Abstract
A robust reliable method for the analysis of residues of pesticides in citrus groves was developed. Residues of twelve pesticides were extracted from citrus essential oils by SPE, separated by liquid chromatography and analyzed by GC–MS. In addition, ten pesticides were extracted by SPE, separated and analyzed by electrospray HPLC–MS. In the case of lemon essential oils, all twenty residues were separated by liquid/solid extraction on a mixed Florisil–C18 cartridge. The method enabled the analysis of the twenty pesticide residues at levels of 2 to 30 ppm with limits of detection ranging between 0.02 to 0.50 mg L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cultivation of citrus crops commonly involves the use chemicals such as fertilizers and pesticides. Essential oils are used in food, pharmaceutical and cosmetic industries. Regulations are increasingly more strict in terms of the residual levels of chemicals used for treatment because of their impact on public health and on the environment. Several methods have been developed to control products on the market. They involve primarily liquid/liquid or liquid/solid extraction (SPE) for sample preparation, and chromatography (gas and liquid) coupled with mass spectrometry for analysis [1, 2, 3, 4, 5, 6, 7, 8].
Fernandez et al. [1] developed an extraction procedure for pesticides based on partition between the matrix (ground) and ethyl acetate. After extraction, the pesticides were analyzed by GC–MS and HPLC–MS. The simple and rapid method developed enabled pesticide residues to be analyzed with good limits of detection because of the large quantity of citrus fruit used, but it also required the use of relatively large quantities of ethyl acetate (about 300 mL per extraction).
In addition, Valenzuela et al. [2] investigated the extraction yields of residual pesticides in lemons with dichloromethane, after their adsorption on a solid phase. The solid phases tested were C18, C8, C2 bonded silica, and silica. The results showed that the C18 and C8 solid supports were the most efficient. The yields obtained with C18 were comparable to those obtained with C8, with the advantage that C8 resulted in a cleaner extract (a minimum of interference from the compounds of the matrix). These results were subsequently used by Valenzuela et al. to analyze pesticide residues in oranges [3].
The work described above was carried out on whole fruit. Other authors [4, 5, 6, 7] considered that pesticides are mainly located in the fruit peel which is considered as a protective layer. The essential oils were extracted from the peel. It was necessary to extract several kilograms of fruit in order to obtain several milliliters of essential oils. The concentration of pesticides in the essential oil is thus much higher than in the fruit.. For this purpose, Saitta et al. [4] developed a liquid/solid extraction procedure to separate organochlorine pesticides from essential oils of citrus fruit. This extraction was carried out on a silica stationary phase using dichloromethane as elution solvent, the extract was then analyzed by GC–MS. This method was used to analyze not more than ten pesticide residues in essential oils of lemons, oranges, tangerines and bergamot by these authors and was subsequently used by Giuseppa et al. [5].
This paper describes a new, simple and rapid extraction and analysis procedure for residues of twenty different pesticides in lemon essential oils. The method involves solid/liquid extraction, separation and cleanup by SPE coupled with GC–MS and HPLC–MS.
Material and methods
Solvents and reagents
The solvents used were HPLC grade water (Millipore), pentane (Merck), dichloromethane and methanol (Prolabo). These HPLC grade solvents were used both in liquid chromatography and for the liquid/solid extraction steps. Pesticide standards were purchased from Sigma–Aldrich.
Liquid/solid extraction procedure
Citrus essential oil (0.5 mL) spiked with twenty pesticides at concentrations ranging from 2 to 30 ppm was dissolved in 0.5 mL of pentane. The mixture was homogenized in an ultrasonic bath for 15 min, after which 400 µL of the solution were deposited on a Florisil cartridge (Maxi-Clean, FL-PR marketed by Alltech), with an HPLC syringe, at a rate of 200 µL min−1. (The cartridge had previously been equilibrated with 5 mL of pentane at a flow-rate of 1 mL min−1.) The solution analyzed was then eluted with 5 mL of pentane at the same flow rate. This led to the elimination of a large part of the essential oil, and pesticides were trapped on the stationary phase of the cartridge. The residues were then eluted with 5 mL of dichloromethane at the same flow-rate.
The preparation procedure we developed is based on liquid/solid extraction. An FL-PR extraction cartridge, specific for pesticides was used, the majority of the stationary phase composed of Florisil. Extraction conditions (liquid/solid) were developed using citrus oil solutions spiked with pesticides. Several solvents were tested, in particular for the desorption of pesticides from the stationary phase. The best compromise between elution volume and the separation of pesticides from polar compounds in the matrix was obtained with dichloromethane. Extracts were concentrated under a nitrogen stream at 30 °C and analyzed by (GC–MS).
In order to determine the total recovery yield of the preparation (cartridge extraction and evaporation), the complete procedure was run three times on three consecutive days.
Analysis techniques
Gas chromatography–mass spectrometry (GC–MS) operating conditions
An HP 6890 chromatograph GC equipped with an HP 7683 autosampler and an HP 5973 mass detector was fitted with a BPX5 column (50 m×0.25 mm i.d. with a 0.25 µm thick film). Conditions: injector 250 °C, column oven: 50 °C (1 min), 30 ° min−1 to 150 °C, 4 ° min−1 to 300 °C (10 min), injected volume 1 µL in splitless mode, carrier gas (He) flow rate 1.2 mL min−1. The transfer line and the quadrupole were maintained at 280 °C and 150 °C, respectively. The filament (70 eV) was turned on after 7 min run of GC program.
Liquid chromatography–mass spectrometry (HPLC–MS) operating conditions
HPLC–MS analyses were carried out using an HP 1100-MSD (Agilent) apparatus. The chromatographic system was composed of an HP 1100 chromatograph equipped with a C18 column (XTerra MS C18), 150 mm×2.1 mm, packed with a 3.5 µm diameter particle stationary phase. The column was fitted with a pre-column, 10 mm×2.1 mm. The UV detector (Diode Array Detector DAD) was set at a wavelength λ=210 nm. The mobile phase was composed of water (adjusted to pH 10 with ammonium formate and ammonia) and methanol. The elution gradient described in Table 1 was used. Samples were filtered before analysis on a 0.45 µm pore diameter Teflon filter.
Mass detection carried out with pesticide standards showed that all the pesticides were detected in positive electrospray mode. The basic mobile phase used was a medium unfavorable for the formation of positive ions. To overcome this problem, a 1% (v/v) solution of formic acid in methanol was added at the UV detector output by means of a T-connector and a pump operating at a flow-rate of 0.15 mL min−1. Mass spectrometer conditions were as follows: nebulizer pressure (60 psig), gas nebulizer temperature (60 °C), ion extraction voltage (−3 kV), fragmentor voltage was maintained at 60 V (0–15 min), at 80 V (15–20 min), at 60 V (20–23.5 min), at 80 V (23.5–25 min) and 60 V (25–35 min).
Results and discussion
The aim of this work was to develop a procedure for the analysis of pesticide residues in lemon essential oils. Based on published data [1, 2, 3, 4, 5, 6, 7, 8], we divided the pesticides used in the citrus industry into two groups. The first included twelve pesticides that could be analyzed by gas chromatography and the second ten pesticides that could be analyzed by liquid chromatography. Table 2 displays these pesticides, their molecular weight (MW) and selected ions (m/z) for both GC–MS and HPLC–MS. We noted that bifenthrin and chlorpyrifos can be analyzed by both techniques.
GC–MS results
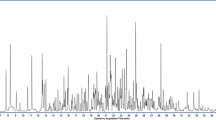
Optimized temperature programming led to the separation of 12 pesticides. Figure 1 shows the gas chromatogram in SIM mode for the spiked sample of lemon essential oils. The temperature ramp (from 50° to 150 °C) is an important step because it allowed elimination of residual volatile constituents of the matrix remaining after SPE extraction. Pesticide residues were then separated in the second temperature ramp (from 150 ° to 300 °C). Retention times were determined by means of pesticide standards. The limits of detection (concentration determined for a signal to noise ratio equal to 3) and the range of quantification (where the calibration curve is linear and for a correlation coefficient ranging between 0.991 and 0.999) were also calculated. for each compound (within the range of concentrations we examined). Table 3 gives retention times, limits of detection in mg L−1, the range of quantification, extraction recoveries in % and % RSD.
Besides, as the complete procedure was run three times a day and on three consecutive days, the results allowed determination of the reproducibility of the sample preparation method developed.
The extraction yields of pesticide residues obtained from lemon essential oils were acceptable (ranging between 67 and 95%) for most of the compounds. The yield obtained with carbofuran was 106.7% The slight retention of this compound on the GC column and its co-elution with residual matrix compounds could explain this high recovery, Furthermore, the mass spectrum did not enable selection of selective ions of carbofuran.
The limits of detection calculated for each compound ranged from 0.03 mg L−1 for chlorpyrifos to 0.50 mg L−1 in the case of dimethoate. These values depend on the structures of the different pesticides. The results are obtained with good % RSD (below 7%).
HPLC–MS Results
Ten pesticides were separated and identified by HPLC–MS. Figure 2 shows the HPLC–MS chromatogram for the spiked sample of lemon essential oils after SPE extraction. Table 4 gives retention times, limits of detection in mg L−1, the range of quantification, extraction recoveries in % (determined in the same way as for GC–MS) and % RSD for each compound.
The background of the HPLC chromatogram increased in comparison with standard solutions previously analyzed in the same analysis sequence. This could be due to the presence of residual matrix compounds. This background increase could explain the lower yields (ranging between 50 and 94%) obtained in HPLC–MS analysis as compared with those obtained in GC–MS. Indeed, we noted that the yields obtained by HPLC–MS for chlorpyrifos (58.2%) and bifenthrin (50.2%) were much lower than those obtained with GC–MS (79.7% and 86.5% respectively), confirming our hypothesis about matrix effects, since the samples were prepared in the same way. Furthermore, co-elution, observed on DAD trace, of matrix compounds with benomyl (at 11.53 min) made the determination of the real extraction yield for this pesticide difficult. On the other hand, the limits of detection were rather good and in the range of 0.02 to 0.06 mg L−1. All the results are obtained with good relative standard deviation (below 2.0%).
Conclusion
The SPE extraction procedure employed in this work is based on a mixed Florisil C18 cartridge that allowed extraction of pesticides belonging to different families of compounds. This procedure is simple and rapid and does not consume large volumes of solvents.
The chromatographic separation methods described in this work, allowed determination of reproducibility. Results obtained with GC–MS and HPLC–MS were rather good for most of the pesticides. Indeed, limits of detection determined for each compound ranged from 0.03 and 0.50 mg L−1 and the quantification range was broad such as for imidacloprid (from 0.1 to 13 mg L−1). In addition, chlorpyriphos and bifenthrin were analyzed by both GC–MS and HPLC–MS with similar limits of detection (about 0.04 mg L−1).
It is well known that tandem mass spectrometry can differentiate between co-eluting compounds since specific ions of each one can be studied in a selective way. It would be interesting to develop in future work a tandem HPLC–MS–MS method which could overcome the matrix effect observed in HPLC–MS analysis of citrus essential oils.
References
Fernandez M, Pico Y, Manes J, (2001) Chromatographia 54:302–308
Valenzuela AI, Lorenzini R, Redondo ML, Font G (1999) J Chromatogr A 839:101–107
Valenzuela AI, Pico Y, Font G (2001) J AOAC Int 84:901–909
Saitta M, Di Bella G, Salvo F, Lo Curto S, Dugo G (2000) J Agric Food Chem 48 797–801
Di Bella G, Luppino R, Faranda MC, Saitta M, Russo MT, Dugo G (2001) Essenze Deriv Agrum 71:133–146
Gazea F, Cicciarello G, (2001) Essenze Deriv Agrum 71:71–75
Gazea F, Cicciarello G (2001) Essenze Deriv Agrum 71:247–251
Fernandez M, Pico Y, Manes J (2001) Food Addit Contam 18:615–624
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrek, S., Paisse, O. & Grenier-Loustalot, MF. Analysis of pesticide residues in essential oils of citrus fruit by GC–MS and HPLC–MS after solid-phase extraction. Anal Bioanal Chem 376, 157–161 (2003). https://doi.org/10.1007/s00216-003-1899-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-1899-9