Abstract
Rationale
Preliminary evidence suggests that cannabidiol (CBD) may be effective in the treatment of neurodegenerative disorders; however, CBD has never been evaluated for the treatment of cognitive impairments associated with schizophrenia (CIAS).
Objective
This study compared the cognitive, symptomatic, and side effects of CBD versus placebo in a clinical trial.
Methods
This study was a 6-week, randomized, placebo-controlled, parallel group, fixed-dose study of oral CBD (600 mg/day) or placebo augmentation in 36 stable antipsychotic-treated patients diagnosed with chronic schizophrenia. All subjects completed the MATRICS Consensus Cognitive Battery (MCCB) at baseline and at end of 6 weeks of treatment. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) at baseline and biweekly.
Results
There was no main effect of time or drug on MCCB Composite score, but a significant drug × time effect was observed (p = 0.02). Post hoc analyses revealed that only placebo-treated subjects improved over time (p = 0.03). There was a significant decrease in PANSS Total scores over time (p < 0. 0001) but there was no significant drug × time interaction (p = 0.18). Side effects were similar between CBD and placebo, with the one exception being sedation, which was more prevalent in the CBD group.
Conclusions
At the dose studied, CBD augmentation was not associated with an improvement in MCCB or PANSS scores in stable antipsychotic-treated outpatients with schizophrenia. Overall, CBD was well tolerated with no worsening of mood, suicidality, or movement side effects.
Trial registration
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function (Heinrichs and Zakzanis 1998; Keefe et al. 2006). The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness, are not simply the result of the symptoms or the treatments and are thought to represent a core feature of the illness that persist even after other symptoms have been effectively treated (Riley et al. 2000). CIAS are more strongly predictive of functional outcome than any other symptom measure, including psychotic symptoms (Hughes et al. 2003). Most patients (~ 70%) appear to have moderate to severe cognitive impairments (Heinrichs and Zakzanis 1998; Keefe et al. 2005). Since existing antipsychotic drugs, all of which block dopamine (D2) receptors, have limited efficacy for CIAS (Buchanan et al. 2007), there is a need to develop treatments for CIAS that target other non-dopaminergic neurotransmitter systems. Several novel approaches have or are being tested for CIAS including pharmacological approaches targeting the glutamatergic system, cholinergic system including specific nicotinic receptors and non-pharmacological cognitive retraining (CRT) (Boggs et al. 2014; Bradley et al. 2010; D’Souza and Markou 2012; O’Donnell et al. 2010; Radek et al. 2010).
One potential target for improving CIAS is the endocannabinoid system that has been implicated in schizophrenia and in cognition (Leweke et al. 1999; Riedel and Davies 2005). The endocannabinoid system is comprised of two G-coupled receptors referred to as the cannabinoid 1 receptor (CB1R) and the cannabinoid 2 receptor (CB2R). While both are present in the brain and periphery, the former is primarily localized in the brain and the latter in the periphery (Devane et al. 1988; Schatz et al. 1997). The primary endocannabinoid ligands are anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and the primary catabolic enzymes for these ligands are fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively (Mechoulam and Parker 2013). CB1Rs are highly prevalent in areas associated with cognition including the hippocampus, cerebral cortex, basal ganglia, and cerebellum (Eggan and Lewis 2007). Studies in animals and humans have demonstrated that both synthetic and phytocannabinoids produce impairments in memory and attention. Furthermore, chronic cannabis exposure is known to disrupt attention, behavioral inhibition, verbal memory, and working memory/executive function (Ranganathan and D’Souza 2006). Acute administration of delta-9-tetrahydrocannabinol (THC), the main psychoactive component of cannabis and CB1R partial agonist, produces robust deficits in verbal learning, attention, and working memory (D’Souza et al. 2004), and schizophrenia patients are more vulnerable to the cognitive-impairing effects of THC compared to healthy controls (D’Souza et al. 2005). In summary, given that the endocannabinoid system is implicated in schizophrenia and CB1R agonists produce robust cognitive deficits, we hypothesized that manipulation of brain endocannabinoid function via CB1R antagonism/inverse agonism or modulating endocannabinoid levels might offer a novel target for reducing CIAS.
Cannabidiol (CBD), one of over 100 plant cannabinoids or phytocannabinoids isolated from Cannabis sativa, (ElSohly et al. 2017), is a constituent in herbal cannabis (Izzo et al. 2009), and unlike THC, it does not produce any psychotomimetic effects (Zuardi et al. 1993).THC, and other CB1Rs agonists, reliably produce robust deficits in verbal memory on the HVLT (D’Souza et al. 2004; Ranganathan and D’Souza 2006) in healthy volunteers and schizophrenia patients (D’Souza et al. 2005), and verbal memory deficits also occur in chronic users of cannabis (Morgan et al. 2010). Further, amongst cannabis users, a higher concentration of CBD in the cannabis used was correlated with lesser verbal memory impairments (Morgan et al. 2010) representing either a neuroprotective or pro-cognitive effect. In addition, non-human primate studies have also shown a protective effect of CBD for acute cognitive deficits produced by THC (Murphy et al. 2017). However, the effects of CBD on CIAS has not been studied to our knowledge.
Preclinical studies suggest that CBD may also have antipsychotic properties (Gomes et al. 2015; Moreira and Guimaraes 2005). Consistent with this, Leweke et al. 2012 demonstrated that CBD was as effective as amisulpride in ameliorating psychotic symptoms in decompensated patients with schizophrenia (Leweke et al. 2012). Further, Leweke et al. suggest that FAAH inhibition may underlie CBD’s antipsychotic effects (Hallak et al. 2010; Leweke et al. 2012; Zuardi et al. 2006; Zuardi et al. 1995). However, CBD may have many different pharmacological effects on the endocannabinoid system (Ibeas Bih et al. 2015). Further, whether CBD has antipsychotic effects in patients with schizophrenia that are psychiatrically stable has not been studied in placebo controlled trials.
The current study aimed to examine the effects of CBD on CIAS using the MATRICS Consensus Cognitive Battery (MCCB) and on psychotic symptoms using the Positive and Negative Syndrome Scale (PANSS).
Methods
Study design
In this 6-week, randomized, placebo-controlled, parallel group, double-blinded, fixed-dose trial, the effects of oral CBD 300 mg BID, (600 mg/daily), added to a stable dose of antipsychotic medication were studied in 36 patients diagnosed with schizophrenia. Adherence to study medication was monitored each week by self-report. The study was conducted in the Schizophrenia Neuropharmacology Research Group at Yale (SNRGY) spanning VA Connecticut Healthcare System, West Haven, Connecticut, and the Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven, Connecticut. The study was conducted under the purview of the Institutional Review Boards of both Yale University and VA Connecticut Healthcare System, and the US FDA (IND #101,185) and in compliance with ICH guidelines. CBD was obtained from STI Pharmaceuticals. The study was registered with clinicaltrials.gov (NCT 00588731). Subjects were recruited using local advertisements and word of mouth and were paid for their participation in the research.
Consent process
Subjects who met entry criteria were invited to meet with the research staff, who explained risks and procedures as outlined in the consent form. After reviewing this information and answering questions, informed consent was obtained from all subjects. A copy of the consent form was provided to all subjects.
Sample size
In the absence of controlled data showing medication-induced improvement in HVLT total immediate recall improvement in schizophrenia, we determined, based on the mean total recall scores from the CATIE study, that for a two-tailed independent t test with alpha = 0.05 and 80% statistical power to detect a 12% or greater increase in total recall due to add-on CBD treatment compared to the levels with antipsychotic treatment alone (18.7 ± 2.11), we would need 15 subjects per group. With an expected dropout rate of 20%, a total number of 36 subjects were studied over 3 years.
Screening
During screening, cognitive ability was measured using the Hopkins Verbal Learning Test (HVLT) (Brandt 1991), and IQ was measured by the Wechsler Adult Intelligence Scale (WAIS) (Wechsler 1955). Verbal memory deficits represent the largest effect size difference in schizophrenia compared to the general population (Heinrichs and Zakzanis 1998); the CATIE study found that the mean HVLT total immediate recall score was 18.73 (out of a maximum possible score of 36) in antipsychotic-treated patients with schizophrenia as compared to 28.16 for the general population (Keefe et al. 2006; Riley et al. 2000; Rund et al. 2004). Subjects who scored less than or equal to 1 standard deviation below the mean for the general population on HVLT (Brandt 1991) total immediate recall were included in the study. Since the composite MCCB score is recommended as the gold standard for determining cognitive-enhancing effects of medications (Harvey and Bowie 2012), it was used as the primary cognitive outcome.
Inclusion/exclusion criteria
Male and female subjects 18–65 years of age, with a DSM-IV-TR diagnosis of schizophrenia, were included in the study. Subjects had at least 3 months of treatment with stable doses (no dose change in 4 weeks) of antipsychotic medication. Subjects were excluded for any other past or current DSM-IV-TR axis I diagnosis that required pharmacologic treatment, DSM-IV-TR diagnosis of substance abuse in the past 3 months or dependence in the past 6 months (excluding nicotine), any serious medical or neurological disorder, pregnant or nursing females or those not willing to use appropriate birth control, history of electroconvulsive treatment in the past 3 months, currently enrolled in a weight loss program, previous recent exposure to the HVLT, and treatment with clozapine, cognitive enhancers, or other investigational agents during the study.
Outcome measures
Cognitive Assessments Cognition was assessed using the T score of the MCCB composite and subscales at baseline and end of study after 6 weeks of study medication (Nuechterlein et al. 2008). An increase in MCCB T score indicates an improvement in cognitive ability.
Psychiatric Assessments Psychotic symptoms were assessed using the PANSS at baseline, week 2, week 4, and week 6 to measure symptom severity (Kay et al. 1987).
Safety Assessments Motor side effects were measured using the Barnes Akathisia Scale (BAS) (Barnes 1989), Simpson Angus Scale (SAS) (Simpson and Angus 1970), and the Abnormal Involuntary Movements Scale (AIMS) (Guy 1976) every 2 weeks. Additional side effects were measured weekly using the UKU-Side Effect Scale (Lingjaerde et al. 1987).
Statistical analysis
Linear mixed models with treatment (placebo, CBD) included as a between-subject factor and time as a within-subject factor were used to analyze each outcome. The interaction between treatment and time was also modeled. PANSS outcomes were analyzed at baseline, 2, 4, and 6 weeks; all other outcomes were analyzed at baseline and 6 weeks. The best-fitting variance-covariance structure was chosen based on the Schwartz-Bayesian Information Criterion (BIC). Least square means were estimated and plotted to assess significant effect. All analyses were conducted using SAS, version 9.4 (Cary, NC).
Results
Subjects
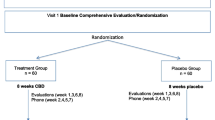
The study was conducted between September 2009 and May 2012. In total, 41 participants were randomized in the study and 39 received study medication: CBD (n = 20), placebo (n = 19) (see Fig. 1). Subject demographics and ratings during screening, for each arm of the study, can be seen in Table 1. In the CBD arm, one subject had a medication change in his antipsychotic after randomization, a protocol violation, and was thus withdrawn from the study before week 2. A second subject in the CBD group reported significant sedation and withdrew before week 2 assessments. In the placebo arm, two subjects voluntarily withdrew, one before week 2 assessments and another before week 6 assessments. This resulted in 18 participants in each arm having at least one assessment after baseline. The progression of subjects from recruitment through the end of study is shown in Fig. 1.
Cognition
Baseline HVLTs, the primary variable for randomization, were similar at baseline between the two groups (see Fig. 2a). There was no main effect of Drug or Time on MCCB Composite score, but a significant drug × time effect was observed (F (1, 32) = 5.94; p = 0.02)(see Fig. 2b). Post hoc analyses revealed that only placebo-treated subjects improved over time (F (1, 32) = 4.84; p = 0.03).
For MCCB subscales, on the Reasoning and Problem Solving domain, there was a trend toward a main effect of time (F (1, 33) = 3.48; p = 0.07) and a drug × time interaction (F (1, 33) = 4.47; p = 0.04) (see Table 2). Post hoc analyses revealed that only placebo-treated subjects improved over time (F (1, 33) = 7.71; p = 0.009).
Psychopathology
Overall, there was a main effect of time, such that there was a significant decrease in PANSS Total scores over time (F (3, 101) = 10.62; p < 0.0001) but there was no significant drug × time interaction (F (3, 101) = 1.66; p = 0. 18) (see Fig. 3). Similarly, there was a significant effect of time for PANSS General (F (3, 101) = 4.55; p = 0.005), PANSS Negative (F (3, 101) = 2.63; p = 0.05), and PANSS Positive (F (3, 101) = .11; p < 0.001) scores, such that the scores decreased with time, but there was no drug × time interaction (p = 0.56, p = 0.26, p = 0.55; respectively).
Positive and Negative Syndrome Scale (PANSS) scores over 6 weeks with cannabidiol or placebo. a PANSS Positive subscale mean (SD) scores. b PANSS Negative subscale mean (SD) scores. c PANSS General subscale mean (SD) scores. d PANSS Total score mean (SD) scores; green square (cannabidiol), red diamond (placebo)
Side effects
There were no significant time, drug, or drug × time interactive effects on the movement side effects as measured by the SAS: CBD (baseline 2.6 ± 2.9, endpoint 2.3 ± 2.8; mean ± SD) versus placebo (baseline 2.9 ± 3.4, endpoint 1.6 ± 1.9); (F (8, 129) = 0.35; p = 0.94); BAS: CBD (baseline 0.58 ± 0.96, endpoint 0.35 ± 0.86) versus placebo (baseline 0.44 ± 0.78, endpoint 0.07 ± 0.26); (F (8, 130) = 0.857; p = 0.55); or AIMS: CBD (baseline 6.6 ± 8.4, endpoint 4.5 ± 7.2) versus placebo (baseline 6.8 ± 8.1, endpoint 5.5 ± 6.5); (F (7, 131) = 0.32; p = 0.94). Other reported sided effects were similar between CBD and placebo, with the one exception being sedation (see Supplemental Table 1), which was more prevalent in the CBD group. One subject in the CBD arm withdrew early in the study due to sedation. During the study, approximately 20% of participants in the CBD arm reported sedation (mild) and 5% reported sedation in the placebo arm.
Discussion
This study was designed to examine the effects of augmentation of CBD on CIAS and psychotic symptoms in chronically ill, stable outpatients with schizophrenia. At the dose tested, CBD augmentation for 6 weeks did not improve MCCB performance or psychotic symptoms in this sample of patients.
Although patients in the CBD arm had no change in MCCB performance, patients in the placebo arm did show improvements on the MCCB Composite Score and Reasoning and Problem Solving domain (see Table 2). These improvements were small (Cohen’s d: MCCB Composite = 0.28, Reasoning and Problem Solving = 0.33) and of questionable clinical significance. Both the MCCB Composite Score and Reasoning and Problem Solving domain scores were higher at baseline and endpoint for the CBD-treated group, suggesting that the observed improvement in the placebo arm could also represent a regression to the mean. A second explanation for the improvement on placebo may be practice effects that have been previously noted on the MCCB (Nuechterlein et al. 2008). The fact that CBD treatment was not associated with a similar improvement could be related to the greater sedation (20% of subjects) observed with CBD as compared to placebo (5%). Although the presence/absence of sedation was noted in this study, the degree of sedation was not systematically quantified during cognitive testing and therefore not covaried for, which should be assessed in future studies. An alternative explanation is that CBD hampered the expected practice-related learning on the MCCB unrelated to its effects on sedation. This, however, is contrary to the expected effects of CBD based on preclinical and clinical data (Fagherazzi et al. 2012; Magen et al. 2010; Morgan et al. 2010) suggesting that CBD may have pro-cognitive effects. For instance, CBD has been shown to attenuate induced cognitive deficits both in mice (Magen et al. 2010; Murphy et al. 2017) and rats (Fagherazzi et al. 2012) and to enhance the expression of hippocampal brain-derived neurotropic factor (BDNF) and has been shown to have benefits in neurodegenerative conditions (Iuvone et al. 2009).
Although limited, the existing preclinical and epidemiological data suggest that CBD may improve cognition. That CBD did not improve MCCB performance in this study, however, is consistent with data from other clinical trials with CBD in schizophrenia. For instance, Hallak et al. examined the effects of a single dose of CBD (600 mg, 300 mg, or placebo) on the STROOP color word task in a randomized study in patients with schizophrenia (Hallak et al. 2010) and found no benefits. Further, a recently published study of CBD (1000 mg) in schizophrenia over 6 weeks also failed to demonstrate cognitive benefits on the Brief Assessment of Cognition in Schizophrenia (BACS) (McGuire et al. 2017). Thus, the current data suggest that CBD at a wide range of doses tested does not have beneficial effects on cognition in schizophrenia.
CBD did not improve psychotic symptoms in the subjects in our study. These results are in contrast to the published case reports (Zuardi et al. 2006; Zuardi et al. 1995), and the two published clinical trials in schizophrenia (Leweke et al. 2012)(McGuire et al. 2017). Leweke et al. found CBD (800 mg) to be as efficacious as amisulpride in reducing positive psychotic symptoms in 42 acutely decompensated patients with schizophrenia (Leweke et al. 2012). More recently, in a larger study in antipsychotic-treated outpatients with schizophrenia (n = 86), McGuire et al. found that CBD augmentation resulted in a small although statistically significant improvement in PANSS positive scores (1.5 points) with CBD compared to placebo (McGuire et al. 2017). However, our results are similar to a separate study also by Leweke et al. who tested the effects of the same dose (600 mg) of CBD in schizophrenia. At this dose, CBD only produced very small improvements in PANSS total scores (~ 2.4) that were not statistically significant (Leweke et al. 2014). Although, this dose (600 mg/day) has been shown to attenuate psychosis-like effects in acute laboratory studies (Bhattacharyya et al. 2010), it appears that a higher dose may be needed to produce beneficial effects on psychotic symptoms in schizophrenia.
A second consideration worth discussing is the stage of illness being tested. Our study included patients with chronic schizophrenia unlike those studied in Leweke et al. who have also demonstrated that patients demonstrate alterations in endocannabinoid levels during early psychosis (Koethe et al. 2009; Leweke et al. 2012). Thus, it is possible that CBD may be even more effective during this critical period rather than in chronic schizophrenia and more studies are needed on the benefits of CBD earlier in the course of psychosis, perhaps even during the prodromal stage. This may be particularly relevant to CIAS. In our study, the mean age of participants was in their mid- to late-40s and mean illness duration was greater than 25 years similar to McGuire et al. 2017 (mean age 41 years) who also failed to demonstrate any benefits on CIAS in their study (McGuire et al. 2017). Interestingly, given the data that cannabis use during adolescence may be associated with a less cognitively severe form of schizophrenia (Yucel et al. 2012), more studies are needed to fully examine the endocannabinoid system as a potential target for the cognitive deficits of schizophrenia.
Overall, subjects in our study tolerated CBD treatment well with no worsening of psychosis, mood, or suicidality. One subject in the CBD arm withdrew within the first 2 weeks due to sedation, and overall, more subjects reported sedation with CBD than placebo. These data are consistent with the previous studies with CBD, suggesting that CBD is well tolerated and not associated with significant motor side effects or laboratory abnormalities (Leweke et al. 2012; McGuire et al. 2017).
Strengths and limitations
A major strength of the study is the design and use of the widely accepted MCCB to measure cognitive deficits. However, there are some limitations to this study. While the CBD dose of 600 mg/day has been used in previous studies, it is lower than Leweke et al. 2012 and McGuire et al. 2017 who used 800 and 1000 mg, respectively. Given that CBD has a low oral bioavailability of about 15% (Scuderi et al. 2009) and higher doses have been tolerated in other studies (Zuardi et al. 2006; Zuardi et al. 1995), it is possible that higher doses may have had beneficial effects on psychosis and possibly CIAS. Second, it is possible that pharmacotherapy alone may be insufficient to produce improvements in CIAS and recent studies combine pharmacotherapy with a cognitive remediation strategy; however, this was not included in this study (D’Souza et al. 2013).
Conclusions
While adjunctive CBD with antipsychotic treatment was well tolerated, it was not effective in treating CIAS. Future studies should assess if CBD treatment earlier in the course of illness is beneficial. Finally, more studies should be conducted to determine an optimal oral dose of CBD for treatment of symptoms and cognitive deficits in schizophrenia.
References
Barnes TR (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676
Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, O'Carroll CM, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK (2010) Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 35:764–774
Boggs DL, Carlson J, Cortes-Briones J, Krystal JH, Cyril D’Souza D (2014) Going up in smoke? A review of nAChRs-based treatment strategies for improving cognition in schizophrenia. Curr Pharm Des 20:5077–5092
Bradley SR, Lameh J, Ohrmund L, Son T, Bajpai A, Nguyen D, Friberg M, Burstein ES, Spalding TA, Ott TR, Schiffer HH, Tabatabaei A, McFarland K, Davis RE, Bonhaus DW (2010) AC-260584, an orally bioavailable M(1) muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology 58:365–373
Brandt J (1991) The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 5:125–142
Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA (2007) Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull 33:1120–1130
D’Souza MS, Markou A (2012) Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology 62:1564–1573
D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH (2004) The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 29:1558–1572
D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH (2005) Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry 57:594–608
D’Souza DC, Radhakrishnan R, Perry E, Bhakta S, Singh NM, Yadav R, Abi-Saab D, Pittman B, Chaturvedi SK, Sharma MP, Bell M, Andrade C (2013) Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 38:492–503
Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613
Eggan SM, Lewis DA (2007) Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex 17:175–191
ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A (2017) Phytochemistry of Cannabis sativa L Phytocannabinoids. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J (eds) Progress in the chemistry of organic natural products, vol. 103. Phytocannabinoids: unraveling the complex chemistry and pharmacology of Cannabis sativa. Springer International Publishing, New York, pp 1–36
Fagherazzi EV, Garcia VA, Maurmann N, Bervanger T, Halmenschlager LH, Busato SB, Hallak JE, Zuardi AW, Crippa JA, Schroder N (2012) Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology 219:1133–1140
Gomes FV, Llorente R, Del Bel EA, Viveros MP, Lopez-Gallardo M, Guimaraes FS (2015) Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr Res 164:155–163
Guy W (1976) Abnormal involuntary movement scale (AIMS). ECDEU Assess Manual Psychopharmacol 338:534–537
Hallak JE, Machado-de-Sousa JP, Crippa JA, Sanches RF, Trzesniak C, Chaves C, Bernardo SA, Regalo SC, Zuardi AW (2010) Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr 32:56–61
Harvey PD, Bowie CR (2012) Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am 35:683–698
Heinrichs RW, Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12:426–445
Hughes C, Kumari V, Soni W, Das M, Binneman B, Drozd S, O’Neil S, Mathew V, Sharma T (2003) Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res 59:137–146
Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ (2015) Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12:699–730
Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L (2009) Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther 15:65–75
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30:515–527
Kay SR, Flszbein A, Opfer LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261
Keefe RS, Eesley CE, Poe MP (2005) Defining a cognitive function decrement in schizophrenia. Biol Psychiatry 57:688–691
Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, del Miller D, Canive JM, Adler LW, Manschreck TC, Swartz M, Rosenheck R, Perkins DO, Walker TM, Stroup TS, McEvoy JP, Lieberman JA (2006) Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology 31:2033–2046
Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, Klosterkotter J, Piomelli D, Leweke FM (2009) Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry J Ment Sci 194:371–372
Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D (1999) Elevated endogenous cannabinoids in schizophrenia. Neuroreport 10:1665–1669
Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkotter J, Hellmich M, Koethe D (2012) Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2:e94
Leweke FM, Hellmich M, Pahlisch F, Kranaster L, Koethe D (2014) Modulation of the endocannabiniod system as a potential new target in the treatment of schizophrenia. Schizophr Res 153:S47
Lingjaerde O, Ahlfors U, Bech P, Dencker S, Elgen K (1987) The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand 76:1–100
Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM (2010) Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol 159:950–957
McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S (2017) Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a Multicenter Randomized Controlled Trial. Am J Psychiatry 175(3):225–231
Mechoulam R, Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47
Moreira FA, Guimaraes FS (2005) Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur J Pharmacol 512:199–205
Morgan CJ, Schafer G, Freeman TP, Curran HV (2010) Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry J Ment Sci 197:285–290
Murphy M, Mills S, Winstone J, Leishman E, Wager-Miller J, Bradshaw H, Mackie K (2017) Chronic adolescent Δ9-tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis Cannabinoid Res 2:235–246
Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR (2008) The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165:203–213
O’Donnell CJ, Rogers BN, Bronk BS, Bryce DK, Coe JW, Cook KK, Duplantier AJ, Evrard E, Hajos M, Hoffmann WE, Hurst RS, Maklad N, Mather RJ, McLean S, Nedza FM, O’Neill BT, Peng L, Qian W, Rottas MM, Sands SB, Schmidt AW, Shrikhande AV, Spracklin DK, Wong DF, Zhang A, Zhang L (2010) Discovery of 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane (CP-810,123), a novel alpha 7 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders in schizophrenia: synthesis, SAR development, and in vivo efficacy in cognition models. J Med Chem 53:1222–1237
Radek RJ, Kohlhaas KL, Rueter LE, Mohler EG (2010) Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des 16:309–322
Ranganathan M, D’Souza DC (2006) The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology 188:425–444
Riedel G, Davies SN (2005) Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol 168:445–477
Riley EM, McGovern D, Mockler D, Doku VC, OCeallaigh S, Fannon DG, Tennakoon L, Santamaria M, Soni W, Morris RG, Sharma T (2000) Neuropsychological functioning in first-episode psychosis—evidence of specific deficits. Schizophr Res 43:47–55
Rund BR, Melle I, Friis S, Larsen TK, Midboe LJ, Opjordsmoen S, Simonsen E, Vaglum P, McGlashan T (2004) Neurocognitive dysfunction in first-episode psychosis: correlates with symptoms, premorbid adjustment, and duration of untreated psychosis. Am J Psychiatry 161:466–472
Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE (1997) Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol 142:278–287
Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (2009) Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res: PTR 23:597–602
Simpson G, Angus J (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 45:11–19
Wechsler D (1955) Manual: Wechsler adult intelligence scale. Psychological Corp, New York
Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD, Pantelis C (2012) The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull 38:316–330
Zuardi AW, Guimaraes FS, Moreira AC (1993) Effect of cannabidiol on plasma prolactin, growth hormone and cortisol in human volunteers. Braz J Med Biol Res 26:213–217
Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R (1995) Antipsychotic effect of cannabidiol. J Clin Psychiatry 56:485–486
Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, Crippa JA (2006) Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol 20:683–686
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Support granted by the Stanely Medical Research Institute.
Mohini Ranganathan has in the past 3 years or currently received research grant support administered through Yale University School of Medicine from Insys Therapeutics and Pfizer Inc. Deepak Cyril D’Souza has in the past 3 years or currently received research grant support administered through Yale University School of Medicine from Pfizer Inc. Pfizer, Inc. had a role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication. Andrew Davies is a founder and full time employee of STI Pharmaceuticals Ltd (UK). Douglas Boggs, Aarti Gupta, John Cahill, Brian Pittman, Ashley Schnakenberg Martin, Halle Thurnauer, Swapnil Gupta, and Toral Surti report no financial relationships with commercial interests.
Electronic supplementary material
Supplemental Table 1
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Boggs, D.L., Surti, T., Gupta, A. et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 235, 1923–1932 (2018). https://doi.org/10.1007/s00213-018-4885-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4885-9