Abstract
Rationale
Cannabis use is common among adolescents and some research suggests that adolescent cannabis use increases the risk for depression, anxiety, and cognitive impairments in adulthood. In human studies, however, confounds may affect the association between cannabis use and the development of brain disorders.
Objectives
These experiments investigated the effects of adolescent exposure to either cannabis smoke or THC on anxiety- and depressive-like behavior and cognitive performance in adulthood in Long-Evans rats.
Methods
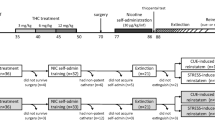
Adolescent rats of both sexes were exposed to either cannabis smoke from postnatal days (P) 29–49 or ascending doses of THC from P35–45. When the rats reached adulthood (P70), anxiety-like behavior was investigated in the large open field and elevated plus maze, depressive-like behavior in the sucrose preference and forced swim tests, and cognitive function in the novel object recognition test.
Results
Despite sex differences on some measures in the open field, elevated plus maze, forced swim, and novel object recognition tests, there were no effects of either adolescent cannabis smoke or THC exposure, and only relatively subtle interactions between exposure conditions and sex, such that sex differences on some performance measures were slightly attenuated.
Conclusion
Neither cannabis smoke nor THC exposure during adolescence produced robust alterations in adult behavior after a period of abstinence, suggesting that adverse effects associated with adolescent cannabis use might be due to non-cannabinoid concomitants of cannabis use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis use is common among adolescents and young adults, but the long-term consequences of such use are a topic of debate. Cannabis use typically starts during early adolescence and peaks when users are in their mid-20s (Hasin et al. 2015). In a large US survey, 7.4% of adolescents reported cannabis use during the past month and 13.1% during the past year (Azofeifa 2016). Cannabis use can have adverse health effects, including increased risks for lung, cardiovascular, and periodontal diseases (Gordon et al. 2013; Jouanjus et al. 2017). Its effects on development of cognitive and affective dysfunction, however, have been less conclusive. An initial study reported that cannabis use, particularly during adolescence, contributes to a lasting neurocognitive decline including an 8-point drop in IQ from childhood to adulthood (Meier et al. 2012). More recent studies, however, do not support this conclusion. For example, cannabis users perform worse on cognitive tests than non-users, but the performance of users is comparable with their non-using twins (Jackson et al. 2016; Meier et al. 2018). Such data suggest that the relatively poor cognitive performance of cannabis users may be due more to familial factors than to cannabis use. On a similar note, earlier studies suggested that adolescent cannabis use can play a causal role in anxiety and mood disorders (Patton et al. 2002; Rey et al. 2002). More recent work suggests that depressed individuals are more likely to use cannabis, but evidence for a causal relationship between cannabis use and depression is lacking (Feingold et al. 2015; Horwood et al. 2012; Moore et al. 2007; Quinn et al. 2008).
These recent data casting doubt on the causal relationship between adolescent cannabis use and long-term cognitive/affective dysfunction stand in contrast to a large body of research in animals showing that adolescent exposure to Δ9-tetrahydrocannabinol (THC) or other cannabinoid receptor agonists does produce adverse effects in adulthood. Many animal studies suggest that exposure to THC during adolescence causes anxiety- and depressive-like behavior in adulthood, as well as impairments in spatial and non-spatial learning and working memory (Cha et al. 2006; O’Shea et al. 2004; Quinn et al. 2008; Realini et al. 2011; Rubino et al. 2009b; Rubino et al. 2008). One possible explanation for the disparity between findings in humans and animals is that subjects in animal studies (e.g., mice and rats) typically receive high doses of cannabinoids, whereas humans inhale cannabis smoke in which THC levels are likely lower. Thus, animals may be exposed to higher doses of cannabinoids than humans would typically consume, which could potentially explain the differences between human and animal studies (Huestis 2007; Rubino et al. 2009a; Zgair et al. 2015). Furthermore, cannabis smoke contains numerous cannabinoids aside from THC, which might counteract potentially deleterious effects of THC (Curran et al. 2016; Niesink and van Laar 2013).
The data reviewed above suggest that experimental designs typically employed in animal studies of adolescent cannabinoid exposure may fail to model potentially critical aspects of human cannabis use. To address this issue, we used a rat model of cannabis smoke exposure developed in our laboratory (Bruijnzeel et al. 2016). In previous work with this model, we showed that in adult rats, acute cannabis smoke exposure alters exploratory behavior and working memory, and that chronic exposure leads to dependence (Blaes et al. 2018; Bruijnzeel et al. 2016). In the present studies, we determined how smoke exposure during adolescence affects anxiety-like behavior, depressive-like behavior, and cognitive function. Male and female rats were exposed to cannabis smoke from postnatal day (P) 29–49, which corresponds to the period of early and mid-adolescence (Tirelli et al. 2003). For comparison, a similar set of experiments was conducted with rats that received THC injections during adolescence (P35–45), using a protocol shown previously to produce adult affective/cognitive dysfunction (Rubino et al. 2008).
Materials and methods
Subjects
Long-Evans rats were obtained from Charles River at P22. In experiment 1 (cannabis smoke exposure), the rats were housed in single sex groups with 3 per cage throughout the smoke exposure period. Shortly, after the smoke sessions were completed, several rats engaged in fighting with their cagemates. To address this, all rats were single housed for the remainder of this experiment. In experiment 2 (THC administration), the rats were housed in single sex groups with 2 per cage for the first part of the study. The rats were single housed before the sucrose preference test and remained single housed afterwards. All rats were housed in the vivarium in the McKnight Brain Institute at the University of Florida on a 12 h light/dark cycle (lights off at 8 AM), with food and water available ad libitum except as noted below. Seventy-two rats (36 male, 36 female) were used for experiment 1 (cannabis smoke exposure) and 40 rats (20 male, 20 female) were used for experiment 2 (THC exposure). Body weights were recorded for each rat on a daily basis during the period of smoke or THC exposure. Estrous phase was not assessed. All animal procedures were performed in accordance with the University of Florida Institutional Animal Care and Use Committee as well as National Institutes of Health guidelines.
Drugs
Cannabis cigarettes, placebo cigarettes, and THC were obtained from the NIDA Drug Supply Program. The cannabis cigarettes were the size of standard tobacco cigarettes (approximately 700 mg each), and contained approximately 5.6% THC, 0% cannabidiol (CBD), and 0.4% cannabinol (CBN) as per the analytical data accompanying the cigarettes. The placebo cigarettes were composed of cannabis plant material from which cannabinoids were extracted, and contained 0.002% THC, 0.001% CBD, and 0.004% CBN. THC was obtained in 100% ethanol. The ethanol was evaporated under a light stream of nitrogen gas applied indirectly to the solution-containing vial inside a fume hood, and the remaining THC was dissolved in a vehicle containing 5% kolliphor, 5% ethanol, and 90% saline (Blaes et al. 2018). In experiment 2, the rats received THC (or an equal volume of vehicle) from P35–45. During this period, the rats received injections (i.p.) twice a day according to the following schedule: P35–37, 2.5 mg/kg; P38–41, 5 mg/kg; P42–45, 10 mg/kg (Rubino et al. 2008).
Smoke exposure
Freely moving rats were exposed to smoke from burning cannabis or placebo cigarettes in two separate cohorts (with equivalent numbers of males and females) as described in our previous work (Blaes et al. 2018; Bruijnzeel et al. 2016; Ravula et al. 2018). Briefly, smoke exposure was conducted in a microprocessor-controlled cigarette smoking-machine (model TE-10, Teague Enterprises, Davis, CA) that burned cigarettes using a standardized smoking procedure (35 cm3 puff volume, 1 puff per minute, 2 s per puff)(Teague et al. 1994). Mainstream and sidestream smoke were transported to a mixing and diluting chamber, where the smoke was diluted with air to a concentration of about 250 mg of total suspended particles (TSP) per cubic meter before being introduced into the exposure chamber. Just prior to the exposure sessions, rats were moved from their home cages and placed into clean, standard polycarbonate rodent cages (38 × 28 × 20 cm; L × W × H) with corncob bedding, wire tops, and a water bottle (rats from the same home cage were moved into the same exposure cage). This whole-body exposure regimen allowed for free movement (i.e., not restrained) and social housing (3 rats/cage) during the exposure sessions. During the sessions, exposure conditions were monitored for carbon monoxide (CO) and TSP levels. CO levels were assessed using a continuous CO analyzer that measures CO levels between 0 and 2000 ppm (Monoxor III, Bacharach, New Kensington, PA USA). In order to measure TSP levels, smoke was pumped out of the chamber through a pre-weighed filter for 5 min (Pallflex Emfab Filter, Pall Corporation, Port Washington, NY USA). The TSP level was calculated by dividing the weight increase of the filter by the volume of airflow through the filter. Rats in the smoke exposure groups were exposed to smoke for 1 h per day. During this period, 5 cannabis cigarettes were burned (10 min per cigarette with a 2 min break between cigarettes). TSP and CO levels are reported in Table S1. Rats in the clean air condition were placed in the smoke exposure chamber as described above with the machine on to allow continuous airflow, but no cigarettes were loaded and no smoke was delivered. Exposure sessions were conducted daily from P29 to P49 for a total of 21 days.
Behavioral testing
Behavioral testing began after P70 and was conducted at the following ages for rats in both the smoke exposure (experiment 1) and THC (experiment 2) studies: large open field, P77; elevated plus maze, P85; sucrose preference, P93; forced swim, P98; novel object recognition, P129. Each behavioral test was completed in 1–2 days.
Large open field test
The large open field test is used to assess anxiety-like behavior (Liebsch et al. 1998). The test was conducted in a dimly lit room (75 lx) as described previously (Bruijnzeel et al. 2016; Qi et al. 2016). The open field apparatus consisted of a large arena measuring 120 × 120 × 60 cm (L × W × H). The arena was made of black high-density polyethylene panels that were fastened together and placed on a plastic bottom plate (Faulkner Plastics, Miami, FL). The rats’ behavior was recorded with a camera mounted above the arena and analyzed with EthoVision XT 11.5 software (Noldus Information Technology, Leesburg, VA). The open field was divided into two zones: a border zone (20-cm wide) and a center zone (80 × 80 cm; L × W). The following behaviors were analyzed: total distance traveled, distance traveled in the border and center zone, latency to enter the center zone, number of center zone entries, and duration in the center zone. The open field was cleaned with a Nolvasan solution between rats.
Elevated plus maze test
The elevated plus maze test is used to assess anxiety-like behavior and was conducted as described previously (Qi et al. 2016; Rylkova et al. 2009). The test apparatus consisted of four black polypropylene arms (Coulbourn Instruments, Whitehall, PA). The two “open” arms had 0.5-cm ledges and the two “closed” arms had 30-cm walls. The open arms were placed opposite of each other. The arms were 10-cm wide, 50-cm long, and were placed on 55-cm tall acrylic legs. Testing occurred in a quiet, dimly lit (75 lx) room. At the beginning of each test, the rats were placed in the center of the apparatus facing an open arm. Rats were allowed to explore the apparatus for 5 min, and their behavior was recorded with a camera mounted above the maze. The elevated plus maze was divided into 5 zones (two open arms, two closed arms, and center). Behavior was scored manually using EthoVision XT 11.5 software by an experienced observer who was blind to the treatment conditions. The following behaviors were analyzed: duration in the open and closed arms and the number of open and closed arm entries. An arm entry was counted when the rat had all four paws on that arm. The apparatus was cleaned with a Nolvasan solution between rats.
Sucrose preference test
The sucrose preference test was conducted in the rats’ home cages in the vivarium (Coelho et al. 2014). Before testing began, the rats were acclimated to the bottles containing the 2% sucrose solution for 48 h in the absence of tap water. The sucrose solution was provided in an identical pair of 6-oz. glass bottles with stainless steel drip-resistant sipper tubes (Kaytee Chew Proof Bottle), which were fastened to the home cage lid to minimize movement during liquid ingestion. During the 24-h two-bottle choice test, one bottle was filled with a 2% sucrose solution and the other with tap water (the left/right position of the bottles was randomly counterbalanced across rat cages to minimize effects of cage side preference). Bottles were weighed before and after the 24-h choice test. The difference in bottle weight was used as an indicator of sucrose and water intake during the 24-h test and was converted to a sucrose preference index (Coelho et al. 2014). A reduced preference for the sucrose solution over water is considered indicative of anhedonia (Willner et al. 1987).
Forced swim test
The forced swim test was conducted as described previously (Detke et al. 1995; Slattery and Cryan 2012) and consisted of two stages: a 15-min pre-test followed by a 5-min test 24 h later. Immediately before each stage, a large Pyrex cylinder (21 × 46 cm; Fisher Scientific) was filled with tap water (23–25 °C) to a depth of 30 cm. The rats’ behavior was recorded with a digital camcorder that was placed 1 m above the cylinder. An experienced observer who was blind to the treatment conditions scored the behavior from the DVD recordings. Three behaviors were scored continuously: immobility, swimming, and climbing. Swimming was defined as horizontal movements across the cylinder. Climbing was defined as upward-directed movement of the forepaws against the cylinder wall. The rats were considered immobile if they were floating in the water and made only the movements necessary to keep their heads above the water. After each test phase was completed, the rats were removed from the water and dried with clean towels.
Novel object recognition test
The novel object recognition test was conducted in the large open field apparatus (described above). This test was conducted in three stages: habituation, familiarization, and novel object recognition testing (Bevins and Besheer 2006; Rojas et al. 2016). During the habituation phase, the rats explored the empty open field for 5 min. After the habituation session was completed, 2 sample objects were placed in the open field (20 cm apart and 10 cm from the wall) and the rats were placed in the open field for the 5-min familiarization stage. The novel object recognition test was conducted 2 h after the familiarization stage. This test session was identical to the familiarization stage except that one of the sample objects was replaced by a novel object. During the familiarization stage, all the rats were exposed to the same two sample objects, which were almost identical but had a slightly different shape and color. In the recognition test session, for half the rats the left sample object was replaced with a novel object and for the other half the right sample object was replaced with a novel object. The novel objects were of a similar size as the sample objects but had a different shape and color (Felix-Ortiz and Febo 2012). The floor of the arena and the objects were cleaned with a Nolvasan solution between rats. The rats’ behavior was recorded during the novel object recognition test with a digital camcorder suspended above the arena. The time spent with the sample and novel objects was scored manually from the DVD recordings using Ethovision XT 11.5 by an observer who was blind to the treatment conditions. A rat was considered to be exploring an object when its head was pointed towards the object and its nose was within 1 cm of the object (sniffing and interacting with the object). A discrimination index (time with novel object – time with familiar object) / total time with objects) was calculated on the basis of the time spent interacting with the two objects.
Statistics
Data were analyzed with multi-factor ANOVA appropriate for each experimental design, using IBM SPSS Statistics version 25 or GraphPad Prism version 7. Body weight data were analyzed using a three-factor ANOVA, with exposure condition (clean air, placebo, and cannabis in experiment 1; THC and vehicle in experiment 2) and sex as between-subjects factors, and day as a within-subjects factor. Behavioral test data were analyzed using two-factor ANOVA, with exposure condition and sex as between-subjects factors. For all statistical analyses, significant interactions in the ANOVA were followed by Bonferroni’s post hoc tests to determine which groups differed from each other. Partial eta-squared (η2p) values are provided as a measure of effect size (Cohen 1973). P values less or equal to 0.05 were considered significant. Significant main effects, interactions, and post hoc comparisons are reported in the “Results” section.
Results
Experiment 1: effects of adolescent cannabis smoke exposure
Cannabis smoke and body weights
Across all groups of rats, males weighed more than the females (F1,66 = 39.38, P < .0001, η2p = 0.374, Figure S1a). There was a significant increase in body weight across smoke exposure days (day, F20,1320 = 3883.95; P < 0.0001; η2p = 0.983), and the magnitude of this increase was significantly greater in males than females (day × sex, F20,1320 = 145.23; P < .0001; η2p = 0.688; Figure S1a). There were, however, no main effects or interactions involving exposure condition. Males continued to weigh more than females at P70 (sex, F1, 66 = 261.45; P < 0.0001; η2p = 0.798), but there was no effect of exposure condition (Figure S1b).
Cannabis smoke and the large open field test
In the large open field test, there were no main effects or interactions involving smoke exposure condition on total distance traveled, distance traveled in the border zone, distance traveled in the center zone, number of center zone entries, or time spent in the center zone (Figs. 1 and S2). There was a significant interaction between exposure condition and sex on latency to enter the center zone (exposure condition × sex, F2,66 = 5.246; P < 0.01; η2p = 0.137; Figure S2a). Post hoc analyses indicated that the sex difference observed under clean air control conditions was significantly attenuated in the placebo and cannabis smoke conditions. In addition, there were robust sex differences in total distance traveled (F1,66 = 9.028, P < 0.01, η2p = 0.120, Fig. 1a), distance traveled in the center zone (F1,66 = 25.9, P < 0.0001, η2p = 0.282, Fig. 1a), number of entries into the center zone (F1,66 = 19.99, P < 0.0001, η2p = 0.232, Figure S2b), and total time in the center zone (F1,66 = 32.71, P < 0.0001, η2p = 0.331, Figure S2c). In all instances, these sex differences were in the direction of greater locomotor activity and/or less anxiety-like behavior in females compared with males.
Exposure to cannabis smoke or THC during adolescence does not affect behavior in the large open field test. Male and female rats were exposed to cannabis smoke (a) or THC (b) and tested in the large open field in adulthood. Asterisks (*P < 0.05) indicate a greater distance traveled in the females compared with the corresponding male group. Cannabis smoke, placebo smoke, and air: n = 12/group/sex. THC and vehicle: n = 10/group/sex. A, air; C, cannabis smoke; P, placebo smoke; T, THC; V, vehicle. Data are expressed as means ± SEM
Cannabis smoke and the elevated plus maze test
Consistent with the data from the open field test, there were no main effects or interactions involving exposure condition on any of the measures in the elevated plus maze test, including percentage of open arm entries and percentage of time spent on the open arms (Fig. 2) and the number of open arm entries or closed arm entries (Figure S3). There were, however, robust sex differences, such that female rats had a greater percentage (F1,66 = 8.676, P < 0.01, η2p = 0.116, Fig. 2a) and number (F1,66 = 5.044, P < 0.05, η2p = 0.071, Figure S3a) of open arm entries compared with male rats and spent a greater percentage of time on the open arms (F1,66 = 10.29, P < 0.01, η2p = 0.135, Fig. 2b). There were no effects of sex on the number of closed arm entries (Figure S3b), suggesting that the greater number of open arm entries in females compared with males was not due to greater locomotor activity, but instead due to less anxiety-like behavior.
Exposure to cannabis smoke or THC during adolescence does not affect behavior in the elevated plus maze test. Male and female rats were exposed to cannabis smoke (a, b) or THC (c, d) and tested in the large open field in adulthood. Cannabis smoke, placebo smoke, and air: n = 12/group/sex. THC and vehicle: n = 10/group/sex. Data are expressed as means ± SEM
Cannabis smoke, sucrose preference, and the forced swim test
There was no effect of exposure condition or sex on sucrose preference (Table 1). However, total fluid intake (F1,66 = 5.351, P < 0.05, η2p = 0.075, Table S2) and water intake (F1,66 = 8.687, P < 0.05, η2p = 0.116) were greater in the males than the females and there was a trend towards greater sucrose intake in the males (F1,66 = 3.297, P = 0.07, η2p = 0.048). There were no effects of exposure condition or sex in the forced swim test (Fig. 3a).
Exposure to cannabis smoke or THC during adolescence does not affect behavior in the forced swim test. Male and female rats were exposed to cannabis smoke (a) or THC (b) and tested in the forced swim test in adulthood. Cannabis smoke, placebo smoke, and air: n = 12/group/sex. THC and vehicle: n = 10/group/sex. A, air; C, cannabis smoke; P, placebo smoke; T, THC; V, vehicle. Data are expressed as means ± SEM
Cannabis smoke and the novel object recognition test
There were no effects of exposure condition or sex on the novel object recognition task either on the discrimination index measure (Fig. 4a) or on any of the measures of time spent interacting with the objects (Figure S4).
Exposure to cannabis smoke or THC during adolescence does not affect the discrimination index in the novel object recognition test. Male and female rats were exposed to cannabis smoke (a) or THC (b) and tested in the novel object recognition test in adulthood. Cannabis smoke, placebo smoke, and air: n = 12/group/sex. THC and vehicle: n = 10/group/sex. Data are expressed as means ± SEM
Experiment 2: effects of adolescent THC exposure
THC and body weights
Across both groups of rats, there was a significant increase in body weight across the days of THC or vehicle administration (day, F10,360 = 1038.47; P < 0.0001; η2p = 0.966), and the magnitude of this increase was significantly greater in males than females (day × sex, F10,360 = 102.55; P < 0.0001; η2p = 0.74; Figure S5). Against this background, THC administration caused a significant reduction in body weight gain (F1,36 = 4.68, P < 0.05, η2p = 0.115), which became larger across days of drug administration (day × drug condition, F10,360 = 33.37; P < 0.0001; η2p = 0.481) and differed by sex (day × sex × drug condition, F10,360 = 2.59; P < 0.01; η2p = 0.067). Post hoc analyses indicate that at treatment day 6 and later, the body weights of the male-vehicle rats were greater than those of the female-vehicle rats, and at day 9 and later, the body weights of the male-vehicle rats were greater than those of the male-THC rats. Follow-up two-factor ANOVAs comparing drug conditions separately in each sex revealed a significant attenuation in body weight gain in both males (day, F10,180 = 661.2; P < 0.0001; η2p = 0.973; day × drug condition, F10,180 = 19.34; P < 0.0001; η2p = 0.518) and females (day, F10,180 = 380.5; P < 0.0001; η2p = 0.955; day × drug condition, F10,180 = 15.12; P < 0.0001; η2p = 0.457), indicating that despite overall sex differences in body weight, THC robustly attenuated normal growth in both males and females.
THC and the large open field test
In the large open field test, there were no main effects or interactions involving drug condition on distance traveled in the border zone, distance traveled in the center zone, number of center zone entries, or time spent in the center zone (Figs. 1b and S6). There was a significant interaction between drug condition and sex on total distance traveled in the open field (sex × drug condition, F1,36 = 4.745; P < 0.05; η2p = 0.116), and post hoc comparisons revealed that the sex difference in total distance traveled in vehicle-treated rats (females greater than males) was diminished in THC-treated rats (Fig. 1b). In addition, the overall ANOVA revealed significant main effects of sex on distance traveled in the center of the open field (F1,36 = 9.421, P < 0.01, η2p = 0.207, Fig. 1b), number of entries into the center zone (F1,36 = 10.18, P < 0.01, η2p = 0.22, Fig. S6b), and time in the center (F1,36 = 6.811, P < 0.05, η2p = 0.159, Figure S6c). As in experiment 1, these sex differences were all in the direction of greater locomotor activity and/or less anxiety-like behavior in females compared with males.
THC and the elevated plus maze test
As was the case with cannabis smoke in experiment 1, THC had no effect (no main effects or interactions) on any of the measures in the elevated plus maze. There were, however, robust sex differences on several of the measures. Compared with males, females spent a greater percentage of time on the open arms (F1,36 = 6.105, P < 0.05, η2p = 0.145, Fig. 2c) and had a higher percentage of open arm entries (F1,36 = 16.64, P < 0.001, η2p = 0.316, Fig. 2d). There was a trend towards a greater number of open arm entries in females compared with the males (F1,36 = 3.086, P = 0.088, η2p = 0.079, Figure S7a), whereas males had a greater number of closed arm entries (F1,36 = 18.29, P < 0.0001, η2p = 0.337, Figure S7b).
THC, sucrose preference, and the forced swim test
Neither THC nor sex produced significant effects on sucrose preference (Table 1) or total fluid intake (Table S2). In the forced swim test, there were no main effects of either drug condition or sex on swimming or climbing (Fig. 3b), but there was a sex × drug condition interaction for immobility (F1,36 = 4.304, P < 0.05, η2p = 0.107). Post hoc tests indicated that the sex difference in immobility in the vehicle-treated rats was diminished in THC-treated rats. There was also a trend towards a sex × drug condition interaction for climbing behavior (F1,36 = 3.519, P = 0.069, η2p = 0.089). In the vehicle condition, the females spent more time climbing than the males but in the THC condition the males spent more time climbing.
THC and the novel object recognition test
In the novel object recognition test, there were no main effects of either drug condition or sex on the discrimination index (Fig. 4b). There was a trend towards an interaction between sex and drug condition on the discrimination index measure (F1,36 = 4.029, P = 0.052, η2p = 0.101), with a trend towards a larger discrimination index (indicative of better retention) in THC- compared with vehicle-treated males. Analyses of the other performance measures during the novel object recognition test showed that females spent more time with the objects than males (F1,36 = 33.99, P < 0.0001, η2p = 0.486, Figure S8a), and that this difference was evident in both time spent with the novel objects (F1,36 = 19.64, P < 0.0001, η2p = 0.353, Figure S8b) and time spent with the familiar objects (F1,36 = 31.94, P < 0.0001, η2p = 0.47, Figure S8c). In addition, there was a significant interaction between sex and drug condition on time spent with the familiar objects (sex × THC, F1,36 = 4.15; P < 0.05; η2p = 0.103; Figure S8c). Post hoc comparisons revealed that the female-THC rats spent more time with the familiar object than the male-THC rats.
Discussion
In the present studies, we evaluated the effects of adolescent exposure to cannabis smoke or THC on anxiety-like behavior, depressive-like behavior, and cognition in adulthood. In contrast to previous work on adult outcomes of adolescent cannabinoid exposure in rodent models, we observed few and only small effects of either cannabis smoke or THC exposure on any of the behavioral tests. These data suggest that adverse adult outcomes are not inevitable consequences of even relatively extensive regimens of adolescent cannabinoid exposure.
Numerous prior studies have shown that dysregulated affect and impaired cognition in adulthood can be caused by a variety of adolescent cannabinoid exposure regimens in rats, using both THC and synthetic cannabinoids such as WIN 55,212-2 or CP 55,940 (Cha et al. 2006; O’Shea et al. 2004; Quinn et al. 2008; Realini et al. 2011; Rubino et al. 2009b; Rubino et al. 2008; Schneider and Koch 2003). For example, adolescent THC exposure decreased sucrose preference in adult male and female rats and led to increased immobility in the forced swim test in adult female rats (Rubino et al. 2008). Findings of adverse effects of adolescent cannabinoid exposure are, however, not consistent in the published literature. For example, adolescent treatment with THC did not affect spatial and nonspatial learning in the water maze in adult male rats (Cha et al. 2006). In addition, even in studies in which adverse effects of cannabinoid exposure are reported on some measures, there are often no effects on other measures in the same animals, suggesting that cannabinoid effects on affect and cognition are by no means universal (Rubino et al. 2008).
Given that there was no evidence for adverse effects on affective/cognitive outcomes in the present experiments, it is important to consider potential explanations. One possibility is that the THC doses to which the adolescent rats were exposed were too low to produce lasting behavioral alterations. The smoke exposure study was designed to model the most frequent route of human cannabis ingestion (inhalation of smoked cannabis), and exposure to smoke from 5 cannabis or placebo cigarettes/day (across 1 h/day) took place over 21 days. Recent work from our labs using a highly sensitive and selective liquid chromatography-tandem mass spectrometry method showed that these same cannabis smoke exposure conditions yielded plasma THC levels of 12 ng/ml, 10–30 min after exposure (Ravula et al. 2018). As plasma THC levels drop rapidly after cessation of cannabis smoke inhalation (Heuberger et al. 2015), it is likely that peak levels are even higher. Indeed, non-compartmental analysis of our prior pharmacokinetic data suggests that the maximum THC concentration (Cmax) after exposure to 5 cannabis cigarettes is approximately 18 ng/ml (Gabrielsson and Weiner 2012). The maximum THC concentration in humans after smoking one 5.9% THC cannabis cigarette varies between 1.9 and 43.6 ng/ml (average 28.3 ng/ml)(Lee et al. 2015). Thus, the maximum THC level in our cannabis smoke study is in the low to middle range of what has been observed after cannabis smoking in humans. In addition, we showed in previous work in adult rats that the cannabis smoke exposure regimen used in the present study (5 consecutively smoked cannabis cigarettes) produces both acute (locomotor activity changes, alterations in working memory) and chronic (dependence) effects on behavior. Hence, irrespective of plasma THC levels, the exposure regimen is sufficient to yield behaviorally active THC levels (Blaes et al. 2018; Bruijnzeel et al. 2016).
In order to address issues of potentially insufficient dosing following smoke exposure, however, experiment 2 employed an adolescent THC exposure regimen that was identical to that used in a series of experiments by Rubino and colleagues that caused depressive-like behavior and cognitive impairments in adulthood (Rubino et al. 2009b; Rubino et al. 2008). As with cannabis smoke, this THC regimen did not affect adult behavior in this present study; it is important to consider, however, that the doses in this regimen (2.5–10.0 mg/kg) are higher than those that would be consumed voluntarily. Place conditioning and taste aversion experiments in rats indicate that THC doses higher than 1–3 mg/kg produce aversive effects (Braida et al. 2004; Elsmore and Fletcher 1972; Parker and Gillies 1995). Furthermore, rats that self-administer THC consume 0.5 mg/kg or less in 1.5–2-h sessions (Spencer et al. 2018; Wakeford et al. 2017). In addition, THC doses in the range employed here and by others yield plasma THC levels that are 10–100 times greater than those observed in humans smoking cannabis. For example, plasma THC levels are roughly 9000 ng/ml after an i.v. bolus of 5 mg/kg THC in rats, whereas they are roughly 80 ng/ml after ad libitum cannabis smoking in humans (Lee et al. 2015; Rubino et al. 2008). Even accounting for differences in species and routes of administration, it seems likely that the THC doses employed in the present study more closely model conditions of cannabis toxicity or overdose than recreational use. The fact that THC significantly attenuated normal increases in body weight during the exposure period (as it did in previous work with this regimen; Rubino et al. 2008) is consistent with this interpretation.
Aside from doses, another potential account for the absence of behavioral consequences of the adolescent exposure regimens in the present study concerns the rat strains used. Previous studies assessing the effects of adolescent cannabinoid exposure (including the work of Rubino and colleagues) have employed Sprague-Dawley rats, whereas the present study employed Long-Evans rats. There is some evidence for strain differences in responses to cannabinoids (Deiana et al. 2007; Lepore et al. 1996). In addition, Sprague-Dawley and Long-Evans rats exhibit significant differences in their response to stressors, such that chronic mild stress diminishes stress-induced corticosterone release in Long-Evans rats but not in Sprague-Dawley rats (Bielajew et al. 2002). As both smoke inhalation and THC administration are likely to be perceived as stressful, it is possible that Long-Evans rats are more protected from the effects of developmental stressors (Zuardi et al. 1984). Such differences might account for the failure to observe adverse effects of smoke or THC in the present study. It is also unlikely that age differences at the time of testing could account for differences between our study and those previous. In the present study, the sucrose preference test and forced swim test were conducted from P93–P98, while Rubino and colleagues conducted these tests from P75–P100 (Realini et al. 2011; Rubino et al. 2008).
Although there were no main effects of cannabis smoke or THC on any of the behaviors tested, a close look at the findings revealed that exposure to either cannabis smoke or THC attenuated sex differences on several of the task performance measures. In both experiments 1 and 2, rats in the control conditions showed large sex differences in performance in the open field, such that females had more center zone entries and spent more time in the center zone than males, potentially indicative of less anxiety-like behavior in females. This sex difference was diminished after exposure to cannabis smoke or THC. Exposure to cannabis smoke also attenuated the sex difference in latency to enter the center zone of the open field, and exposure to THC attenuated the sex difference in immobility in the forced swim test. Notably, similar sex-specific behavioral changes have been observed in mice after exposure to estrogenic endocrine disruptors during pregnancy and the early postpartum period, such that males exposed to endocrine disruptors (e.g., the plastic derivative bisphenol A and the insecticide methoxychlor) display more female-like behavior, and females exposed to endocrine disruptors display more male-like behavior (Gioiosa et al. 2007). Rubino and colleagues measured plasma estradiol levels immediately following the THC regimen used in the present study and found no changes in estradiol levels (Rubino et al. 2015). It is possible, however, that THC and other cannabinoids affect estrogen receptor (ER) signaling in the brain. ER-deficient male mice exhibit female-type behavior in the open field test (e.g., increase in center entries) (Ogawa et al. 1997). Furthermore, there is evidence that THC may cause anti-estrogenic effects by upregulating ERβ receptor expression and the formation of ERα/ERβ dimers (Takeda 2014). Considering these similarities in the effects of endocrine disruptors and cannabinoids, it is possible that some of the effects of cannabis smoke and THC exposure in the present study are due to disruption of ER signaling mechanisms. Arguing against this possibility, however, is the fact that the effects of placebo smoke in the open field test were somewhat similar to those of cannabis smoke (e.g., Figure S2). This suggests that, at least in the smoke exposure study, factors aside from THC might affect the expression of sex differences. Burning cannabis plant material generates high levels of polycyclic aromatic hydrocarbons, which are also potent endocrine disruptors (Moir et al. 2007; Santodonato 1997). Therefore, smoking cannabis might lead to inhalation of compounds that could disrupt endocrine signaling and ultimately the expression of sex-specific behaviors later in life.
Aside from effects of cannabinoid exposure, the present studies revealed the presence of robust sex differences on several of the behavioral tests. In the cannabis smoke experiment, compared with males, females traveled a greater distance in the center of the open field, had shorter latencies to enter the center zone, spent more time in the center zone, and made more entries into the center zone. A similar pattern of results was observed in the vehicle-control males and females in the THC experiment. This finding is in line with previous studies of open field locomotion showing that female rats travel a greater distance in the open field than male rats (Brotto et al. 2000; Masur et al. 1980; Romero and Chen 2004; Slob et al. 1986). As anxiolytic drugs such as chlordiazepoxide and diazepam increase entries into the center zone of the open field and time spent in the center of the open field (Britton and Britton 1981; Gentsch et al. 1987; Prut and Belzung 2003), these data could indicate that females are less anxious than males in this test. A similar pattern of results was observed in the elevated plus maze test, in which females spent a greater percentage of time on the open arms and had a higher percentage of open arm entries. Anxiolytic drugs increase open arm time and open arm entries, and therefore this pattern of results suggest that the females are less anxious than the males. The results of the present study are in line with a previous study in which adult female rats made a greater percentage of entries into the open arms and showed a trend towards an increase in the percentage of time spent on the open arms (Johnston and File 1991).
In the forced swim test, there were no main effects of sex or exposure condition on immobility, swimming, or climbing, although in experiment 2 there was a sex × drug condition interaction. Follow-up analyses indicated that vehicle females displayed less immobility than the vehicle males, and that this effect was diminished after THC treatment. Immobility in the forced swim test has traditionally been interpreted as a state of “behavioral despair” and resignation (Castagné et al. 2009), although more recent studies have questioned the validity of this interpretation (De Pablo et al. 1989; Molendijk and de Kloet 2015). It has also been suggested that increasing immobility is an adaptive coping strategy that helps to save energy and increase chance of survival. Several studies have compared sex differences in the forced swim test. Interestingly, some of these studies reported that females display less immobility than males (Alonso et al. 1991a; Alonso et al. 1991b) although other studies have found no sex differences (Armario et al. 1995; Frye and Walf 2002).
In conclusion, these studies found no evidence that repeated exposure to either cannabis smoke or THC during adolescence in rats produces adverse affective or cognitive outcomes during adulthood, although cannabinoid exposure appeared to attenuate sex differences in some aspects of anxiety- and depressive-like behavior. Although the failure here to observe such adverse effects may have been due to the particular rat strain employed, it was unlikely due to insufficient dosing, particularly because the THC regimen was identical to one used previously to produce such adverse effects. In addition, it is important to note that although rats underwent a fairly extensive battery of affective testing, cognitive function was tested with only a single measure (novel object recognition, which is sensitive to some forms of hippocampal and prefrontal cortical disruption) (Cohen and Stackman Jr 2015). Finally, although adolescent cannabinoid exposure did not robustly affect adult behavior in the current studies, it is important to appreciate the limitations of rodents with respect to modeling human development and substance use, including the duration of substance intake during adolescence, the self-selected nature of the human cannabis-using population, and frequent concomitants of human cannabis use including psychiatric disorders, environmental stressors, and polysubstance use (Liu et al. 2018). Going forward, it will be important to address such limitations experimentally (e.g., by using cannabinoid self-administration models and studying the interactions between cannabis use and comorbidities), in order to better model the impact of cannabis on the developing brain and behavior.
References
Alonso S, Arevalo R, Afonso D, Rodriguez M (1991a) Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol Behav 50:511–517
Alonso SJ, Castellano MA, Afonso D, Rodriguez M (1991b) Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav 49:69–72
Armario A, Gavaldà A, Martí J (1995) Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology 20:879–890
Azofeifa A (2016) National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill Summ 65
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1:1306–1311
Bielajew C, Konkle A, Merali Z (2002) The effects of chronic mild stress on male Sprague–Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav Brain Res 136:583–592
Blaes SL, Orsini CA, Holik HM, Stubbs TD, Ferguson SN, Heshmati SC, Bruner MM, Wall SC, Febo M, Bruijnzeel AW (2018) Enhancing effects of acute exposure to cannabis smoke on working memory performance. Neurobiol Learn Mem 157:151–162
Braida D, Iosue S, Pegorini S, Sala M (2004) Δ9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol 506:63–69
Britton DR, Britton KT (1981) A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav 15:577–582
Brotto LA, Barr AM, Gorzalka BB (2000) Sex differences in forced-swim and open-field test behaviours after chronic administration of melatonin. Eur J Pharmacol 402:87–93
Bruijnzeel AW, Qi X, Guzhva LV, Wall S, Deng JV, Gold MS, Febo M, Setlow B (2016) Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS One 11:e0153327
Castagné V, Moser P, Porsolt RD (2009) Behavioral assessment of antidepressant activity in rodents
Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder H (2006) Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav 83:448–455
Coelho JE, Alves P, Canas PM, Valadas JS, Shmidt T, Batalha VL, Ferreira DG, Ribeiro JA, Bader M, Cunha RA (2014) Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front Psych 5:67
Cohen J (1973) Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ Psychol Meas 33:107–112
Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117
Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH (2016) Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci 17:293–306
De Pablo JM, Parra A, Segovia S, Guillamón A (1989) Learned immobility explains the behavior of rats in the forced swimming test. Physiol Behav 46:229–237
Deiana S, Fattore L, Spano MS, Cossu G, Porcu E, Fadda P, Fratta W (2007) Strain and schedule-dependent differences in the acquisition, maintenance and extinction of intravenous cannabinoid self-administration in rats. Neuropharmacology 52:646–654
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72
Elsmore TF, Fletcher GV (1972) Δ9-Tetrahydrocannabinol: aversive effects in rat at high doses. Science 175:911–912
Feingold D, Weiser M, Rehm J, Lev-Ran S (2015) The association between cannabis use and mood disorders: a longitudinal study. J Affect Disord 172:211–218
Felix-Ortiz AC, Febo M (2012) Gestational valproate alters BOLD activation in response to complex social and primary sensory stimuli. PLoS One 7:e37313
Frye CA, Walf AA (2002) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41:306–315
Gabrielsson J, Weiner D (2012) Non-compartmental analysis computational toxicology. Springer, pp 377–389
Gentsch C, Lichtsteiner M, Feer H (1987) Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behav Brain Res 25:101–107
Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P (2007) Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav 52:307–316
Gordon AJ, Conley JW, Gordon JM (2013) Medical consequences of marijuana use: a review of current literature. Curr. Psychiatry Rep 15:419
Hasin DS, Wall M, Keyes KM, Cerdá M, Schulenberg J, O’Malley PM, Galea S, Pacula R, Feng T (2015) Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry 2:601–608
Heuberger JA, Guan Z, Oyetayo O-O, Klumpers L, Morrison PD, Beumer TL, van Gerven JM, Cohen AF, Freijer J (2015) Population pharmacokinetic model of THC integrates oral, intravenous, and pulmonary dosing and characterizes short-and long-term pharmacokinetics. Clin Pharmacokinet 54:209–219
Horwood LJ, Fergusson DM, Coffey C, Patton GC, Tait R, Smart D, Letcher P, Silins E, Hutchinson DM (2012) Cannabis and depression: an integrative data analysis of four Australasian cohorts. Drug Alcohol Depend 126:369–378
Huestis MA (2007) Human cannabinoid pharmacokinetics. Chem Biodivers 4:1770–1804
Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, McGue M, Raine A, Baker LA (2016) Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc Natl Acad Sci 113:E500–E508
Johnston AL, File SE (1991) Sex differences in animal tests of anxiety. Physiol Behav 49:245–250
Jouanjus E, Raymond V, Lapeyre-Mestre M, Wolff V (2017) What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr Atheroscler Rep 19:26
Lee D, Bergamaschi MM, Milman G, Barnes AJ, Queiroz RH, Vandrey R, Huestis MA (2015) Plasma cannabinoid pharmacokinetics after controlled smoking and ad libitum cannabis smoking in chronic frequent users. J Anal Toxicol 39:580–587
Lepore M, Liu X, Savage V, Matalon D, Gardner EL (1996) Genetic differences in Δ9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci 58:PL365–PL372
Liebsch G, Montkowski A, Holsboer F, Landgraf R (1998) Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res 94:301–310
Liu Y, Williamson V, Setlow B, Cottler LB, Knackstedt LA (2018) The importance of considering polysubstance use: lessons from cocaine research. Drug Alcohol Depend 192:16–28
Masur J, Schutz MT, Boerngen R (1980) Gender differences in open-field behavior as a function of age. Developmental Psychobiology. The Journal of the International Society for Developmental Psychobiology 13:107–110
Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife. ProcNatlAcadSciUSA 109:E2657–E2664
Meier MH, Caspi A, Danese A, Fisher HL, Houts R, Arseneault L, Moffitt TE (2018) Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction 113:257–265
Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S (2007) A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 21:494–502
Molendijk ML, de Kloet ER (2015) Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62:389–391
Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G (2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370:319–328
Niesink RJ, van Laar MW (2013) Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry 4:130
O’Shea M, Singh ME, McGregor IS, Mallet PE (2004) Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol 18:502–508
Ogawa S, Lubahn DB, Korach KS, Pfaff DW (1997) Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci 94:1476–1481
Parker LA, Gillies T (1995) THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci 109:71–78
Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W (2002) Cannabis use and mental health in young people: cohort study. BMJ 325:1195–1198
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33
Qi X, Guzhva L, Yang Z, Febo M, Shan Z, Wang KK, Bruijnzeel AW (2016) Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Eur Neuropsychopharmacol 26:1378–1389
Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA (2008) Adolescent rats find repeated Δ 9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33:1113–1126
Ravula A, Chandasana H, Setlow B, Febo M, Bruijnzeel AW, Derendorf H (2018) Simultaneous quantification of cannabinoids tetrahydrocannabinol, cannabidiol and CB1 receptor antagonist in rat plasma: an application to characterize pharmacokinetics after passive cannabis smoke inhalation and co-administration of rimonabant. J Pharm Biomed Anal 160:119–125
Realini N, Vigano D, Guidali C, Zamberletti E, Rubino T, Parolaro D (2011) Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology 60:235–243
Rey JM, Sawyer MG, Raphael B, Patton GC, Lynskey M (2002) Mental health of teenagers who use cannabis. Results of an Australian survey. Br J Psychiatry 180:216–221
Rojas A, Ganesh T, Manji Z, O’neill T, Dingledine R (2016) Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats. Neuropharmacology 110:419–430
Romero RD, Chen W-JA (2004) Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol Biochem Behav 78:675–681
Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, Sagheddu C, Ligresti A, Tonini R, Di Marzo V (2015) Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 73:60–69
Rubino T, Realini N, Braida D, Alberio T, Capurro V, Vigano D, Guidali C, Sala M, Fasano M, Parolaro D (2009a) The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res 15:291–302
Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D (2009b) Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19:763–772
Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D (2008) Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33:2760–2771
Rylkova D, Shah HP, Small E, Bruijnzeel AW (2009) Deficit in brain reward function and acute and protracted anxiety-like behavior after discontinuation of a chronic alcohol liquid diet in rats. Psychopharmacology 203:629–640
Santodonato J (1997) Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: relationship to carcinogenicity. Chemosphere 34:835–848
Schneider M, Koch M (2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28:1760–1769
Slattery DA, Cryan JF (2012) Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014
Slob AK, Huizer T, Ten Bosch JVDW (1986) Ontogeny of sex differences in open-field ambulation in the rat. Physiol Behav 37:313–315
Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, Allen N, Scofield MD, Ortiz-Ithier T, Kalivas PW (2018) A model of Δ9-tetrahydrocannabinol self-administration and reinstatement that alters synaptic plasticity in nucleus accumbens. Biol Psychiatry 84:601–610
Takeda S (2014) Δ9-Tetrahydrocannabinol targeting estrogen receptor signaling: the possible mechanism of action coupled with endocrine disruption. Biol Pharm Bull 37:1435–1438
Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH (1994) A sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol 6:79–93
Tirelli E, Laviola G, Adriani W (2003) Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev 27:163–178
Wakeford AG, Wetzell BB, Pomfrey RL, Clasen MM, Taylor WW, Hempel BJ, Riley AL (2017) The effects of cannabidiol (CBD) on Δ9-tetrahydrocannabinol (THC) self-administration in male and female Long-Evans rats. Exp Clin Psychopharmacol 25:242–248
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364
Zgair A, Wong JC, Sabri A, Fischer PM, Barrett DA, Constantinescu CS, Gershkovich P (2015) Development of a simple and sensitive HPLC–UV method for the simultaneous determination of cannabidiol and Δ9-tetrahydrocannabinol in rat plasma. J Pharm Biomed Anal 114:145–151
Zuardi A, Teixeira N, Karniol I (1984) Pharmacological interaction of the effects of delta 9-trans-tetrahydrocannabinol and cannabidiol on serum corticosterone levels in rats. Arch Int Pharmacodyn Ther 269:12–19
Acknowledgments
We thank the NIDA Drug Supply Program for kindly providing the cannabis and placebo cigarettes and THC, and Shelby Blaes for assistance with THC preparation.
Funding
This work was supported by funding from the National Institutes of Health (R21 DA039349) to BS and AWB and the McKnight Brain Institute to BS, AWB, and MF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1231 kb)
Rights and permissions
About this article
Cite this article
Bruijnzeel, A.W., Knight, P., Panunzio, S. et al. Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology 236, 2773–2784 (2019). https://doi.org/10.1007/s00213-019-05255-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05255-7