Abstract
Rationale and objectives
Stress-induced alterations in oxidative and inflammatory parameters have been implicated in the pathophysiology of mood disorders. Based on the antioxidant and anti-inflammatory properties of the selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole (CMI), we assessed its ability to reverse depression-like behavioral alterations, neuroinflammation, and oxidative imbalance induced by acute restraint stress.
Methods
Mice submitted to restraint for 240 min received CMI (1 or 10 mg/kg, orally) 10 min after the end of the stress induction. Behavioral and biochemical tests were carried out after further 30 min.
Results
Restraint-induced depression-like behavior in the tail suspension test (TST), splash test, and new object exploration test was reversed by CMI. None of the treatments evoked locomotor alteration. In addition, CMI abrogated restraint-induced increases in plasma levels of corticosterone and in markers of oxidative stress and impaired superoxide dismutase and catalase activity in the prefrontal cortex (PFC) and hippocampus (HC). CMI also blocked stress-induced downregulation of mRNA levels of glucocorticoid receptor and brain-derived neurotrophic factor and upregulation of nuclear factor kappa B, inducible nitric oxide synthase, tumor necrosis alpha, indoelamine-2,3-dioxygenase, and glycogen synthase kinase 3 beta in PFC and HC.
Conclusions
These preclinical results indicate that administration of selenium-containing compounds might help to treat depression associated with inflammation and oxidative stress.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to stress induces behavioral, emotional, and cognitive modifications that can increase the risk of progression of neuropsychiatric disorders such as major depressive disorder (Joëls et al. 2006). Although glucocorticoids (GCs) released by the adrenal cortex in response to stress are claimed to have immunosuppressive and anti-inflammatory properties, recent data indicate they can also have pro-inflammatory influence on the immune system (Elenkov 2008).
Stress can activate the innate immune system through the activation of Toll-like receptor 4 (TLR4) by damage-associated molecular patterns (DAMPs) that are generated from insults to tissues, or by pathogen-associated molecular patterns (PAMPs), which can be a result of bacterial translocation. Activated TLR4 promotes the activation of glycogen synthase kinase 3 beta (GSK-3β) (Jope et al. 2017), which can also be activated by increases in cortisol (Dobarro et al. 2013). Among its many actions, activation of GSK-3β by phosphorylation at tyrosine 216 residue prevents the translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) from the cytosol to the nucleus (Kensler et al. 2007). Consequently, Nrf2 does not bind to the antioxidant response element (ARE), thereby reducing the transcription of antioxidant enzymes, such as heme oxygenase-1 (HO-1), superoxide dismutase (SOD), and catalase (CAT) (Kensler et al. 2007). Additionally, GSK-3β promotes the activation of transcription factors in the inflammatory response, such as nuclear factor kappa B (NF-κB), thereby increasing the production of pro-inflammatory cytokines in the central nervous system, especially by microglia and astrocytes (Jope et al. 2017). Pro-inflammatory cytokines are also responsible for the activation of the enzymes indoleamine-2,3-dioxygenase (IDO) (Schwarcz and Pellicciari 2002) and inducible nitric oxide synthase (iNOS), further contributing to impaired neurotransmission and oxidative imbalance by increased reactive species (RS) generation. Excessive formation of free radicals and/or defects in the antioxidant defense can damage lipids, proteins, and nucleic acids, which in turn are implicated in major depressive disorder (Maes et al. 2011), highlighting the importance of the antioxidant balance in the central nervous system.
Selenium is an essential trace element for human health and exerts most of its action as integral constituent of selenoproteins that are widely implicated in redox signaling (Brigelius-Flohé and Flohé 2017). In turn, selenium-containing organic compounds have emerged as promising biologically active molecules (Casaril et al. 2015; Domingues et al., 2018 ; Singh et al. 2016; Masaki et al. 2016; Kil et al. 2017; Gandin et al. 2018; Sudati et al. 2018). Recently, Vieira et al. (2015) reported that the synthetic organoselenium compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole (CMI) has in vitro antioxidant activity, while Casaril et al. (2017b) showed that it potently inhibits inflammation-associated oxidative stress (i.e., hypochlorous acid, peroxynitrite, and hydrogen peroxide). In addition, Birmann et al. (2018) have demonstrated that the antinociceptive effect of CMI is mediated by the monoaminergic, opioidergic, and adenosinergic systems, pointing out the promising role of CMI in modulating different pathways for the treatment of pain and inflammation. Further studies revealed that CMI prevents the depressive-like behavior induced by lipopolysaccharide (LPS) in mice (Casaril et al. 2017a).
In light of the potential antioxidant and anti-inflammatory profile of CMI, we hypothesized that this organoselenium compound should be able to counteract stress-induced depression-like behavior in mice. We used for this purpose a model of acute restraint stress in mice that had already been validated (Pesarico et al. 2015; Thakare et al. 2016; Surkin et al. 2018). We show here that CMI has antidepressant properties in this model that are associated with downregulation of stress-induced inflammation and oxidative stress.
Materials and methods
Drugs and reagents
CMI (Fig. 1A) was prepared and characterized at the Laboratory of Clean Organic Synthesis at the Federal University of Pelotas, according to Vieira et al. (2015). The RNA extraction reagent was purchased from Ambion (Life Technology, USA). The oligonucleotides were synthesized by Exxtend Biotecnologia Ltda (Campinas, Brazil). All other chemicals were of analytical grade and were obtained from Servylab and WF Científica (Brazil). CMI was dissolved in canola oil (a non-polar and inert substance) and administered intragastrically (i.g.) at a constant volume of 10 ml/kg body weight.
Animals
Behavioral experiments were performed in Swiss male mice (25–30 g; 10–13-week-old), maintained at 22–25 °C and 40–60% relative humidity with free access to water and food, under a 12:12-h light/dark cycle (lights on at 7:00 a.m.). The studies were performed in accordance with protocols approved by the Committee on the Care and Use of Experimental Animal Resources at the Federal University of Pelotas, Brazil (4034-2017).
Acute restraint stress
The physical restraint was performed in mice as previously reported by Pesarico et al. (2015), with minor modifications. Briefly, mice were subjected to immobilization for 240 min using an individual rodent restraint device made of Plexiglas fenestrate, restraining all physical movement and causing minimum pain. Mice were deprived of food and water during the physical stress. After the restraint stress, mice were put back in their home cage and received canola oil or CMI 10 min later. They were submitted to behavioral testing 30 min later and euthanized immediately after.
Experimental design
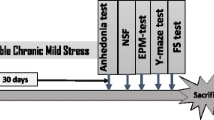
Animals were randomly divided into six experimental groups (n = 6/group) and the physical stress was performed for 240 min (Fig. 1B). The behavioral tasks—open field test (OFT), tail suspension test (TST), new object exploration test (NOET), and splash test—were carried out 280 min after the beginning of the acute restraint stress (ARS) protocol, and the vehicle or CMI (1 or 10 mg/kg, orally) was given 10 min after the physical restraint (Pesarico et al. 2015). Previous data also support the 30-min pre-treatment to evaluate the antidepressant-like activity of CMI (Casaril et al. 2017a) and other selenium-containing compounds (Martinez et al. 2014; Pinto Brod et al. 2016). All the observations were done by an observer blinded to the treatment and the behavioral tests were scored manually. Following the behavioral assessment, mice were anesthetized (inhalation of isoflurane) before blood collection by cardiac puncture (Parasuraman et al. 2010). After that, mice were killed by cervical dislocation, followed by brain removal (residual blood and blood cells can be present in the samples) and isolation of the prefrontal cortex (PFC) and total hippocampus (HC) for analysis (Sunkin et al. 2013).
Behavioral tests
Open field test
The open field test (OFT) was carried out before the other behavioral tests (Walsh and Cummins 1976) to assess the possible effect of the treatments on the locomotor activity. Mice were placed individually in the center of a box (30 × 30 × 15 cm) divided into nine quadrants of equal areas, and observed for 5 min to report their locomotor (scored by the number of segments crossed with the four paws) and exploratory activities (expressed by the number of time the mice stood on rear limbs).
Tail suspension test
The total duration of immobility in the tail suspension test (TST) was measured according to the method described by Steru et al. (1985). Mice that were both acoustically and visually isolated from each other were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of their tail. During the first 2 min of habituation, the latency time to immobility was recorded (i.e., time for the first immobility episode), and during the last 4 min, the immobility duration (defined as the absence of the escape attempt behavior) was observed.
New object exploration test
For this test, mice were allowed to explore a novel object for 15 min in an arena 18 × 25 cm2 surrounded by plastic walls (Strekalova et al. 2004). The object had a complex texture surface (artificial flower, 2.5 × 2.5 × 4 cm3) and it was fixed to the center of the arena. The total duration of time spent exploring the object was scored, and at the end of each test session, the arena was cleaned with a 70% ethanol solution.
Splash test
The grooming behavior of mice was observed as a measurement of motivational and self-care activities, which are considered to be deficient in depressive patients. A 10% sucrose solution was squirted on the dorsal coat of each mice and the grooming activity (including nose/face grooming, head washing, and body grooming) was recorded for 5 min (Freitas et al. 2013).
Biochemical evaluation
Tissue processing
The PFC and HC were separated in two hemispheres in order to submit each sample to all biochemical determinations (Casaril et al. 2017a). The right hemispheres were immersed in TRIzol, maintained at − 80 °C, and were submitted to the quantitative real-time polymerase chain reaction (qRT-PCR). The left hemispheres were homogenized in 50 mM Tris-HCl, pH 7.4 (1:10, w/v). The homogenate was centrifuged at 2500g for 10 min at 4 °C, and the supernatant fraction was used for the determination of reactive oxygen species (ROS) formation, thiobarbituric acid reactive species (TBARS) levels, nitric oxide metabolites (NOx), catalase (CAT), and superoxide dismutase (SOD) activities.
Plasma corticosterone assay
Determination of plasma corticosterone levels was performed according to Zenker and Bernstein (1958). Briefly, aliquots of plasma were incubated with chloroform and centrifuged for 5 min at 2500 rpm, followed by addition of 0.1 M NaOH and another round of centrifugation. After the addition of the fluorescence reagent (H2SO4 and ethanol 50%), samples were centrifuged (5 min at 10,000g) and incubated at room temperature for 2 h. Fluorescence intensity emission, corresponding to plasma corticosterone levels, was recorded at 540 nm (with 247-nm excitation) and corticosterone levels were expressed as nanogram per milliliter.
Determination of the reactive oxygen species formation
Quantification of reactive oxygen species (ROS) levels in the PFC and HC of mice was performed according to Loetchutinat et al. (2005). Briefly, aliquots of the homogenate supernatant were incubated with 1 mM dichloro-dihydro-fluorescein diacetate (DCHF-DA) and 10 mM Tris-HCl pH 7.4. The oxidation of DCFH-DA to fluorescent dichlorofluorescein (DCF) is measured for the detection of intracellular ROS. The DCF fluorescence intensity emission was recorded at 520 nm (with 480-nm excitation) and ROS levels were expressed as arbitrary units (AU) of fluorescence.
Thiobarbituric acid reactive species assay
Lipid peroxidation in the PFC and HC was measured by the formation of thiobarbituric acid reactive species (TBARS) during an acid-heating reaction, as described by Ohkawa et al. (1979). An aliquot of the homogenate supernatant was incubated with 8.1% SDS, 0.8% TBA, and acetic acid/HCl (pH 3.4) at 95 °C during 2 h. Malondialdehyde (MDA) was used as a biomarker of lipid peroxidation. Absorbance was measured at 532 nm, and the results were expressed as nanomoles of MDA per gram of tissue.
Nitric oxide in neural tissue
The total nitric oxide (NO) metabolites, NOx, in the PFC and HC of mice were determined by the Griess reaction as an indicator of nitrate/nitrite production (Lima-Junior et al. 2013). Briefly, aliquots of the homogenate were incubated with equal volume of the Griess reagent for 5 min at 25 °C, and the nitrite concentration was determined by measuring the optical density at 550 nm in reference to a standard curve of NaNO2 solution. Results were expressed as nanomoles of NOx per gram of tissue.
Evaluation of superoxide dismutase activity
The measurement of superoxide dismutase (SOD) activity is based on the capacity of SOD to inhibit autoxidation of adrenaline to adrenochrome, as described by Misra and Fridovich (1972). The color reaction was detected spectrophotometrically at 480 nm and the enzymatic activity was expressed as units per milligram of protein.
Evaluation of catalase activity
Catalase (CAT) activity was assessed spectrophotometrically by the method described by Aebi (1984), which involves monitoring the disappearance of H2O2 in the presence of S1 at 240 nm. Enzymatic activity was expressed in units per milligram of protein (1 U decomposes 1 μmol of H2O2 per minute at pH 7 at 25 °C).
Quantitative real-time polymerase chain reaction
Total mRNA was extracted in the HC and PFC right hemispheres using TRIzol (Invitrogen™, Carlsbad, USA) followed by DNase treatment with DNA-free® kit (Ambion™, USA) and mRNA quantification. The cDNA synthesis was performed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems™, UK) according to the manufacturer’s protocol. The amplification was made with UltraSYBR Mix (COWIN Bioscience Co., Beijing, China) using the Stratagene Mx3005P and the oligonucleotides were obtained from Exxtend Biotecnologia Ltda, Campinas, Brazil. Gene expressions were normalized using GAPDH as a reference gene and the conditions for the reaction involved 95 °C for 15 s, 60 °C for 60 s, and 72 °C for 30 s. The 2ΔΔCT (delta-delta comparative threshold) method was used to normalize the fold change in gene expressions. The following genes were analyzed: glucocorticoid receptor (GR; fwd 5′-ACA GCA ACG GGA CCA CCT C-3′, rev 5′-ATG GCA TCC CGA AGC TTC-3′), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB; fwd 5′-GCT TTC GCA GGA GCA TTA AC-3′, rev 5′-CCG AAG CAG GAG CTA TCA AC-3′), inducible nitric oxide synthase (iNOS; fwd 5′-GTG GTG ACA AGC ACA TTT GG-3′, rev 5′-AAG GCC AAA CAC AGC ATA CC-3′), tumor necrosis factor alpha (TNF-α; fwd 5′-CAT CTT CTC AAA ATT CGA GTG ACA A-3′, rev 5′-TGG GAG TAG ACA AGG TAC AAC CC-3′), indoleamine-2,3-dioxygenase (IDO; fwd 5′-AAT CAA AGC AAT CCC CAC TG-3′, rev 5′-AAA AAC GTG TCT GGG TCC AC-3′), glycogen synthase kinase 3 beta (GSK-3β; fwd 5′-CGG GAC CCA AAT GTC AAA CT-3′, rev 5′-TCC GAG CAT GTG GAG GGA TA-3′), brain-derived neurotrophic factor (BDNF; fwd 5′-CCA TAA GGA CGC GGA CTT GTA C-3′, rev 5′-AGA CAT GTT TGC GGC ATC CAG G-3′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; fwd 5′-AGG TCG GTG TGA ACG GAT TTG-3′, rev 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′).
Protein determination
Protein concentration was measured according to the method of Lowry et al. (1951) using serum bovine albumin as a standard.
Statistical analysis
All experimental data are presented as mean ± standard error of the mean (SEM). Comparisons between stress exposure and treatment were performed by two-way analysis of variance (ANOVA). When ANOVA revealed a significant main effect, Tukey’s post hoc test was used for between-group comparisons. Pearson’s correlation analysis was performed to investigate any possible relationship between the immobility time in the TST and neurochemical data. Probability values less than 0.05 (p < 0.05) were considered statistically significant. The statistical analysis was accomplished using GraphPad Prism version 7.0 for Windows, GraphPad Software (San Diego, CA, USA).
Results
CMI abrogates restraint-induced depressive-like behavior without altering locomotor activity
The exploratory and locomotor activities of mice submitted to the experimental protocol of this study were assessed in order to confirm the absence of false positive results in the other behavioral tests. Figure 2 A and B show that none of the treatments altered locomotor and exploratory activities in the OFT, respectively. A two-way ANOVA revealed no significant ARS × CMI interaction (F(2,30) = 0.65, p = 0.53) for the number of crossings and no ARS × CMI interaction (F(2,30) = 0.82, p = 0.45) for the number of rearings.
Effect of CMI (1 and 10 mg/kg; i.g.) on behavioral tasks and corticosterone levels in mice submitted to the ARS (240 min). (A) Number of crossings and (B) number of rearings evaluated in the OFT. (C) Immobility time and (D) latency time on the TST. (E) Exploratory time in the NOET. (F) Grooming time in the splash test. (G) Circulating levels of corticosterone. Data are expressed as mean ± SEM of six independent animals. ##p < 0.01 and ###p < 0.001 when compared to non-stressed vehicle group. ***p < 0.001 when compared to stressed mice
The TST is widely used to measure depression-like behavior in rodents, while assessing the antidepressant-like response from several pharmacological classes of drugs (Browne and Lucki 2013). As depicted in Fig. 2C, mice submitted to physical stress showed increased duration of immobility when compared to the vehicle-treated group. Noteworthy, this depressive-like behavior was reversed by the administration of CMI independently of the dose (ARS × CMI interaction; F(2,30) = 16.70, p < 0.001). Administration of CMI in non-stressed mice did not influence immobility time.
Corroborating with this result, stressed mice showed reduced latency for the first immobility episode, when compared to the vehicle-treated group (Fig. 2D). On the contrary, treatment with CMI at both doses was able to reverse the decreased latencies (ARS × CMI interaction; F(2,30) = 15.4, p < 0.001). Administration of CMI to non-stressed mice did not influence latency time.
Regarding the new object exploration test (NOET) (Fig. 2D), a two-way ANOVA of the exploratory time revealed a significant ARS × CMI interaction (F(2,30) = 13.20, p < 0.001). Concerning self-care and grooming (Fig. 2E), a two-way ANOVA revealed a significant ARS × CMI interaction (F(2,30) = 7.06, p = 0.003). Administration of CMI to non-stressed mice did not influence these behaviors.
Increased circulating glucocorticoid levels induced by ARS were restored by treatment with CMI
To document the involvement of the HPA axis in our experiments, plasma levels of corticosterone were measured in mice submitted to ARS. The results presented in Fig. 2G show that the restraint-induced increase in corticosterone levels was significantly reversed by CMI (ARS × CMI interaction; F(2,30) = 23.90, p < 0.001) while the administration of CMI to non-stressed mice had no effect.
Acute treatment with CMI reversed oxidative stress in the PFC and HC of stressed mice
As depicted in Fig. 3 A and B, the increased ROS production elicited by ARS was significantly abolished by CMI in the PFC (ARS × CMI interaction; F(2,30) = 16.00, p < 0.001) and HC (ARS × CMI interaction; F(2,30) = 13.50, p < 0.001) of mice. Similarly, CMI treatment abrogated the lipid peroxidation (Fig. 3 C and D) induced by physical stress in the PFC (ARS × CMI interaction; F(2,30) = 7.81, p = 0.002) and HC (ARS × CMI interaction; F(2,30) = 3.69, p = 0.04) of mice. Excessive nitrite production (Fig. 3 E and F) on mice submitted to ARS was also reversed by CMI treatment in both the PFC (ARS × CMI interaction; F(2,30) = 10.20, p < 0.001) and HC (ARS × CMI interaction; F(2,30) = 10.20, p < 0.001). Administration of CMI to non-stressed mice did not alter the oxidative stress parameters evaluated here.
Effect of CMI (1 and 10 mg/kg; i.g.) on markers of oxidative stress in mice submitted to the ARS (240 min). RS levels in (A) PFC and (B) HC; TBARS levels in (C) PFC and (D) HC; NOx levels in (E) PFC and (F) HC. Data are expressed as mean ± SEM of six independent animals. ###p < 0.001 when compared to non-stressed vehicle group. **p < 0.01 and ***p < 0.001 when compared to stressed mice
CMI abrogates restraint-induced increases in SOD and decreases in CAT activities
As depicted in Fig. 4 A and B, the increased SOD activity in the PFC and HC elicited by physical stress was significantly reversed by CMI treatment in the PFC (ARS × CMI interaction; F(2,30) = 4.96, p = 0.01) and HC (ARS × CMI interaction; F(2,30) = 6.89, p = 0.003) while the administration of CMI to non-stressed mice had no effect. The results presented in Fig. 4 C and D show that CMI was able to block restraint-induced decreases in CAT activity in the HC of mice (ARS × CMI interaction; F(2,30) = 9.10, p < 0.001). None of the treatments had any effect on CAT activity in the PFC of mice.
Effect of CMI (1 and 10 mg/kg; i.g.) in the activity of antioxidant enzymes in mice submitted to the ARS (240 min). SOD activity in (A) PFC and (B) HC; CAT activity in (C) PFC and (D) HC. Data are expressed as mean ± SEM of six independent animals. ##p < 0.01 and ###p < 0.001 when compared to non-stressed vehicle group. **p < 0.01 and ***p < 0.001 when compared to stressed mice
CMI blocks restraint-induced alterations in gene expression
Considering the similar effect of CMI administered at 1 and 10 mg/kg in the behavioral tests and in the abovementioned assays to address the oxidative stress, only the lowest dose of the compound was chosen to assess the effect of CMI on restraint-induced alterations in gene expression.
Restraint downregulated GR expression in the PFC (Fig. 5A) and HC (Fig. 5B) of mice, when compared to the non-stressed ones. CMI treatment reversed the decreased GR expression in both the PFC (ARS × CMI interaction; F(1,20) = 765.00, p < 0.001) and HC (ARS × CMI interaction; F(1,20) = 22.90, p < 0.001) of mice. Administration of CMI to non-stressed mice increased the expression of GR in the PFC (p < 0.001) when compared to the control group, while no alteration was found in the HC.
Effect of CMI (1 mg/kg, i.g.) in the mRNA expression of target genes in mice submitted to the ARS (240 min). Expression of GR, NF-κB, iNOS, TNF-α, IDO, GSK-3β, and BDNF in (A) PFC and (B) HC of mice. Data are expressed as mean ± SEM of six independent animals. ^p < 0.01 and #p < 0.001 when compared to non-stressed + vehicle. ***p < 0.001 when compared to ARS + vehicle
Similar results were found for NF-κB mRNA expression in the PFC (ARS × CMI interaction; F(1,20) = 897.00, p < 0.001; Fig. 5A) and HC (ARS × CMI interaction; F(1,20) = 141.00, p < 0.001; Fig. 5B). In this case, administration of CMI to non-stressed mice did not influence NF-κB expression in the PFC and HC.
Treatment with CMI abrogated stress-induced increase in iNOS mRNA expression in the PFC (ARS × CMI interaction; F(1,20) = 261.00, p < 0.001; Fig. 5A) and HC (ARS × CMI interaction; F(1,20) = 23.20, p < 0.001; Fig. 5B) of mice. Administration of CMI to non-stressed mice downregulated the expression of iNOS in the PFC (p < 0.001) when compared to the control group, while no alteration was found in the HC.
Similarly, treatment with CMI abrogated stress-induced upregulation of TNF-α mRNA expression in the PFC (ARS × CMI interaction; F(1,20) = 20.00, p < 0.001; Fig. 5A) and HC (ARS × CMI interaction; F(1,20) = 193.00, p < 0.001; Fig. 5B) of mice. Administration of CMI to non-stressed mice did not alter the expression of this pro-inflammatory cytokine in both brain structures.
Similar results were obtained for IDO mRNA expression in the PFC (ARS × CMI interaction; F(1,20) = 5.47, p = 0.03; Fig. 5A) and HC (F(1,20) = 20.30, p < 0.001; Fig. 5B) of stressed mice treated with CMI. Administration of CMI to non-stressed mice downregulated the expression of IDO in the PFC (p < 0.001) when compared to the control group, while no alteration was found in the HC.
Mice submitted to physical stress also exhibited upregulated mRNA expression of GSK-3β in the PFC (Fig. 5A) and HC (Fig. 5B), which were abrogated by treatment with CMI (ARS × CMI interaction in the PFC; F(1,20) = 143.00, p < 0.001; ARS × CMI interaction in the HC; F(1,20) = 25.60, p < 0.001). Administration of CMI to non-stressed mice downregulated the expression of GSK-3β in the PFC (p < 0.001) when compared to the control group, while no alteration was found in the HC.
The two-way ANOVA on BDNF mRNA expression revealed a significant ARS × CMI interaction in the PFC (F(1,20) = 12,895.00, p < 0.001; Fig. 5A) and HC (F(1,20) = 28.90, p < 0.001; Fig. 5B). Administration of CMI to non-stressed mice downregulated the expression of IDO in the PFC (p < 0.001) when compared to the control group, while no alteration was found in the HC.
Correlations between behavioral and biochemical effects in the PFC and HC of mice
Considering that the administration of CMI reduced the increased immobility time in the TST (Fig. 2C) and improved several biochemical endpoints altered by restraint (Figs. 3, 4, and 5), we analyzed if these effects were correlated using Pearson’s correlation analysis (Table 1). The results demonstrated significant positive correlation between the immobility time and ROS formation, NO generation, TBARS levels, SOD activity, and NF-κB, iNOS, TNF-α, IDO, and GSK-3β expressions in the PFC and HC of mice. A significant negative correlation was found between the immobility time and CAT activity in the HC and GR and BDNF expressions in the PFC and HC of mice. No significant correlation was found between the immobility time and CAT activity in the PFC.
Discussion
The present study shows for the first time that a single administration of CMI is able to reverse the behavioral response to restraint in the TST, splash test, and NOET by a mechanism dependent, at least in part, on the modulation of oxidative stress and neuroinflammation. Indeed, we observed that CMI regulated the SOD and CAT activities in the PFC and HC of stressed mice, alongside with a decrease in plasma levels of corticosterone. Additionally, we reported here that CMI reversed the increases in TBARS, ROS, and NOx formation in the PFC and HC of mice submitted to ARS. Noteworthy, CMI was also able to restore the mRNA expression of GR, NF-κB, iNOS, TNF-α, IDO, GSK-3β, and BDNF in the PFC and HC of mice. A summary of these results is shown in Table 2. Thus, the beneficial effects of CMI on behavior are associated with its capacity to modulate oxidative stress and neuroinflammation caused by immobilization stress. The hypothesis of a causal role of oxidative stress and neuroinflammation in the behavioral effects of restraint is supported by the significant correlations between the biochemical endpoints and immobility time in the TST.
Despite several reports having examined the ability of promising drugs to protect behavioral and biochemical modifications induced by ARS (Moretti et al. 2013; Freitas et al. 2014; Ai et al. 2017), few positive results have been obtained so far (Pesarico et al. 2015). Therefore, our study was designed to help to fulfill this gap and if possible increase the range of potential treatments for major depressive disorder. We show here that CMI has clearly the ability to treat stress-induced depression. This effect is independent of the dose at least in the range of doses tested in the present experiments. This lack of a dose-response effect has already been documented for other selenium-containing compounds (Pinto Brod et al. 2016; Domingues et al. 2018) and neuroprotective agents (Rosa et al. 2018).
The ARS is a widely used animal model to induce depressive-like behavior (Thakare et al. 2016; Surkin et al. 2018) akin to major depressive disorder symptoms, partially by targeting the brain antioxidant and inflammatory systems (Buynitsky and Mostofsky 2009; Spiers et al. 2016; Jope et al. 2017; Surkin et al. 2018). Indeed, the establishment of brain oxidative stress as a result of increased activity of nicotinamide adenosine dinucleotide phosphate (NADPH) oxidase (NOX) (Schiavone et al. 2009) and/or mitochondrial impairment (Jevtić et al. 2016) has been linked to neuropathogenetic alterations induced by different types of stress in rodents. In addition, stress procedure has been shown to cause alterations in amyloid beta (Aβ) release, contributing to the induction of depressive-like behavior in rodents (Morgese et al. 2017). The depressive-like behaviors induced by Aβ occur via impact on serotonergic and neurotrophin levels, HPA axis activation, and microglial TLR4 signaling (Justice et al. 2015; Morgese et al. 2014; Ledo et al. 2016). In the present study, we focused on the ability of CMI to counteract depressive-like behaviors via modulation of oxidative alterations and neuroinflammation, opening a window for future investigations related to mitochondrial function and Aβ signaling.
Hyperactivity of the HPA axis is the most common alteration found in patients with major depressive disorder (Stetler and Miller 2011). Increased levels of circulating corticosterone with concentrations in the range of those reported in other studies (Spiers et al. 2016) confirm the effectiveness of the ARS protocol employed in the present study. Additionally, the downregulation of GR mRNA expression in the PFC and HC of mice submitted to ARS could contribute to the reduced negative feedback loop that controls GC secretion. In accordance with this interpretation, we observed that the acute administration of CMI reversed the increasing plasmatic levels of corticosterone and upregulated the GR mRNA expression in the PFC and HC of mice submitted to ARS. These findings, together with the significant negative correlation with immobility time in the TST, show the ability of CMI to modulate hyperactivity of the HPA axis, an effect that could play a role in its antidepressant properties.
Stress is known to activate intracellular pathways involved in increasing free radical production. GC can induce neuronal oxidative stress by enhancing mitochondrial respiration and oxidative phosphorylation (You et al. 2009). NO is a neuromodulator able to control the release of corticosterone (Rettori et al. 2009), thus further influencing the stress response (Gądek-Michalska et al. 2016), including in the model of ARS (Chen et al. 2016; Surkin et al. 2018). Overall, RS produced during the stress promote lipid peroxidation, as evidenced by increased MDA formation (Maes et al. 2011; Niki 2012), that in fact is the major consequence of the oxidative stress in the brain (Niki 2012). In line with this, the present study showed a higher production of ROS, TBARS, and NOx and decreased mRNA expression of iNOS in the PFC and HC of mice submitted to the ARS, when compared to the non-stressed control. Interestingly, these oxidative alterations in mice subjected to ARS were reversed by a single dose of CMI. Considering that CMI was already capable of preventing oxidative alterations in LPS-challenged mice (Casaril et al. 2017a), this antioxidant effect of CMI is perhaps not surprising, but still very promising in terms of recovery from acute stressors. Since behavioral disturbances may be a reflection of increased oxidative alterations in the brains of stressed mice, we propose that the blockade of depression-like behavior by CMI treatment is mediated by its ability to decrease ROS, NOx, and TBARS generation, a hypothesis that is supported by the significant correlation among these variables.
Further analysis of oxidative stress endpoints confirmed alterations of SOD and CAT activities in the PFC and HC of mice submitted to the ARS protocol. We found significant increases in the SOD activity in the PFC and HC and a reduced CAT activity in the HC of mice submitted to the ARS. Previous studies have shown contradictory results about the activity of antioxidant enzymes in major depressive disorder (Selek et al. 2008; Kotan et al. 2011; Lukic et al. 2014; Tsai and Huang 2016). The reduced CAT activity in the HC of stressed and increased SOD activity observed in the present study are in agreement with data previously reported by others (Moretti et al. 2013; Freitas et al. 2014; Pesarico et al. 2015; Tsai and Huang 2016). Increased SOD activity accompanied by reduced CAT activity is an indicator of a pro-oxidative state, since SOD converts superoxide anion to hydrogen peroxide (H2O2), but CAT does not metabolize H2O2 to water (Halliwell 2007). This excessive production of H2O2 may favor the Fenton reaction and the generation of hydroxyl radical, which in turn triggers lipid peroxidation. In this way, SOD and CAT activities in stressed mice treated with CMI (1 and 10 mg/kg) were restored to values similar to those in the control group, pointing out the ability of CMI to normalize oxidative alterations in depressive mice. This effect of CMI could help to explain its ability to reverse the increased expression of NF-κB. H2O2 formed in the brain can rapidly diffuse to extracellular spaces, affecting neighboring cells and contributing to oxidative stress and activation of NF-κB. Therefore, the restauration of SOD and CAT activities after CMI administration could result in less H2O2 available to diffuse and influence the oxidative pathways. Another possible explanation for the ability of CMI to reduce the oxidative imbalance in the PFC and HC of mice submitted to ARS is related to the downregulation of the GSK-3β mRNA expression, which in turn could increase the synthesis of antioxidant enzymes (such as SOD and CAT) mediated by Nrf2.
In addition to this antioxidant effect, decreased GSK-3β signaling by CMI could reduce the expression of pro-inflammatory cytokines (such as TNF-α) through the reduction of NF-κB activation, explaining the downregulation of IDO mRNA in the PFC and HC after CMI treatment. Reduction of GSK-3β signaling may also increase BDNF expression by facilitating the cyclic AMP response element-binding protein (CREB) activity (Grimes and Jope 2001). It is important to note that stress can increase pro-inflammatory cytokines (García-Bueno et al. 2008), and the main consequence of a dysregulation of cytokine levels in the brain is the establishment of neuroinflammation and oxidative and nitrosative stress that could affect neurogenesis (Dantzer et al. 2008; Stepanichev et al. 2014). The antioxidant activity of CMI alongside with its ability to downregulate the expression of NF-κB and TNF-α induced by physical stress and IL-1β and IL-6 induced by LPS (Casaril et al. 2017a) and to upregulate BDNF mRNA indicates that CMI may be able to prevent impairment of neurogenesis. In addition, the reduction of pro-inflammatory cytokine expression evoked by the CMI treatment could account for the decreased corticosterone levels on stressed mice. Additionally, mitochondrial ROS (mtROS) can induce inflammatory cytokine responses (notably IL-1β and IL-6) and antioxidants that act within mitochondria, such as melatonin and indole-3-carboxylic acid, can reduce IL-1β and IL-6 levels via downregulation of NF-κB expression in endothelial cells (Lowes et al. 2011). In this regard, downregulation of NF-κB, IL-1β, and IL-6 mRNA by CMI could contribute to its antioxidant profile. Nonetheless, more studies are warranted to confirm the effect of CMI on mtROS and on the reduction in adult neurogenesis that takes place in response to inflammatory stimuli or environmental stress.
Alongside with the well-known effects of physical stress in the brain antioxidant and inflammatory systems, new evidences have suggested that increased intestinal permeability and consequently translocation of intestinal bacteria may influence TLR4 signaling pathway activation after acute restraint stress exposure (Gárate et al. 2013, 2014; MacDowell et al. 2015). Indeed, Gárate et al. (2014) have demonstrated that bacterial translocation is partially responsible for the physical stress-induced TLR4 mRNA upregulation and protein expression in rat brain frontal cortices. Therefore, the results presented here agree with previous evidence (Casaril et al. 2017b) suggesting that, by modulating the effects of inflammatory and oxidative mediators produced after TLR4 activation by DAMPs and PAMPs in an acute stress response, CMI can reverse the depression-like behaviors in mice (Fig. 6).
Summary of the effects of acute restraint stress (ARS) on brain oxidative and inflammatory pathways and possible targets of CMI. Administration of CMI after ARS downregulated the mRNA expression of GSK-3β, NF-kB, iNOS, and IDO in prefrontal cortex (PFC) and hippocampus (HC), while increased the expression of GR and BDNF in those brain structures. Treatment with CMI reversed the increased generation of RS, NOx metabolites, and lipid peroxidation in PFC and HC of mice. CMI also modulated the activity of SOD and CAT in PFC and HC and the plasmatic levels of corticosterone. Together, these effects contributed to improve the behavioral phenotype altered by ARS in mice
Conclusion
The inhibition of GSK-3β (Jope et al. 2017; Rosa et al. 2018; Rana and Singh 2018) and IDO (Felger and Lotrich 2013; Felger and Treadway 2017) and the reduction of neuroinflammation and oxidative stress (Maes et al. 2011; Felger and Lotrich 2013) have already been shown to represent novel treatment strategies to major depressive disorders beyond the classical therapies aimed at monoaminergic neurotransmission. Altogether, our data demonstrated that the oral administration of a selenium-containing compound with anti-oxidative and anti-inflammatory properties is able to reverse stress-induced increases in depression-like behavior. These data support the antidepressant properties of this type of compound and clearly encourage further studies to elucidate the pathways underlying these properties. Nevertheless, although we provided evidence of the involvement of the TLR4-NF-κB and Nrf2-GSK-3β pathways in the antidepressant-like effect of CMI through evaluation of mRNA expression, the present study is not sufficient to provide direct evidence supporting this hypothesis, which is the major limitation of this study. Therefore, the role of each of these targets deserves further studies to determine their relation to the antidepressant-like effect of CMI.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ai H, Shi XF, Hu XP, Fang WQ, Zhang B, Lu W (2017) Acute stress regulates phosphorylation of N-methyl-d-aspartate receptor GluN2B at S1284 in hippocampus. Neuroscience 351:24–35. https://doi.org/10.1016/j.neuroscience.2017.03.029
Birmann PT, Sousa FSS, de Oliveira DH, Domingues M, Vieira BM, Lenardão EJ, Savegnago L (2018) 3-(4-Chlorophenylselanyl)-1-methyl-1H-indole, a new selenium compound elicits an antinociceptive and anti-inflammatory effect in mice. Eur J Pharmacol 827:71–79. https://doi.org/10.1016/j.ejphar.2018.03.005
Brigelius-Flohé R, Flohé L (2017) Selenium and redox signaling. Arch Biochem Biophys 617:48–59. https://doi.org/10.1016/j.abb.2016.08.003
Browne CA, Lucki I (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. https://doi.org/10.3389/fphar.2013.00161
Buynitsky T, Mostofsky DI (2009) Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev 33:1089–1098. https://doi.org/10.1016/j.neubiorev.2009.05.004
Casaril AM, Domingues M, Fronza MG, Vieira B, Begnini K, Lenardão EJ, Seixas FK, Collares T, Nogueica C, Savegnago L (2017a) Antidepressant-like effect of a new selenium-containing compound is accompanied by a reduction of neuroinflammation and oxidative stress in lipopolysaccharide-challenged mice. J Psychoneuropharmacol
Casaril AM, Ignasiak MT, Chuang CY, Vieira B, Padilha NB, Carroll L, Lenardão EJ, Savegnago L, Davies MJ (2017b) Selenium-containing indolyl compounds: kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free Radic Biol Med 113:395–405. https://doi.org/10.1016/j.freeradbiomed.2017.10.344
Casaril AM, Martinez DM, Ricordi VG, Alves D, Lenardão EJ, Schultze E, Collares T, Seixas FK, Savegnago (2015) Evaluation of the toxicity of α-(phenylselanyl) acetophenone in mice. Regul Toxicol Pharmacol 73:868–874. doi: https://doi.org/10.1016/j.yrtph.2015.10.004
Chen H-JC, Spiers JG, Sernia C, Lavidis NA (2016) Acute restraint stress induces specific changes in nitric oxide production and inflammatory markers in the rat hippocampus and striatum. Free Radic Biol Med 90:219–229. https://doi.org/10.1016/j.freeradbiomed.2015.11.023
Dantzer R, O’Connor JC, Freund GG, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. https://doi.org/10.1038/nrn2297
Dobarro M, Orejana L, Aguirre N, Ramírez MJ (2013) Propranolol reduces cognitive deficits, amyloid β levels, tau phosphorylation and insulin resistance in response to chronic corticosterone administration. Int J Neuropsychopharmacol 16:1351–1360. https://doi.org/10.1017/S1461145712001393
Domingues M, Casaril AM, Birmann PT, de Lourenço DA, Vieira BM, Begnini K, Seixas FK, Collares T, Lenardão EJ, Savegnago L (2018) Selanylimidazopyridine prevents lipopolysaccharide-induced depressive-like behavior in mice by targeting neurotrophins and inflammatory/oxidative mediators. Front Neurosci 12:486. https://doi.org/10.3389/fnins.2018.00486
Elenkov IJ (2008) Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int 52:40–51. https://doi.org/10.1016/j.neuint.2007.06.037
Felger JC, Lotrich FE (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246:199–229. https://doi.org/10.1016/j.neuroscience.2013.04.060
Felger JC, Treadway MT (2017) Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42:216–241. https://doi.org/10.1038/npp.2016.143
Freitas AE, Bettio LEB, Neis VB, Santos DB, Ribeiro CM, Rosa PB, Farina M, Rodrigues AL (2014) Agmatine abolishes restraint stress-induced depressive-like behavior and hippocampal antioxidant imbalance in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 50:143–150. https://doi.org/10.1016/j.pnpbp.2013.12.012
Freitas AE, Machado DG, Budni J, Neis VB, Balen GO, Lopes MW, de SLF, Dalfre AL, Leal RB, Rodrigues AL (2013) Fluoxetine modulates hippocampal cell signaling pathways implicated in neuroplasticity in olfactory bulbectomized mice. Behav Brain Res 237:176–184. https://doi.org/10.1016/j.bbr.2012.09.035
Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J (2016) Psychosocial stress inhibits additional stress-induced hyperexpression of NO synthases and IL-1β in brain structures. Pharmacol Reports 68:1178–1196. https://doi.org/10.1016/J.PHAREP.2016.09.003
Gandin V, Khalkar P, Braude J, Fernandes AP (2018) Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic Biol Med 127:80–97. https://doi.org/10.1016/j.freeradbiomed.2018.05.001
Gárate I, García-Bueno B, Madrigal JL, Caso JR, Alou L, Gómez-Lus ML, Leza JC (2014) Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J Neuroinflammation 11:8. https://doi.org/10.1186/1742-2094-11-8
Gárate I, Garcia-Bueno B, Madrigal JLM, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC (2013) Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry 73:32–43. https://doi.org/10.1016/j.biopsych.2012.07.005
García-Bueno B, Caso JR, Leza JC (2008) Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 32:1136–1151. https://doi.org/10.1016/j.neubiorev.2008.04.001
Grimes CA, Jope RS (2001) CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem 78:1219–1232
Halliwell B (2007) Biochemistry of oxidative stress: Figure 1. Biochem Soc Trans 35:1147–1150. doi: https://doi.org/10.1042/BST0351147
Jevtić G, Nikolić T, Mirčić A, Stojković T, Velimirović M, Trajković V, Marković I, Trbovich AM, Radonjić NV, Petronijević N (2016) Mitochondrial impairment, apoptosis and autophagy in a rat brain as immediate and long-term effects of perinatal phencyclidine treatment — influence of restraint stress. Progr Neuro-Psychopharmacol Biol Psychiatry. 66:87–96
Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ (2006) Learning under stress: how does it work? Trends Cogn Sci 10:152–158. https://doi.org/10.1016/j.tics.2006.02.002
Jope RS, Cheng Y, Lowell JA, Worthen RJ, Sitbon YH, Beurel E (2017) Stressed and inflamed, can GSK3 be blamed? Trends Biochem Sci 42:180–192. https://doi.org/10.1016/j.tibs.2016.10.009
Justice NJ, Huang L, Tian JB, Cole A, Pruski M, Hunt AJ Jr, Flores R, Zhu MX, Arenkiel BR, Zheng H (2015) Posttraumatic stress disorder-like induction elevates β-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci 35:2612–1623. https://doi.org/10.1523/jneurosci.3333-14.2015
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116. https://doi.org/10.1146/annurev.pharmtox.46.120604.141046
Kil J, Lobarinas E, Spankovich C, Griffiths SK, Antonelli PJ, Lynch ED, Le Prell CG (2017) Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 390:969–979. https://doi.org/10.1016/S0140-6736(17)31791-9
Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S (2011) Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Prog Neuro-Psychopharmacol Biol Psychiatry 35:1284–1290. https://doi.org/10.1016/j.pnpbp.2011.03.021
Ledo JH, Azevedo EP, Beckman D, Ribeiro FC, Santos LE, Razolli DS, Kincheski GC, Melo HM, Bellio M, Teixeira AL, Velloso LZ, Foguel D, De Felice FG, Ferreira ST (2016) Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by Alzheimer’s amyloid-β oligomers in mice. J Neurosci 36:12106–12116. https://doi.org/10.1523/JNEUROSCI.1269-16.2016
Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva ALN, Mineo TWP, Gutierrez FR, Bellio M, Bortoluci KR, Flavell RA, Bozza MT, Silva JS, Zamboni DS (2013) Inflammasome-derived IL-1β production induces nitric oxide–mediated resistance to Leishmania. Nat Med 19:909–915. https://doi.org/10.1038/nm.3221
Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin JP, Mankhetkorn S (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat Phys Chem 72:323–331. https://doi.org/10.1016/J.RADPHYSCHEM.2004.06.011
Lowes DA, Almawash AM, Webster NR, Reid VL, Galley HF (2011) Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br J Anesthesia 107:193–201. https://doi.org/10.1093/bja/aer149
Lowry OH, Rosebrough NJ, Farr AL, RandalL RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lukic I, Mitic M, Djordjevic J, Tatalovic N, Bozovic N, Soldalovic I, Mihaljevic M, Pavlovic Z, Radojcic MB, Maric NP, Adzic M (2014) Lymphocyte levels of redox-sensitive transcription factors and antioxidative enzymes as indicators of pro-oxidative state in depressive patients. Neuropsychobiology 70:1–9. https://doi.org/10.1159/000362841
MacDowell K, Caso J, Martín-Hernández D, Madrigal JL, Leza JC, García-Bueno B (2015) Paliperidone prevents brain Toll-like receptor 4 pathway activation and neuroinflammation in rat models of acute and chronic restraint stress. Int J Neuropsychopharmacol 18. https://doi.org/10.1093/ijnp/pyu070
Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry 35:676–692. https://doi.org/10.1016/j.pnpbp.2010.05.004
Martinez DM, Barcellos A, Casaril AM, Savegnago L, Lenardão EJ (2014) Antidepressant-like activity of dehydrozingerone: involvement of the serotonergic and noradrenergic systems. Pharmacol Biochem Behav 127:111–117. https://doi.org/10.1016/j.pbb.2014.10.010
Masaki C, Sharpley AL, Cooper CM, Godlewska BR, Singh N, Vasudevan SR, Harmer CJ, Churchill CG, Sharp T, Rogers RS, Cowen PJ (2016) Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacology 233:2655–2661. https://doi.org/10.1007/s00213-016-4319-5
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moretti M, Budni J, dos Santos DB, Antunes A, Daufenbach JF, Manosso LM, Farina M, Rodrigues AL (2013) Protective effects of ascorbic acid on behavior and oxidative status of restraint-stressed mice. J Mol Neurosci 49:68–79. https://doi.org/10.1007/s12031-012-9892-4
Morgese MG, Tucci P, COlaianna M, Zotti M, Cuomo V, Schiavone S, Trabace L (2014) Modulatory activity of soluble beta amyloid on HPA axis function in rats. Curss Pharm Des 20:2539–2546. https://doi.org/10.2174/13816128113199990500
Morgese MG, Schiavone S, Trabace L (2017) Emerging role of amyloid beta in stress response: implication for depression and diabetes. E J Pharmacol 817:22–29. https://doi.org/10.1016/j.ejphar.2017.08.031
Niki E (2012) Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett 586:3767–3770. https://doi.org/10.1016/j.febslet.2012.09.025
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1:87–93. https://doi.org/10.4103/0976-500X.72350
Pesarico AP, Stangherlin EC, Mantovani AC, Zeni G, Nogueira CW (2015) 7-Fluoro-1,3-diphenylisoquinoline-1-amine abolishes depressive-like behavior and prefrontal cortical oxidative damage induced by acute restraint stress in mice. Physiol Behav 149:294–302. https://doi.org/10.1016/j.physbeh.2015.06.018
Pinto Brod LM, Fronza MG, Vargas JP, Ludtke DS, Luchese C, Wilhelm EA, Savegnago L (2016) Involvement of monoaminergic system in the antidepressant-like effect of (octylseleno)-xylofuranoside in the mouse tail suspension test. Prog Neuro-Psychopharmacol Biol Psychiatry 65:201–207. https://doi.org/10.1016/j.pnpbp.2015.10.008
Rana AK, Singh D (2018) Targeting glycogen synthase kinase-3 for oxidative stress and neuroinflammation: opportunities, challenges and future directions for cerebral stroke management. Neuropharmacology 139:124–136. https://doi.org/10.1016/J.NEUROPHARM.2018.07.006
Rettori V, Fernandez-Solari J, Mohn C, Zubilete MAZ, De La Cal C, Prestifilippo JP, De Laurentiis A (2009) Nitric oxide at the crossroad of immunoneuroendocrine interactions. Ann N Y Acad Sci 1153:35–47. https://doi.org/10.1111/j.1749-6632.2008.03968.x
Rosa JM, Pazini FL, Cunha MP, Colla ARS, Manosso LM, Mancini G, Souza ACG, de Bem AF, Prediger RS, Rodrigues ALS (2018) Antidepressant effects of creatine on amyloid β 1–40-treated mice: the role of GSK-3β/Nrf 2 pathway. Prog Neuro-Psychopharmacol Biol Psychiatry doi: https://doi.org/10.1016/j.pnpbp.2018.05.001, 86, 270, 278
Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause K-H (2009) Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66:384–392. https://doi.org/10.1016/j.biopsych.2009.04.033
Schwarcz R, Pellicciari R (2002) Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303:1–10. https://doi.org/10.1124/jpet.102.034439
Selek S, Savas HA, Gergerlioglu HS, Bulvul F, Uz E, Yumru M (2008) The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord 107:89–94. https://doi.org/10.1016/j.jad.2007.08.006
Singh N, Sharpley AL, Emir UE, Masaki C, Herzallah MM, Gluck MA, Sharp T, Harmer CJ, Vasudeven SR, Cowen PJ, Churchill GC (2016) Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep and emotional processing in humans. Neuropsychopharmacology 41:1768–1778. https://doi.org/10.1038/npp.2015.343
Spiers JG, Chen H-JC, Cuffe JSM, Sernia C, Lavidis NA (2016) Acute restraint stress induces rapid changes in central redox status and protective antioxidant genes in rats. Psychoneuroendocrinology 67:104–112. https://doi.org/10.1016/j.psyneuen.2016.02.005
Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gylyaeva N (2014) Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int 2014:1–20. https://doi.org/10.1155/2014/932757
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–126. https://doi.org/10.1097/PSY.0b013e31820ad12b
Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017. https://doi.org/10.1038/sj.npp.1300532
Sudati JH, Nogara PA, Saraiva RA, Wagner C, Alberto EE, Braga AL, Fachinetto R, Piquini PC, Rocha JBT (2018) Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: in vitro and in silico studies. Org Biomol Chem 16:3777–3787. https://doi.org/10.1039/c8ob00451j
Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert T, Thompson CL, Hawrylycz M, Dang C (2013) Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acid Res 41:D996–D1008. https://doi.org/10.1093/nar/gks1042
Surkin PN, Gallino SL, Luce V, Correa F, Solari JF, Laurentiis A (2018) Pharmacological augmentation of endocannabinoid signaling reduces the neuroendocrine response to stress. Psychoneuroendocrinology 87:131–140. https://doi.org/10.1016/j.psyneuen.2017.10.015
Thakare VN, Dhakane VD, Patel BM (2016) Potential antidepressant-like activity of silymarin in the acute restraint stress in mice: modulation of corticosterone and oxidative stress response in cerebral cortex and hippocampus. Pharmacol Reports 68:1020–1027. https://doi.org/10.1016/j.pharep.2016.06.002
Tsai M-C, Huang T-L (2016) Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res 235:38–42. https://doi.org/10.1016/j.psychres.2015.12.005
Vieira BM, Thurow S, Brito JS, Perin G, Alves D, Jacob R, Santi C, Lenardão EJ (2015) Sonochemistry: an efficient alternative to the synthesis of 3-selanylindoles using CuI as catalyst. Ultrason Sonochem 27:192–199. https://doi.org/10.1016/J.ULTSONCH.2015.05.012
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
You JM, Yun SJ, Nam KN, Kang C, Won R, Lee EH (2009) Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can J Physiol Pharmacol 87:440–447. https://doi.org/10.1139/y09-027
Zenker N, Bernstein DE (1958) The estimation of small amounts of corticosterone in rat plasma. J Biol Chem 231:695–701
Acknowledgements
The authors are grateful to UFPel and especially to the Biotechnology Graduate Program (UFPel) for providing support to carry out this work. CNPq is also acknowledged for the fellowship to LS, EL, FS, TC, and CWN.
Contributions
AMC and MD performed the experiments and the analysis of data and wrote the manuscript. SRB, DAL, and MS performed the experiments. AMC, MD, and LS designed the project. NBP and EJL synthesized the compound CMI. TC, FKS, LS, and CWN supervised the experiments. RD revised the scientific content of the manuscript and provided valuable intellectual insights. All authors critically reviewed the content and approved the final version for publication.
Role of funding source
This study received financial support and scholarships from the Brazilian agencies CNPq, CAPES, and FAPERGS (PRONEM 16/2551-0000240-1, PqG 17/2551-00011046-9, and FAPERGS/CAPES 04/2018 - DOCFIX 18/2551-0000511-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The studies were performed in accordance with protocols approved by the Committee on the Care and Use of Experimental Animal Resources at the Federal University of Pelotas, Brazil (4034-2017).
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Neuroimmune Signaling in Psychiatric Disease
Rights and permissions
About this article
Cite this article
Casaril, A.M., Domingues, M., Bampi, S.R. et al. The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: modulation of oxido-nitrosative stress and inflammatory pathway. Psychopharmacology 236, 2867–2880 (2019). https://doi.org/10.1007/s00213-018-5151-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5151-x