, which originates manly from adsorbed impurities on the plates. Therefore if more sensitivity were needed, it would be necessary to clean the TLC plate by predevelopment(s) with polar solvents. With high amounts the quality of the extraction is still good but the linearity of the curve is deteriorated by suppression effects of the electrospray ionization.
Together with an internal standard, quantitative results over a range of more than 3 decades can be obtained. When the signal of yohimbine in the upper setup is used as an internal standard, the ratio (ajmalicine signal/yohimbine signal) is 1.3±0.2 as shown in Fig. 11. The increase of the signal ratio at high concentrations is due to the competition effects between the analytes.
The detection limit is mainly determined by the spectrometric properties of the molecules under investigation. The sensitivity can be enhanced when the TLC plates are cleaned by predevelopment. However, with standard identification problems, typical TLC sample amounts, and decent ion formation properties of the compound, TLC plates can be used successfully without pretreatment.
Conclusions
The described TLC extractor is a versatile instrument for the isolation of TLC-separated compounds. The recovery of sample is complete within a broad range of concentrations. Its operation can be optimized for low elution volumes (<50 μL) or for high speed (<15 s) by the choice of solvent flow and plunger size: band-shaped chromatograms can be extracted with rectangular plungers. This device can be coupled directly to a detector or may be used offline.
References
Ismailov NA, Schraiber MS (1938) Famatsija 3:1
Fanibanda T, Milnes J, Gormally J (1994) Int J Mass Spectrom Ion Processes 140:127–132
Orinak A, Arlinghaus HF, Vering G, Justinova M, Orinakova R, Turcaniova L, Halama M (2003) J Planar ChromatogrModern TLC 16(1):23–27
Busch KL, Mullis JO, Carlson RE (1993) Planar Chromatogr 16(8):1695–1713
Busch KL (1989) J Planar Chromatogr Modern TLC 2(5):355–361
Gusev AI (2000) Fresenius J Anal Chem 366(6–7):691–700
Crecelius A, Clench MR, Richards DS (2003) LCGC Europe April:2–6
Guittard JG, Hronowski XL, Costello CE (1999) Rapid Commun Mass Spectrom 13:1838–1849
Mehl JT, Hercules DM (2000) Anal Chem 72:68–73
Esteban J (1977) Tecnicas Laboratorio 5(59):285–292
Anderson RM, Busch KL (1998) Planar Chromatogr 11:336–341
Wilson ID (1999) J Chromatog A 856:429–442
Van Berkel GJ, Sanchez AD, Quirke JM (2002) Anal Chem 74:6216–6223
Hsu FL, Chen CH, Yuan CH, Shiea J (2003) Anal Chem 75(10):2493–2498
Krebs HG, Heusser D, Wimmer H, Stahl E (eds) (1967) Dünnschichtchromatographie. Springer, Berlin Heidelberg New York, p 840
White T, Bursten S, Federighi D, Lewis RA, Nudelman E (1998) Anal Biochem 258:109–117
Luftmann H (2002) German Patent100 36 293. 1–52
Arndt P, Bockholt K, Gerdes R, Huschens S, Pyplo J, Redlich H, Samm K (2003) Cellulose 10:75–83
Christie WW (1990) Lipid Technol 2:22–23
Christie WW, Dobson G (1999) Lipid Technol 11:64–66
Müthing J, Burg M, Möckel B, Langer M, Metelmann-Strupat W, Werner A, Neumann U, Peter-Katalinic J, Eck J (2002) Glycobiology 12(8):485–497
Acknowledgements
The author would like to thank the members of the workshop of the Organisch-Chemische Institut (Münster) for fruitful discussions and technical skills.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luftmann, H. A simple device for the extraction of TLC spots: direct coupling with an electrospray mass spectrometer. Anal Bioanal Chem 378, 964–968 (2004). https://doi.org/10.1007/s00216-003-2293-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2293-3