Abstract
Background
The acute and delayed effect of analgesic-range doses of ketamine on neurocognitive and behavioural outcomes is understudied. Using a non-controlled open-labelled design, three (1-h duration) increasing intravenous (IV) ketamine infusions comprising (i) 30 mg bolus of ketamine + 8 mg/h IV infusion, (ii) 12 mg/h IV infusion and (iii) 20 mg/h infusion were administered to 20 participants (15 male, 5 female, mean age = 30.8 years). Whole-blood ketamine and norketamine concentrations were determined at each treatment step and post-infusion.
Methods
The Cambridge Neuropsychological Test Automated Battery (CANTAB) was used to assess reaction/movement time (RTI, Simple and 5-Choice), visuospatial working memory (SWM), spatial planning (SOC) and subjective effects (visual analogue scale; VAS) during treatment and at post-treatment.
Results
Significant main effects were reported for time (dose) on CANTAB RTI 5-Choice reaction (F(4,18) = 3.41, p = 0.029) and movement time (F(4,18) = 4.42, p = 0.011), SWM (F(4,18) = 4.19, p = 0.014) and SOC (F(4,18) = 4.13, p = 0.015), but not RTI Simple reaction or movement time. Post hoc analyses revealed dose-dependent effects for both RTI 5-Choice reaction and movement time (all p < 0.05). Post-treatment performance on all neurocognitive and behavioural tasks returned to baseline levels. Regression analyses revealed a weak positive linear association between SWM ‘strategy’ score (R2 = 0.103, p < 0.001), all performance-based CANTAB VAS items (R2 range 0.005–0.137, all p < 0.05) and ketamine blood concentrations.
Discussion
The open-label, non-controlled trial design somewhat precludes the ability to adequately account for random treatment effects. Notwithstanding, these results suggest that analgesic doses of ketamine produce acute, selective, dose-dependent deficits in higher-order neurocognitive and behavioural domains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonist utilised in critical care medicine as an adjunct therapy to optimise and maintain analgesia and anaesthesia (Bristow and Orlikowski 1989). At doses indicated for pain relief, ketamine inhibits NMDA activity, inducing an analgesic effect (Hirota and Lambert 1996). At higher doses (between 0.5 and 1.5 mg kg−1 intravenous infusion), division of the thalamo-neocortical regions and the limbic system occur, resulting in a rapid, state-reversible ‘dissociative’ form of anaesthesia (White et al. 1982). Even at subanaesthetic doses, administration of ketamine induces a clinical syndrome characterised by marked impairments in memory (Wong et al. 2016), neurocognition (Olofsen et al. 2012), and psychomotor performance (Krystal et al. 1994) and elicits potent psychotomimetic effects (Bowdle et al. 1998).
Ketamine affects various aspects of neurocognitive and behavioural ability via NMDA receptor hypo-function, specific disruption of long-term potentiation initiation within the hippocampus and the cerebral cortex (Malhotra et al. 1996; Moghaddam et al. 1997; Newcomer et al. 1999), and via targeted NMDA-receptor blockade (Anis et al. 1983; Guillermain et al. 2001). Dose-dependent, additive effects (Guillermain et al. 2001) are observed on tasks assessing balance, simple psychomotor speed (Carter et al. 2013) and reaction time (Rogers et al. 2004). Deficits in foreperiod duration (skills relevant to motor preparation) under ketamine conditions have also been observed in extended assessment protocols (Micallef et al. 2004); however, examination as to these behavioural effects at analgesic-range doses, particularly under acute conditions, is comparatively understudied. Disruption to working memory-specific processes, particularly those involving mental manipulation (but not maintenance), has similarly been observed (Honey et al. 2003). Importantly, these effects appear differentially targeted by ketamine depending on dose (Harborne et al. 1996), task and/or drug timing (Honey et al. 2003), as some inconsistencies remain with regard to the exact domains affected. Finally, ketamine-induced NMDA receptor dysfunction in the frontal cortex has been shown to elicit marked deficits in several executive cognition abilities, including procedural learning (Krystal et al. 2000) and processing speed (Morgan et al. 2003). However, these findings are somewhat limited in their application due the often high-range dosing regimen used and the specific contextualisation of impairment within the schizophrenic syndrome only. As such, it is unclear if these effects are similarly represented at lower, analgesic doses, or whether more general executive function abilities are affected.
Measures of higher-order neurocognitive functioning and behavioural abilities are pivotal in the planning, initiation, sequencing and monitoring of complex goal-directed behaviours (Jurado and Rosselli 2007), and evaluation of functional capacity remains an important component of acute and longer-term patient care (Crisp et al. 2000). At present, some discrepancy remains with regard to the effect of analgesic-range doses of ketamine where select higher-order neurocognitive skills (Morgan and Curran 2006), or behavioural abilities, are assessed (Harborne et al. 1996). Indeed, much of the existing literature examines abstract or nonrepresentational aspects of neurocognitive and behavioural functioning under high-range dosing paradigms only (i.e. contextualised as pathophysiological model of schizophrenia only), and thus it is unclear whether these effects are transferable to more applied aspects of functioning, and if these deficits occur at doses indicated for analgesia. Many of these effects are observed to be dose-dependent and are elicited under stepped-administration (clinical) protocols (Malhotra et al. 1997). Thus, examination as to whether deficits in neurocognitive and behavioural abilities are similarly observed using this dosing paradigm has the potential to inform both clinical and adjunct treatment practices for those receiving ketamine at these therapeutically indicated, analgesic doses.
This study therefore aims to examine the effect of three stepwise, increasing analgesic doses of intravenously administered ketamine on a range of neurocognitive and behavioural functions in a sample of healthy adults. This study incorporates aspects of lower- and higher-order cognitive and behavioural performance assessed at each treatment step and at post-treatment in order to identify specific acute and extended deficits in these domains. It is anticipated that these analgesic-range doses of ketamine will induce dose-dependent impairments in reaction time and will disrupt the target domains of executive function and working memory.
Methods
Participants
A total of 20 volunteers comprising 5 females and 15 males, aged between 23 and 40 years (mean age 30.8, SD ± 4.8, mean weight 79.2 kg, SD ± 12.3) attended a single session in a designed-for-purpose area at the Monash Health Translational Research Precinct, Melbourne. All participants indicated previous use of sedating-type medications with no known allergic or adverse reactions and were screened prior to participation to ensure that they had no history of significant cardiovascular, respiratory, endocrine, gastrointestinal, bleeding, neurological or renal disorders; current or past diagnosis of substance abuse; and other medical illness. Current use of any psychiatric medication was a proxy indicator of significant psychiatric history and was considered cause for exclusion. Alcohol consumption was prohibited 24 h preceding the tests, and caffeine was prohibited on the morning of the study. Prior to testing, participants were screened for evidence of recent use of drugs [amphetamine/d-methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA), cocaine, cannabis (delta-9-tetrahydrocannabinol), opiates and ketamine] using the Securetec DrugWipe 6s device (Aberl and VanDine 2005). Positive results were cause for exclusion from the study. The research was approved by Monash Health Human Research Ethics committee (approval number: HREC/16/MonH/240). All participants provided written informed consent prior to commencing any study procedures. This study was registered on the Australia and New Zealand Clinical Trials registry (www.anzctr.com.au); trial number ACTRN12616001485426.
Acute drug administration and biological sampling
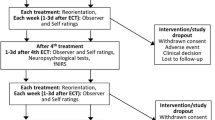
Ketamine solution was infused via a cannula placed in a cubital fossa vein in the non-dominant arm. Whole-blood samples were similarly extracted from this single cannula. S(+)-ketamine was added to 0.9% NaCl to produce 100 mL (200 mg ketamine in 100 ml NaCl = 2 mg/mL) solution and was delivered using a volumetric infusion pump (Volumat MC Agilia; Fresenius Kabi Norge AS, Norway). The use of a stepwise dosing paradigm, dosing amount and treatment timing was selected with the goal of simulating therapeutically indicated levels of ketamine commonly utilised for analgesic purposes (Jouguelet-Lacoste et al. 2015; Kurdi et al. 2014). This approach further facilitates attainment of a series of steady blood concentrations during a single experimental session. The treatment steps comprised of an initial (i: step 1) 30 mg bolus of ketamine infusion, plus 8 mg/h IV infusion, followed by (ii: step 2) 12 mg/h IV infusion and, finally, (iii: step 3) 20 mg/h infusion. The average bolus dose provided was 0.38 mg/kg, and the max infusion was 0.25 mg/kg/h. Each infusion step lasted 1 h, for a total infusion time of 3 h. Venous blood (4 mL) was drawn using a vacuum tube from the cannula at approximately 5 min post-conclusion of each infusion, and again at approximately 1.5 h post-cessation of treatment (see Fig. 1). Discard volume of blood (5 mL) was drawn before each blood collection to preclude any contamination of the IV line with the saline solution. Once the sample was taken, the line was again flushed with 5 mL of 0.9% saline and the IV was reconnected. Blood samples were immediately stored at − 80 °C as whole-blood samples without centrifuge.
Procedure
Participants presented to the hospital at 8:00 a.m. to complete the baseline assessments and remained on-site under observation until discharge at 4:00 p.m. (Fig. 1). The cognitive battery (see “Cognitive assessment”) took approximately 35 min to complete. Task order was non-randomised throughout. Following baseline assessment, participants’ vital signs were assessed (blood pressure, pulse, 02 saturation and temperature) and they were cannulated (as per “Acute drug administration and biological sampling” above). The infusion was commenced at 9:00 a.m. Cognitive performance was assessed thereafter at each treatment step. Vital signs were monitored at half-hourly intervals throughout the infusion period. The infusion was discontinued at 12:00 p.m. and participants were provided with light refreshments. The post-treatment assessments occurred at approximately 1:30 p.m. (Fig. 1). Participants’ vital signs were monitored at half-hourly intervals by nursing staff until discharge.
Ketamine and norketamine analysis
Venous whole-blood samples (0.25 mL) were analysed by Forensic Science South Australia for ketamine and norketamine. Drug quantification was performed by spiking ketamine and norketamine (sourced from Cerilliant, USA) to drug-free whole blood (0.25 mL) obtained from the Australian Red Cross Blood Service. A concentration range of 0.01–4.00 mg/L was used for each analyte using D4-ketamine and D4-norketamine (Cerilliant, USA), each at 0.40 mg/L. The relative response was linear within this range, and the limit of quantitation was set at 0.01 mg/L. Whole-blood samples (0.25 mL) were diluted with 1.5 mL of MilliQ water™ and concentrated ammonia solution (0.25 mL; 30% w/v). The samples were vortexed and butyl chloride (5 mL) was added. The vials were rolled for 15 min prior to centrifugation at 3000 rpm for 10 min. The organic layer was evaporated to dryness under N2 gas at 40 °C and reconstituted in ethanol (0.10 mL). Samples were analysed in duplicate.
Two microlitres of each sample extracts were analysed using an Agilent 1200 LC liquid chromatograph in combination with an Agilent 6520 Q-TOF, in AutoMSMS mode with an electrospray ionisation source. Separation of the drugs was obtained using a Waters Acquity BEH C18 1.7 μm × 3.0 mm × 50 mm column fitted with a Phenomenex C18 4.0 × 3.0 mm guard cartridge. The mobile phase consisted of a gradient of (a) 0.1% v/v formic acid in water and (b) acetonitrile over 12 min. Analytes were identified by retention time, accurate mass and MS/MS spectral match to a standard. Quantification was achieved by measuring the relative response of the M + H ion for each drug to its respective internal standard.
Cognitive assessment
Neurocognitive and behavioural ability was evaluated using a subset of the well-validated computerised tasks from the Cambridge Neuropsychological Test Automated Battery (CANTAB). The primary endpoint with respect to the neurocognitive and behavioural outcomes was the domain scores of each individual task (defined below) at each treatment step and post-treatment when compared to baseline. The CANTAB battery consisted of the visual analogue scale (VAS), reaction/movement time (RTI) (Simple and 5-Choice) task, visuospatial working memory (SWM) and spatial planning task, as assessed by the Stockings of Cambridge (SOC).
Subjective effects (visual analogue scale)
The CANTAB VAS was used to assess performance-based subjective effects among participants at each treatment step and at post-treatment (Bond and Lader 1974). This included the following: alertness (0 = alert, 100 = drowsy), clear-headedness (0 = clear headed, 100 = muzzy), coordination (0 = well-coordinated, 100 = clumsy), mental ability (0 = quick witted, 100 = mentally slow), attention (0 = attentive, 100 = dreamy) and proficiency (0 = proficient, 100 = incompetent).
Reaction/movement time (Simple and 5-Choice) task
The RTI comprises two main tasks: Simple and 5-Choice reaction and movement time. For both tasks, participants are required to respond directly to the stimuli presented on the touch screen (reaction time), or press and hold a button and react in response to the visual stimuli (movement time). The task is divided into five stages, which require increasingly complex chains of responses. The four outcome measures report reaction and movement times (ms), and accuracy.
Visuospatial working memory task
This CANTAB sub-test assesses the ability to retain and manipulate information in working memory, and requires participants to search among a spatial array of on-screen boxes in order to locate the box with the blue tokens hidden within. Participants are instructed that only one taken will be present at any one time for each set, and that once they have found a blue token in a box, it will not be in that box again for the rest of that set. Outcome measures are the following: ‘between-search’ errors, recorded when participants revisit the boxes which have already been found to contain a token; and ‘strategy’, an estimate of the use of the most efficient heuristic search sequence.
Spatial planning: Stockings of Cambridge task
The SOC task is a modified version of the Tower of London (Shallice 1982), which evaluates aspects of spatial planning and problem-solving ability. In this task, participants are required to move the balls in the bottom display window to match those presented at the top of the screen. Participants are encouraged to plan the sequence in which they will execute the move. The task consists of problems increasing in difficulty, from two, three, four, to five moves. The primary outcome measure is ‘problems solved in the minimum number of moves’.
Statistical analysis
A participant pool of N = 20 was selected for comparison to similar cohorts for this type of assessment (Honey et al. 2003). Demographic characteristics are presented as summary statistics for categorical variables only (n, %). Neurocognitive, behavioural and subjective VAS data derived from the CANTAB task was analysed using separate linear fixed-effects models. A compound symmetry covariance structure was interpreted as to be the best fit for the data. Time (dose) was entered into the model as the repeated measures factor (CS covariance structure) and CANTAB VAS and neurocognitive and behavioural domain scores were included as the outcome variable. Where a main effect was observed, post hoc paired t tests with Bonferroni correction for multiple comparisons were conducted to contrast each time point (treatment step 1, 2 and 3 and post-treatment phase) to baseline scores. Linear regression models were used to assess associations between whole-blood concentrations of both ketamine and norketamine and performance on target CANTAB variables. All statistical analyses were conducted with the use of SPSS 24.0 (SPSS Inc., USA), and tests are two-tailed with a conventional level of significance of p < 0.05.
Results
Demographics
Demographic information for the sample is presented in Table 1. A large proportion (95%) of the sample identified as being Caucasian, with the majority of participants speaking English as first language (94.7%). Over half of the sample held a diploma/some tertiary education and were currently employed on a full-time (55%) or part-time basis (10%). All participants (100%) had reported previous (lifetime history) use of any sedating-type analogues (including ketamine). A large proportion (80%) also reported previous (lifetime) use of cannabis, amphetamines, MDMA and cocaine.
Biological data
Ketamine
Mean whole-blood concentrations of ketamine recorded at each time point are presented in Fig. 1. Baseline ketamine concentrations are based on n = 2 observations. Whole-blood concentrations of ketamine increased rapidly from following ketamine infusion at treatment step 1 (8 mg/h IV infusion + one-off 30 mg bolus of ketamine) to treatment step 2 (12 mg/h IV infusion of ketamine), where blood concentrations rose from a mean concentration of 0.34 to 0.48 mg/L, respectively. This concentration was observed to peak at a maximum (Cmax) of 0.82 mg/L following administration of treatment step 3 (20 mg/h IV infusion ketamine). Blood concentrations decreased rapidly following cessation of the treatment to a concentration nadir of 0.21 mg/L at 2-h post-treatment. A weak positive linear association was observed between blood levels of ketamine and CANTAB SWM strategy score (R2 = 0.103, p < 0.001), and all CANTAB VAS items (R2 range 0.005–0.137, all p < 0.05).
Norketamine
Mean whole-blood concentrations of norketamine are recorded at each time point is presented in and Fig. 1. Blood concentrations of norketamine increased from a mean of 0.06 mg/L following the administration of treatment step 1 (8 mg/h IV infusion + one-off 30 mg bolus of ketamine) to a mean concentration of 0.07 mg/L at step 2. Blood concentrations further increased to a mean peak concentration (Cmax) of 0.09 mg/L following treatment step 3 (20 mg/h IV infusion ketamine). Levels dropped to a mean of 0.07 mg/L post-treatment. No correlations were deemed significant (Fig. 2).
Cognitive tasks
Reaction/movement time (Simple and 5-Choice) task
Summary data for all CANTAB outcomes for each testing time point are provided in Table 1. Linear fixed-effects model analyses failed to indicate any main effects of time (dose) on the CANTAB RTI Simple accuracy score (F(4,18) = 0.46, p = 0.763), RTI Simple reaction time (F(4,18) = 1.23, p = 0.332) or RTI Simple movement time (F(2,18) = 0.494, p = 0.740). Linear fixed-effects model analyses indicated a significant main effect of time (dose) for both CANTAB RTI 5-Choice reaction time (F(4,18) = 3.41, p = 0.03) and RTI 5-Choice movement time (F(4,19) = 4.42, p = 0.011), but not for the RTI 5-Choice accuracy score (F(4,18) = 1.96, p = 0.141). Post hoc analyses for RTI 5-Choice reaction time revealed significant differences from baseline for treatment step 1, treatment step 2 and treatment step 3 (all p < 0.05). No significant difference from baseline was noted for RTI 5-Choice reaction time at post-treatment. Post hoc analyses for RTI 5-Choice movement time indicated significant differences from baseline for treatment step 1, treatment step 2 and treatment step 3 (all p < 0.05). No difference from baseline was noted for RTI 5-Choice movement time at time point 4 (post-treatment).
Visuospatial working memory task
Linear fixed-effects model analyses indicated a significant main effects of time (dose) for the CANTAB SWM ‘between-search’ error score (F(4,18) = 4.19, p = 0.014), but not the SWM ‘strategy’ score (F(4,19) = 1.68, p = 0.195). Post hoc analyses did not indicate any significant group differences from baseline.
Spatial planning: Stockings of Cambridge task
Linear fixed-effects model analyses indicated a significant effect for time (dose) for the CANTAB SOC ‘problem solved in minimum moves’ score (F(4,18) = 4.13, p = 0.015). Post hoc analyses did not indicate any significant group differences from baseline.
Subjective effects (VAS)
Summary data for subjective ratings from the performance-related CANTAB VAS scores across for testing session are provided in Table 2. Linear fixed-effects model analyses indicated a significant main effect for time for alertness (F(4,18) = 6.51, p = 0.002), clear-headedness (F(4,19) = 10.0, p < 0.001), coordination (F(4,18) = 13.75, p < 0.001), mental ability (F(4,18) = 5.37, p = 0.005), attention (F(4,18) = 12.71, p < 0.001) and proficiency (F(4,18) = 9.13, p < 0.001). Post hoc analyses for VAS scores revealed significant differences from baseline for all self-reported performance variables following treatment step 1 (all p < 0.05). This remained significant at treatment step 2 for coordination, mental ability and proficiency (all p < 0.05), at treatment step 3 for coordination and proficiency (both p < 0.05). At post-treatment, this was sustained for self-reported proficiency only (p < 0.05) (Table 3).
Discussion
This study reports the acute and residual effect of three increasing analgesic-range doses of ketamine on measures of neurocognitive and behavioural performance. Results indicate that these increasing doses of ketamine elicit acute, dose-dependent and transient effects on tasks engaging typically ‘higher-order’ neurocognitive functions necessitating complex behavioural execution (reaction time). The effect on aspects of higher-order cognitive ability, including visuospatial working memory and spatial planning, was evident; however, the effect of dose was somewhat less clear. Observed correlations between blood levels of ketamine and subjective indicators of competency highlight the potential additional role of perceived capability in executing complex tasks at these doses of ketamine. These findings support the targeted effect of ketamine on neurocognition and behaviour, and provide additional information as to the functional implication of the drug for patients receiving this medication at therapeutic (analgesic) doses.
Ketamine elicits an additive pattern of effects on component measures of neurocognitive and behavioural performance (Guillermain et al. 2001; Micallef et al. 2004; Micallef et al. 2002). Results from the present study outline acute and selective dose-dependent effects on measures of complex psychomotor-based performance, attention, response inhibition, cognitive flexibility and processing speed (5-Choice RTI task). This expands previous observations of ketamine-induced impairments on tasks necessitating higher cognitive load (Guillermain et al. 2001). Mechanistically, behavioural impairments resulting from ketamine use are considered to reflect acute ketamine-induced blockade of NMDA receptors (Guillermain et al. 2001); however, the secondary stimulation of α-amino-3-hydroxy-5-methylisoxasole-4-proprionic acid and activation of glutamatergic neurotransmission at kainate receptors have also been potentiated (Micallef et al. 2004). The resulting increase in dopamine release in the pre-frontal cortex (Rao et al. 1989) may exert effects on behavioural competency on the types of tasks sensitive to these neurochemical alterations (such as 5-Choice reaction) (Robbins 2002). In the current study, no significant effect was detected for the proportion of correct responses (accuracy) as a function of treatment for the 5-Choice RTI task. This supports previous observations of retention of functional specificity (Lofwall et al. 2006) and indicates the potential lack of effect on stimulus selection and pre-cognitive processing abilities. Preservation of performance on tasks of simple reaction/movement time under ketamine conditions is noted elsewhere (Krystal et al. 1994), and thus the observed lack of effect on this outcome may reflect limited impairment in general psychomotor speed at these doses. A transience of behavioural effect was observed for all affected domains, with performance returning to baseline levels at the post-treatment assessment phase. Remission of psychomotor effects post-treatment has been noted by others (Ghoneim et al. 1985; Lofwall et al. 2006) and may reflect the short half-life and rapid pattern of metabolic elimination of ketamine when administered intravenously (Clements and Nimmo 1981). Indeed, whole-blood concentrations of ketamine and norketamine measures here demonstrated a steady increase relative to treatment dose, and featured rapid metabolic elimination post-treatment consistent with a re-distribution rate of 2.5 h (Wieber et al. 1975). Thus, further examination of these behavioural effects under treatment schedules which utilise alternate administration protocols (such as intramuscular route) may lend information to the direct role of ketamine metabolism on performance outcomes during the acute and post-treatment phase.
Findings pertaining to the neurocognitive domains of SWM and spatial planning (SOC) were somewhat inconclusive. A significant main effect for time (dose) was noted for these tasks; however, no specific differences were noted from baseline during post hoc analyses. A similar lack of definitive evidence to reflect true domain-specific impairments in these neurocognitive abilities using comparable tasks has been noted elsewhere (Honey et al. 2003). Indeed, inconsistencies in tasks necessitating select higher-order cognitive skills (Morgan and Curran 2006), or measures of applied neurocognitive abilities (Harborne et al. 1996), are reported by others. This is perhaps further evidenced by the presence of a single weak positive association noted between whole-blood levels of ketamine and cognitive performance only (SWM strategy score). Limited studies similarly indicate an absence of association between blood levels and cognitive performance on comparable test parameters (Honey et al. 2003), suggesting that blood concentrations may also not accurately reflect the degree of cognitive impairment observed. This preservation of neurocognitive abilities suggests that NMDA receptor hypo-function induced by ketamine does not negatively impact these specific domains; however, it must be noted that not all conceivable domains were examined, and so it is unclear if other peripherally related functional domains might be implicated. Measures of higher-order neurocognitive functioning are pivotal in the planning, initiation, sequencing and monitoring of complex goal-directed behaviours (Jurado and Rosselli 2007), and thus further examination of acute and residual neurocognitive deficits in a treatment context, and among potentially at-risk patient groups, may be of high clinical importance with respect to longer-term treatment outcomes.
Subjective feelings of performance-based functional capacity decreased as a function of treatment dose, and some residual effects were also observed (for self-rated proficiency only). Existing literature has evaluated psychotomimetic (Bowdle et al. 1998) or abstract subjective effects related to mood only (Mortero et al. 2001). This research, therefore, provides additional insight as to the subjective effects of therapeutically indicated, analgesic doses of ketamine, particularly for those skills relating to perceived functional ability. Positive linear associations were also observed across all subjective indexes as a function of ketamine-blood concentrations, suggesting close association between objective levels of sedation and subjective symptomology. Similar, albeit stronger, effects of subjective intoxication under ketamine conditions have been noted by others (Bowdle et al. 1998); however, these were related largely to subjective psychotomimetic, not performance-based effects. Poorer perception of functional capacity is associated with poor outcomes post-treatment, particularly among older individuals (Lou et al. 2004). Thus, evaluating self-rated functional or performance-based capacity is likely clinically useful when considering the longer-term follow-up requirements for those undergoing treatment with these doses of ketamine.
Results from the current study are to be considered in light of a few key limitations. The absence of a comparative control group precludes the ability to adequately control for random treatment effects. Nonetheless, the use of a within-subjects design which utilises a stepwise, increasing administration protocol is likely highly clinically relevant due to the similarities with existing treatment protocols using ketamine at similar doses (Petrenko et al. 2003). Somewhat conversely, the open-label, cumulative-dose design used in the trial may have inadvertently impacted the results. It is possible that patients may have anticipated the sequenced dose and altered their responses accordingly. However, given that effects were consistently observed on some (but not all) of the outcomes, and marked changes were noted in VAS scores (which were related to blood concentrations), we do not anticipate that this approach has significantly impacted the findings. By extension, repeated use of the CANTAB battery at each treatment step may have inadvertently induced some practice or learned effects. However, specific effects often vary with task difficulty and have not yet been reported for the specific tasks used here (Lowe and Rabbitt 1998). It is worthwhile considering a potential NMDA adaptation effect, which may hinder some of the interpretations of the neurocognitive and behavioural data. Preclinical studies indicate a degree of NMDA receptor adaptation following chronic administration of select anti-depressant medications (such as imipramine and citalopram) (Skolnick et al. 1996). To our knowledge, however, no such effect has yet been reported under ketamine conditions (in neither anti-depressant nor analgesic context), nor when examined under an acute treatment protocol. Thus, given the acute dosing paradigm employed, we do not consider this to have significantly influenced our findings. The negative value (0.00 mg/L) of ketamine and norketamine blood concentrations at baseline are somewhat implied, as data for this time point was only collected for n = 2 participants due to late-stage protocol variations. Although highly unlikely, we cannot exclude the possibility that some participants may have recorded some blood concentrations of ketamine and norketamine metabolites at baseline. To address this, the use of validated oral-fluid (saliva) drug detection was employed at baseline, and no positive tests were returned.
In conclusion, these three increasing analgesic-range doses of intravenously administered ketamine produced selective, transient, and dose- and time-related effects on several neurocognitive and behavioural domains. Specific additive ketamine-induced deficits were observed for skills relating to complex behavioural planning and execution (reaction time); however, further characterisation is required to elucidate how these doses and treatment regimen impair visuospatial working memory and spatial planning at these doses. Acute impairments evidenced on these tasks have potential clinical implications for patients receiving ketamine infusion at these doses, particularly for individuals who report poorer self-rated competence post-treatment. Additionally, longer-term research may assist in defining the natural course of association between these objective and subjective indicators and global functional health outcomes, particularly among at-risk patient groups.
References
Aberl F, VanDine R (2005) Saliva and sweat testing with Drugwipe®. In: Wong RC, Tse HY (eds) Drugs of abuse: body fluid testing. Humana Press, Totowa, pp 161–175. https://doi.org/10.1007/978-1-59259-951-6_10
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79(2):565–575. https://doi.org/10.1111/j.1476-5381.1983.tb11031.x
Bond A, Lader M (1974) The use of analogue scales in rating subjective feelings. Br J Med 47(3):211–218. https://doi.org/10.1111/j.2044-8341.1974.tb02285.x
Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP (1998) Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology 88(1):82–88. https://doi.org/10.1097/00000542-199801000-00015
Bristow A, Orlikowski C (1989) Subcutaneous ketamine analgesia: postoperative analgesia using subcutaneous infusions of ketamine and morphine. Ann R Coll Surg Engl 71(1):64–66
Carter LP, Kleykamp BA, Griffiths RR, Mintzer MZ (2013) Cognitive effects of intramuscular ketamine and oral triazolam in healthy volunteers. Psychopharmacology 226(1):53–63. https://doi.org/10.1007/s00213-012-2883-x
Clements JA, Nimmo WS (1981) Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth 53(1):27–30. https://doi.org/10.1093/bja/53.1.27
Crisp BR, Swerissen H, Duckett SJ (2000) Four approaches to capacity building in health: consequences for measurement and accountability. Health Promot Int 15(2):99–107. https://doi.org/10.1093/heapro/15.2.99
Ghoneim MM, Hinrichs JV, Mewaldt SP, Petersen RC (1985) Ketamine: behavioral effects of subanesthetic doses. J Clin Psychopharmacol 5(2):70–77. https://doi.org/10.1097/00004714-198504000-00003
Guillermain Y, Micallef J, Possamaï C, Blin O, Hasbroucq T (2001) N-methyl-d-aspartate receptors and information processing: human choice reaction time under a subanaesthetic dose of ketamine. Neurosci Lett 303(1):29–32. https://doi.org/10.1016/S0304-3940(01)01695-0
Harborne GC, Watson FL, Healy DT, Groves L (1996) The effects of sub-anaesthetic doses of ketamine on memory, cognitive performance and subjective experience in healthy volunteers. J Psychopharmacol 10(2):134–140. https://doi.org/10.1177/026988119601000208
Hirota K, Lambert DG (1996) Ketamine: its mechanism(s) of action and unusual clinical uses. Br J Anaesth 77(4):441–444. https://doi.org/10.1093/bja/77.4.441
Honey RAE et al. (2003) Subdissociative dose ketamine produces a deficit in manipulation but not maintenance of the contents of working memory. Neuropsychopharmacology 28: doi:https://doi.org/10.1038/sj.npp.1300272
Jouguelet-Lacoste J, La Colla L, Schilling D, Chelly JE (2015) The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med 16(2):383–403. https://doi.org/10.1111/pme.12619
Jurado MB, Rosselli M (2007) The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 17(3):213–233. https://doi.org/10.1007/s11065-007-9040-z
Krystal JH, Bennett A, Abi-Saab D, Belger A, Karper LP, D’Souza DC, Lipschitz D, Abi-Dargham A, Charney DS (2000) Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry 47(2):137–143. https://doi.org/10.1016/S0006-3223(99)00097-9
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive nmda antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51(3):199–214. https://doi.org/10.1001/archpsyc.1994.03950030035004
Kurdi MS, Theerth KA, Deva RS (2014) Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res 8(3):283–290. https://doi.org/10.4103/0259-1162.143110
Lofwall MR, Griffiths RR, Mintzer MZ (2006) Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharmacol 14(4):439–449. https://doi.org/10.1037/1064-1297.14.4.439
Lou M-F, Yu P-J, Huang G-S, Dai Y-T (2004) Predicting post-surgical cognitive disturbance in older Taiwanese patients. Int J Nurs Stud 41(1):29–41. https://doi.org/10.1016/S0020-7489(03)00112-3
Lowe C, Rabbitt P (1998) Test\re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Neuropsychologia 36(9):915–923. https://doi.org/10.1016/S0028-3932(98)00036-0
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, David Missar C, Pickar D, Breier A (1996) NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 14(5):301–307. https://doi.org/10.1016/0893-133X(95)00137-3
Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A (1997) Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 17(3):141–150. https://doi.org/10.1016/s0893-133x(97)00036-5
Micallef J, Guillermain Y, Tardieu S, Hasbroucq T, Possamaï C, Jouve E, Blin O (2002) Effects of subanesthetic doses of ketamine on sensorimotor information processing in healthy subjects. Clin Neuropharmacol 25:101–106
Micallef J, Gavaudan G, Burle B, Blin O, Hasbroucq T (2004) A study of a topiramate pre-treatment on the effects induced by a subanaesthetic dose of ketamine on human reaction time. Neurosci Lett 369(2):99–103. https://doi.org/10.1016/j.neulet.2004.06.082
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17(8):2921–2927
Morgan CJA, Curran HV (2006) Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology 188(4):408–424. https://doi.org/10.1007/s00213-006-0572-3
Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV (2003) Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology 29(1):208–218. https://doi.org/10.1038/sj.npp.1300342
Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA (2001) The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg 92:1465–1469. https://doi.org/10.1097/00000539-200106000-00022
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW (1999) Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20(2):106–118. https://doi.org/10.1016/S0893-133X(98)00067-0
Olofsen E, NoppersI NM, Kharasch E, Aarts L, Sarton E, Dahan A (2012) Estimation of the contribution of norketamine to ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers. Anesthesiology 117(2):353–364. https://doi.org/10.1097/ALN.0b013e31825b6c91
Petrenko AB, Yamakura T, Baba H, Shimoji K (2003) The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg 97:1108–1116. https://doi.org/10.1213/01.ane.0000081061.12235.55
Rao TS, Kim HS, Lehmann J, Martin LL, Wood PL (1989) Differential effects of phencyclidine (PCP) and ketamine on mesocortical and mesostriatal dopamine release in vivo. Life Sci 45(12):1065–1072. https://doi.org/10.1016/0024-3205(89)90163-X
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacol 163(3-4):362–380. https://doi.org/10.1007/s00213-002-1154-7
Rogers R, Wise RG, Painter DJ, Longe SE, Tracey I (2004) An investigation to dissociate the analgesic and anesthetic properties of ketamine using functional magnetic resonance imaging. Anesthesiology 100(2):292–301. https://doi.org/10.1097/00000542-200402000-00018
Shallice T (1982) Specific impairments of planning. Philos Trans R Soc Lond Ser B Biol Sci 298(1089):199–209. https://doi.org/10.1098/rstb.1982.0082
Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R (1996) Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry 29(01):23–26. https://doi.org/10.1055/s-2007-979537
White PF, Way WL, Trevor AJ (1982) Ketamine—its pharmacology and therapeutic uses. Anesthesiology 56(2):119–136. https://doi.org/10.1097/00000542-198202000-00007
Wieber J, Gugler R, Hengstmann JH, Dengler HJ (1975) Pharmacokinetics of ketamine in man. Anaesthesist 24(6):260–263
Wong JJ, O’Daly O, Mehta MA, Young AH, Stone JM (2016) Ketamine modulates subgenual cingulate connectivity with the memory-related neural circuit—a mechanism of relevance to resistant depression? PeerJ 4:e1710. https://doi.org/10.7717/peerj.1710
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research was approved by Monash Health Human Research Ethics committee (approval number: HREC/16/MonH/240). All participants provided written informed consent prior to commencing any study procedures. This study was registered on the Australia and New Zealand Clinical Trials registry (www.anzctr.com.au); trial number ACTRN12616001485426.
Conflict of interest
Dr. Amie Hayley is supported by a National Health and Medical Council (NHMRC) Peter Doherty Biomedical Early Career Research Fellowship (APP1119960). A/Prof Downey is supported by an NHMRC R.D. Wright Biomedical Career Development Fellowship (CDF: 2017-2020). Dr. Green, Dr. Keane, Ms. Kostakis and Prof Shehabi declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Hayley, A., Green, M., Downey, L. et al. Neurocognitive and behavioural performance of healthy volunteers receiving an increasing analgesic-range infusion of ketamine. Psychopharmacology 235, 1273–1282 (2018). https://doi.org/10.1007/s00213-018-4842-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4842-7