Abstract
Rationale
Environmental stimulus control over drug relapse requires the retrieval of context-response-cocaine associations, maintained in long-term memory through active reconsolidation processes. Identifying the neural substrates of these phenomena is important from a drug addiction treatment perspective.
Objectives
The present study evaluated whether the agranular insular cortex (AI) plays a role in drug context-induced cocaine-seeking behavior and cocaine memory reconsolidation.
Methods
Rats were trained to lever press for cocaine infusions in a distinctive context, followed by extinction training in a different context. Rats in experiment 1 received bilateral microinfusions of vehicle or a GABA agonist cocktail (baclofen and muscimol (BM)) into the AI or the overlying somatosensory cortex (SSJ, anatomical control region) immediately before a test of drug-seeking behavior (i.e., non-reinforced lever presses) in the previously cocaine-paired context. The effects of these manipulations on locomotor activity were also assessed in a novel context. Rats in experiment 2 received vehicle or BM into the AI after a 15-min reexposure to the cocaine-paired context, intended to reactivate context-response-cocaine memories and initiate their reconsolidation. The effects of these manipulations on drug context-induced cocaine-seeking behavior were assessed 72 h later.
Results
BM-induced pharmacological inactivation of the AI, but not the SSJ, attenuated drug context-induced reinstatement of cocaine-seeking behavior without altering locomotor activity. Conversely, AI inactivation after memory reactivation failed to impair subsequent drug-seeking behavior and thus cocaine memory reconsolidation.
Conclusions
These findings suggest that the AI is a critical element of the neural circuitry that mediates contextual control over cocaine-seeking behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapse triggered by exposure to drug-associated environmental contexts is a major challenge for the successful treatment of cocaine use disorder (Rohsenow et al. 1990; Ehrman et al. 1992; Childress et al. 1999; Foltin and Haney 2000). Following retrieval, which can be induced by exposure to a previously drug-paired context, drug-associated memories produce cocaine craving and promote drug-seeking behavior. At the same time, these memories can become unstable and must undergo protein synthesis-dependent reconsolidation in order to be updated or maintained over time (Taylor et al. 2009). Thus, manipulations that disrupt the motivational effects of drug-associated stimuli may interfere with an acute relapse episode, while manipulations that interfere with the reconsolidation of labile drug-associated memories may preempt future relapse to drug-seeking and drug-taking behaviors (Lee et al. 2005; Miller and Marshall 2005; Milekic et al. 2006; Tronson and Taylor 2007). Accordingly, identifying the neural substrates involved in drug-seeking behavior and drug memory reconsolidation is critical for the identification of effective pharmacotherapeutic targets for relapse prevention.
Several subregions of the prefrontal cortex (PFC) have been implicated in drug context-induced reinstatement of cocaine-seeking behavior and some forms of memory reconsolidation in rodents (Grant et al. 1996; Taylor et al. 2009; Fuchs et al. 2009; Ramirez et al. 2009; Sorg 2012). Specifically, drug context-induced cocaine-seeking behavior depends on the functional integrity of the dorsomedial PFC and ventral lateral orbitofrontal cortex (lOFC) (Fuchs et al. 2005; Lasseter et al. 2009), and protein synthesis and/or mechanistic target of rapamycin complex 1 activation in these brain regions is necessary for drug memory reconsolidation under some experimental conditions (Barak et al. 2013; Sorg et al. 2015; but see Ramirez et al. 2009). However, much less is known about the functional contributions of the anterior agranular insular cortex (AI), a brain region that is laterally adjacent and similar in connectivity to the lOFC (Schoenbaum et al. 2009).
The first objective of the present study was to evaluate the putative involvement of the AI in drug context-induced cocaine-seeking behavior. Consistent with this possibility, a meta-analysis of functional neuroimaging studies has indicated that the AI exhibits increased cue-induced activation in cocaine users (Kühn and Gallinat 2011) despite a cocaine-related decrease in gray matter density (Franklin et al. 2002). Furthermore, reduced insular cortex activation during a drug-unrelated decision-making task predicts treatment-seeking methamphetamine users who relapse as opposed to those who remain abstinent over an extended time period (Paulus et al. 2005). In apparent contrast to these findings, work utilizing animal models indicates that AI inactivation disrupts explicit conditioned stimulus (CS)-induced reinstatement of cocaine-seeking behavior (Cosme et al. 2015). To help resolve this discrepancy, it is important to evaluate the generalizablity of these findings to other animal models of drug relapse, including the drug context-induced reinstatement paradigm.
The second objective of the present study was to assess the role of the AI in the reconsolidaiton of cocaine-related memories that promote drug context-induced cocaine-seeking behavior. The insular cortex appears to be functionally heterogenous with respect to its involvement in memory reconsolidation. The granular and dysgranular subregions of the insular cortex, which are located posterior and dorsal relative to the AI, play critical roles in taste aversion memory and fear memory maintenance (Stehberg et al. 2009; Wang et al. 2012; García-DeLaTorre et al. 2009, 2010; Kobilo et al. 2007; Zubedat and Akirav 2017). Furthermore, within the AI, protein synthesis and DNA methyltransferase activity are necessary for the reconsolidation of Pavlovian amphetamine memories (Contreras et al. 2012) and Pavlovian naloxone-precipitated morphine withdrawal memories (Liu et al. 2016), respectively. However, the contributions of the AI to the reconsolidation of drug memories established in an instrumental paradigm have not been investigated.
To investigate these questions in the present study, a cocktail of GABAA and GABAB agonists was microinfused into the AI to temporarily inhibit neuronal activity either immediately before or immediately after reexposure to the cocaine-paired context. We hypothesized that AI neuronal inactivation before drug context reexposure would inhibit the expression of cocaine-seeking behavior. In addition, we postulated that AI inactivation following drug context reexposure would interfere with the reconsolidation of contextual cocaine memories and that the resulting memory impairment would be indicated by a subsequent decrease in drug context-induced reinstatement of cocaine-seeking behavior.
Materials and methods
Animals
Male Sprague-Dawley rats (Harlan/Envigo, Livermore, CA; N = 43; 275–300 g) were individually housed in a temperature- and humidity-controlled vivarium on a reversed light-dark cycle. Rats were maintained on 20–25 g of rat chow per day with water available ad libitum. All protocols for the housing and treatment of animals were approved by the Institutional Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Food training
Rats first received a 16-h overnight food training session to facilitate the acquisition of cocaine self-administration. During the session, food reinforcement was available under a fixed-ratio 1 (FR1) schedule controlled using Graphic State Notation software version 4.1.04 (Coulbourn). Presses on a designated active lever resulted in the delivery of a single food pellet (45 mg; Bio-serve, Flemington, NJ). Presses on a second, inactive lever had no programmed consequences. The session was conducted in sound-attenuated operant conditioning chambers (26 × 27 × 27 cm; Coulbourn Instruments, Allentown, PA) dedicated to food training. Thus, during food training, the rats had no access to the chambers or to the visual, olfactory, tactile, and auditory stimuli that were subsequently paired with cocaine access during self-administration training.
Surgery
Forty-eight hours after food training, rats were fully anesthetized with ketamine and xylazine (100.0 and 5.0 mg/kg, respectively, intraperitoneal (i.p.)). Intravenous (i.v.) catheters were constructed in-house and were inserted into the right jugular vein, as described previously (Fuchs et al. 2007). The catheters ran subcutaneously and exited on the back, between the scapulae. Rats were next placed into a stereotaxic instrument (Stoelting, Wood Dale, IL), and 26-Ga stainless steel guide cannulae (Plastics One, Roanoke, VA) were aimed bilaterally at the AI (+2.8 anterior-posterior (AP), ±4.0 medial-lateral (ML), −4.1 dorsal-ventral (DV), mm relative to bregma) or the jaw region of the somatosensory cortex (SSJ; +2.8 AP, ±4.0 ML, −2.1 DV, mm relative to bregma). Guide cannulae were secured to the skull with stainless steel screws and cranioplastic cement. The catheters were flushed daily with 0.1 mL of an antibiotic solution of cefazolin (100 mg/mL; Henry Schein Animal Health, Tualatin, OR) dissolved in heparinized saline (70 U/mL; Patterson Veterinary Supply, Sterling, MA) followed by 0.1 mL of heparinized saline (10 U/mL), to maintain catheter patency. Catheter patency was assessed periodically using propofol (1 mg/0.1 mL; Abbott Laboratories, North Chicago, IL), which produces rapid and temporary loss of muscle tone when administered intravenously.
Cocaine self-administration training
Cocaine self-administration training was conducted in the operant conditioning chambers configured to one of two contexts. Context 1 consisted of a continuous red house light (0.4 fc brightness), intermittent pure tone (80 dB, 1 kHz; 2-s on, 2-s off), pine-scented air freshener (Car Freshener Corp., Watertown, NY), and wire mesh flooring (26 cm × 27 cm). Context 2 consisted of an intermittent white stimulus light over the inactive lever (1.2 fc brightness; 2-s on, 2-s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener (Sopus Products, Moorpark, CA), and a slanted ceramic tile wall that bisected the bar flooring (19 cm × 27 cm). Rats were randomly assigned to context 1 or context 2 for cocaine self-administration training. Daily training sessions took place during the rats’ dark cycle for a period of 2 h.
During each drug self-administration session, active lever presses resulted in i.v. cocaine infusions (cocaine hydrochloride dissolved in sterile saline; 0.15 mg/0.05 mL per infusion, delivered over 2 s; NIDA Drug Supply System, Research Triangle Park, NC) under a FR1 schedule of reinforcement with a 20-s timeout period. Active lever presses had no programmed consequences during the timeout period. Inactive lever presses were recorded but had no programmed consequences. Training continued until rats reached the acquisition criterion (≥10 cocaine infusions per session) on at least ten training days.
Extinction training
After reaching the acquisition criterion, rats received daily 2-h extinction training sessions. Rats that had access to cocaine in context 1 underwent extinction training in context 2 and vice versa. During the sessions, active and inactive lever presses were recorded but had no programmed consequences. Immediately before (experiment 1) or after (experiment 2) the fourth extinction session, rats were adapted to the intracranial microinfusion procedure. Stainless steel injection cannulae (33 Ga, Plastics One) were inserted 2 mm below the tip of the guide cannulae. The injector cannulae remained in place for 4 min with no infusion of fluids.
Experiment 1: effects of AI or SSJ neural inactivation on drug context-induced reinstatement of cocaine-seeking behavior
After a minimum of seven extinction sessions (plus additional sessions until active lever presses fell to or below 25/session on two consecutive days), rats received bilateral microinfusions of phosphate-buffered saline vehicle (VEH) or baclofen and muscimol (BM; 1.0 mM/0.1 mM; Alexis Biochemicals) at volumes of 0.5 μL per hemisphere into the AI or the SSJ (anatomical control region) over 2 min, using protocols described previously (Arguello et al. 2014). BM infused into the lOFC or basolateral amygdala (BLA) at this dose disrupts the expression of drug-seeking behavior and the reconsolidation of cocaine-associated contextual memories, respectively (Lasseter et al. 2009; Wells et al. 2011). Treatment assignment was counterbalanced based on previous cocaine intake and active lever-pressing history. After the microinfusions, rats were placed into the previously cocaine-paired context for a 2-h test session during which lever presses had no programmed consequences.
Experiment 2: effect of AI neuronal inactivation on cocaine-context memory reconsolidation
After cocaine self-administration training, all rats in experiment 2 received seven extinction sessions in order to keep memory age, a boundary condition of memory reconsolidation (Tronson and Taylor 2007), uniform at the time of memory reactivation and AI manipulation. During the memory reactivation session, rats were reexposed to the cocaine-paired context for 15 min to destabilize cocaine memories and trigger memory reconsolidation (Nader et al. 2000; Tronson and Taylor 2007). This session length was selected because parametric analyses demonstrated that it is sufficient to destabilize cocaine memories without producing overt behavioral extinction (Fuchs et al. 2009). During the session, lever presses had no programmed consequences. Immediately after the session, rats received bilateral intra-AI microinfusions of VEH or BM, as in experiment 1. They were then returned to their home cages. Next, rats received additional extinction training sessions until the total number of active lever presses/session fell to or below 25 on two consecutive training days (i.e., minimum two sessions). Rats were then placed into the previously cocaine-paired context for a 2-h test session during which lever responses had no programmed consequences.
Locomotor activity
To evaluate whether the observed effects of AI inactivation on lever pressing were due to altered motor activity, a subset of rats from experiment 1 was assigned to receive bilateral infusion of VEH or BM into the AI. Locomotor activity was assessed immediately after VEH or BM infusion, 2–4 days after the test of cocaine-seeking behavior. Testing was conducted in novel Plexiglas chambers (42 × 20 × 20 cm) equipped with eight light sources and photodetectors. Photobeam breaks were measured for 1 h by a computerized system (San Diego Instruments, San Diego, CA).
Brain histology
Rats were overdosed with ketamine and xylazine (300 and 15 mg/kg, respectively, i.p. or i.v.). The brains were dissected, flash frozen in isopentane (−20 °C), and stored at −80 °C. Brains were sectioned in the coronal plane at 40 μm using a cryostat. Brain sections were mounted on glass slides and stained with cresyl violet (Fisher Scientific). Using light microscopy, the most ventral portion of each cannula tract was recorded on appropriate plates of the rat brain atlas (Paxinos and Watson 2014). Data of rats with misplaced injection cannulae were excluded from data analysis.
Statistical data analysis
Separate analyses of variance (ANOVAs) or t tests were conducted to test for possible preexisting group differences in cocaine intake and lever responding during cocaine self-administration training (mean of last 3 days), extinction training, and the memory reactivation session and to test for group differences in the number of sessions required to reach the extinction criterion. Non-reinforced lever responses during the test session and preceding extinction session were analyzed using mixed factorial ANOVAs with treatment (VEH or BM) as the between-subject factor and testing context (extinction, cocaine-paired) and time (20-min intervals) as within-subject factors, when appropriate. Significant effects were further probed using Tukey’s HSD post hoc tests, when appropriate. Alpha was set at 0.05.
Results
Cannula placement
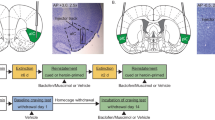
Target brain regions were defined as the anterior agranular insular cortex (AI) and the somatosensory cortex (SSJ). Cannula tracts were located bilaterally within the target regions for the following number of rats per group: experiment 1—VEH AI, n = 8; BM AI, n = 9; VEH SSJ, n = 6; BM SSJ, n = 7 (Fig. 1a) and experiment 2—VEH AI, n = 6; BM AI, n = 7 (Fig. 1b). The microinfusions did not produce unusual gliosis or cell loss visible at ×25 magnification (Fig. 1c).
Photomicrograph and schematics depicting of cannula placements within the agranular insular cortex (AI) and the jaw region of the somatosensory cortex (SSJ). The most ventral point of injector cannula tracts is shown for rats that received phosphate-buffered saline vehicle (VEH) into the AI (open circles) or SSJ (open triangles) and/or for rats that received baclofen and muscimol (BM; 1.0/0.1 mM) into the AI (filled circles) or SSJ (filled triangles) in a experiment 1 or b in experiment 2. Numbers denote distance from bregma in millimeters on the schematics modified from the rat brain atlas of Paxinos & Watson (2014)
Behavioral history
Rats exhibited stable responding on the active lever during the last 3 days of drug self-administration training (≤10% variability in daily cocaine intake). In experiments 1–2, there were no preexisting differences in active lever responding, inactive lever responding, or cocaine intake between the groups that later received VEH or BM into the AI or SSJ (data not shown). Upon exposure to the extinction context, active lever responding gradually declined (data not shown), and the subsequent treatment groups did not differ in active lever responding in the extinction context across the (first) seven extinction training sessions. The 2 × 7 ANOVAs of non-reinforced active lever presses revealed significant time main effects only (F 6,11–15 = 3.84–14.76, P = 0.0001–0.02). Thus, active lever responding gradually decreased during extinction training for both groups (Ext D1 > D7, Tukey’s tests, P < 0.01). In experiment 1, the subsequent treatment groups did not differ in the number of days needed to reach the extinction criterion prior to the test session. Inactive lever presses remained low during extinction training in all groups.
Effects of intra-AI BM administration on drug context-induced reinstatement of cocaine-seeking behavior
AI inactivation impaired the expression of cocaine-seeking behavior in the previously cocaine-paired context relative to VEH pretreatment (Fig. 2b). The ANOVA of active lever presses during the last extinction session and the test session revealed a significant context × treatment interaction effect (F 1,15 = 5.38, P = 0.04), context main (F 1,15 = 36.35, P = 0.0001), and treatment main (F 1,15 = 7.33, P = 0.02) effects. The VEH-pretreated group exhibited more active lever responding in the cocaine-paired context than in the extinction context (Tukey’s test, P < 0.01), whereas the BM-pretreated group did not. In addition, the BM-pretreated group exhibited less active lever responding than the VEH-pretreated group in the cocaine-paired context (Tukey’s test, P < 0.01) but not in the extinction context. A time course analysis of active lever presses in the cocaine-paired context revealed significant time main (F 5,15 = 21.15, P = 0.0001) and treatment main (F 1,15 = 5.53, P = 0.033) effects but no time × treatment interaction. Accordingly, the BM-pretreated group exhibited less active lever responding than the VEH-pretreated group at test, and active lever responding declined at similar rates in both groups after the first 20 min of the test session (Fig. 2c; Bin1 > Bin 2–6, Tukey’s tests, P < 0.01).
AI neural inactivation impairs drug context-induced reinstatement of extinguished cocaine-seeking behavior. a Schematic depicting the timeline for experiment 1. b Active and d inactive lever responses (mean ± SEM/2 h) during self-administration training (SA, mean of last three sessions), extinction training in the extinction context (EXT, last session), and testing in the cocaine-paired context (COC-paired) for rats that received VEH (white bars/symbols) or BM (1.0 mM/0.1 mM; 0.5 μL per hemisphere; black bars/symbols) into the AI immediately before testing. Time course of c active and e inactive lever responses (mean ± SEM/20 min) at test in the cocaine-paired context. Symbols represent difference relative to the EXT context (*Tukey’s test, P < 0.01), relative to the VEH group (a †Tukey’s test, P < 0.01 and b ANOVA treatment main effect, P = 0.03), or relative to the first 20-min interval (#Tukey’s test, P < 0.01)
AI inactivation did not alter inactive lever responding in the cocaine-paired or extinction context relative to VEH treatment (Fig. 2d). The ANOVA of inactive lever presses did not reveal any context or treatment main or interaction effects. Furthermore, the time course analysis of inactive lever presses at test revealed a significant time main effect only (F 5,15 = 4.48, P = 0.001). Thus, inactive lever responding declined after the first 20 min of the session (Fig. 2e; Bin1 > Bin 2–6, Tukey’s tests, P < 0.05).
Effects of intra-SSJ BM administration on drug context-induced reinstatement of cocaine-seeking behavior
Inactivation of the SSJ anatomical control region did not alter cocaine-seeking behavior in the cocaine-paired or the extinction context relative to VEH treatment (Fig. 3b). The ANOVA of active lever presses during the last session of extinction and the test session revealed a context main effect only (F 1,11 = 76.67, P = 0.0001). Both groups exhibited more active lever responding in the cocaine-paired context than in the extinction context. Additionally, the time course analysis of active lever presses in the cocaine-paired context revealed a time main effect only (F 5,11 = 21.76, P = 0.0001), as active lever responding declined after the first 20 min of the session (Fig. 3c; Bin1 > Bin 2–6, Tukey’s tests, P < 0.01).
SSJ neural inactivation, adjacent to the AI, does not alter drug context-induced reinstatement of extinguished cocaine-seeking behavior. a Schematic depicting the timeline. b Active and d inactive lever responses (mean ± SEM/2 h) during self-administration training (SA, mean of last three sessions), extinction training in the extinction context (EXT, last session), and testing in the cocaine-paired context (COC-paired) for rats that received VEH (white bars/symbols) or BM (1.0 mM/0.1 mM; 0.5 μL per hemisphere; black bars/symbols) into the SSJ immediately before testing. Time course of c active and e inactive lever responses (mean ± SEM/20 min) at test. Symbols represent difference relative to the EXT context (*ANOVA context main effect, P = 0.0001) or relative to the first 20-min interval (#Tukey’s test, P < 0.01)
Similarly, SSJ inactivation did not alter inactive lever responding in the cocaine-paired or extinction context relative to VEH treatment (Fig. 3d). Furthermore, the time course analysis of inactive lever presses in the cocaine-paired context revealed a time main effect only (F 5,11 = 16.76, P = 0.001). Thus, inactive lever responding declined after the first 20 min of the test session (Fig. 3e; Bin1 > Bin 2–6, Tukey’s tests, P < 0.01).
Effects of intra-AI BM administration following cocaine memory reactivation on subsequent cocaine-seeking behavior
Prior to intracranial treatment, the groups did not differ in active or inactive lever responding during the 15-min memory reactivation session. Likewise, after memory reactivation and intracranial treatment, there was no difference between the groups in the number of days needed to reach the extinction criterion (mean ± SEM, 2.00 ± 0.00). Thus, testing of cocaine-seeking behavior occurred 72 h after memory reactivation and intracranial manipulation in all subjects.
AI inactivation after cocaine memory reactivation did not alter cocaine-seeking behavior 72 h later (Fig. 4b). The ANOVA of active lever presses during the last extinction session and the test session revealed a context main effect only (F 1,11 = 23.45, P = 0.001). Both groups exhibited more active lever responding in the cocaine-paired context than in the extinction context. The time course analysis of active lever presses at test revealed a time main effect only (F 5,11 = 10.96, P = 0.0001). Thus, active lever responding declined after the first 20 min of the test session (Fig. 3c; Bin1 > Bin 2–6, Tukey’s tests, P < 0.01).
AI neural inactivation following cocaine memory reactivation does not alter subsequent drug context-induced reinstatement of extinguished cocaine-seeking behavior. a Schematic depicting the timeline for experiment 2. b Active and d inactive lever responses (mean ± SEM) during self-administration training (SA, mean of last three 2-h sessions), during the 15-min memory reconsolidation session (MR, 15-min session), during extinction training in the extinction context (EXT, last 2-h session following MR), and at testing in the cocaine-paired context (COC-paired, 2-h session) for rats that had received VEH (white bars/symbols) or BM (1.0 + 0.1 mM/0.5 μL per hemisphere; black bars/symbols) into the AI immediately after the memory reactivation session. Time course of c active and e inactive lever responses (mean ± SEM/20 min) at test. Symbols represent difference relative to the EXT context (a*ANOVA context main effect, P = 0.0001 and b ANOVA context main effect, P = 0.04) or relative to the first 20-min interval (#Tukey’s test, P < 0.01)
AI inactivation after cocaine memory reactivation did not alter inactive lever responding in the cocaine-paired context 72 h later (Fig. 4b). The ANOVA of inactive lever responding revealed a context main effect only (F 1,11 = 5.35, P = 0.04). Both groups exhibited a slight increase in inactive lever responding in the cocaine-paired context relative to responding in the extinction context, similar to earlier studies (e.g., Fuchs et al. 2009). Additionally, the time course analysis of inactive lever presses in the cocaine-paired context revealed a time main effect only (F 5,11 = 7.64, P = 0.001). Thus, inactive lever responding declined after the first 20 min of the test session (Fig. 4e; Bin1 > Bin 2–6, Tukey’s tests, P < 0.01).
Locomotor activity
The ANOVA of photobeam interruptions during the 1-h test revealed a time main effect only (F 2,10 = 87.93, P = 0.0001). Thus, the number of photobeam breaks declined after the first 20 min of the 1-h test session (Tukey’s tests, P < 0.01), and AI inactivation (n = 6) did not alter locomotor activity relative to VEH pretreatment (n = 6) (Fig. 5).
AI neural inactivation fails to alter locomotor activity (mean photobeam breaks/1 h + SEM) in a novel context. VEH (white squares) or BM (1.0 + 0.1 mM/0.5 μL per hemisphere, black squares) into the AI immediately before testing. Symbols represent difference relative to the first 20-min interval (*Tukey test, P < 0.01)
Discussion
In the present study, BM-induced pharmacological inactivation of the AI had different effects on the expression of drug context-induced cocaine-seeking behavior and on cocaine memory reconsolidation. Specifically, AI inactivation attenuated the reinstatement of extinguished cocaine-seeking behavior upon exposure to the cocaine-paired context relative to vehicle pretreatment (Fig. 2b, c). The BM-induced decrease in cocaine-seeking behavior was not due to impairment in general motor activity given that AI inactivation did not attenuate inactive lever responding in the cocaine-paired context (Fig. 2d, e). A floor effect could have prevented the detection of a BM effect on inactive lever responding; however, AI inactivation also failed to alter locomotor activity in a novel context (Fig. 5). The effect of AI inactivation on reinstatement was anatomically selective to the AI in that BM administration into the SSJ, a dorsally adjacent brain region that is in the most likely path of unintended BM diffusion away from the AI, did not alter cocaine-seeking behavior (Fig. 3b–e). Contrary to our hypothesis, BM-induced AI inactivation at the time of memory reconsolidation (i.e., immediately after brief reexposure to the previously cocaine-paired context) failed to inhibit drug context-induced reinstatement of cocaine-seeking behavior approximately 72 h later (Fig. 4b–e). Expanding upon earlier studies that demonstrate the role of the AI in explicit CS-induced reinstatement of cocaine-seeking and nicotine-seeking behaviors (Cosme et al. 2015; Pushparaj et al. 2015a), the present findings indicate that the AI mediates the expression of drug context-induced cocaine-seeking behavior as opposed to the long-term, reconsolidation-dependent maintenance of cocaine memories established in the reinstatement paradigm.
Extant literature suggests that the AI may facilitate the expression of drug context-induced cocaine-seeking behavior through multiple, related mechanisms. Sensory input from the thalamus passes through the granular and dysgranular insular cortices, which encode stimulant reward (Contreras et al. 2007; Pushparaj et al. 2013), before it enters the AI. In turn, the AI is thought to maintain cognitive representations of interoceptive states associated with previous experiences, presumably including context-dependent drug experiences, and combines these into a conscious affective state (Contreras et al. 2007; Naqvi et al. 2014). In line with this, the AI is recruited to control goal-directed behavior upon exposure to stimuli that are more likely to engender a distinct interoceptive state. For instance, when CS salience is limited (i.e., a single vs compound cue is paired with cocaine infusions), lidocaine-induced AI inactivation inhibits the reinstatement of drug-seeking behavior when response-contingent CS presentation occurs in the presence of an olfactory, but not an auditory, background stimulus during training and testing (Di Pietro et al. 2006). This also suggests that AI recruitment was likely optimized in the present study since the cocaine-paired context included tactile and olfactory, in addition to visual and auditory, stimuli. In further support of this possibility, drug-associated multi-modal contextual stimuli in particular elicit insula activation and an associated increase in subsequent smoking behavior in cigarette smokers (McClernon et al. 2016).
Interestingly, chronic and acute AI dysfunctions appear to differently alter drug seeking and impulsivity (a cognitive factor contributing to relapse propensity). Cocaine users exhibit decreases in insular cortex gray matter density (Franklin et al. 2002). Insular cortex gray matter volume reduction in cocaine users (Moreno-López et al. 2012), AI cortical thinness in rats (Belin-Rauscent et al. 2016), and insular cortex lesions in drug-naïve individuals (Clark et al. 2008) and AI lesions in rats (Belin-Rauscent et al. 2016) positively correlate with measures of risky decision making and trait impulsivity. Furthermore, diminished insular cortex activation during a drug-unrelated decision-making task in methamphetamine users (Paulus et al. 2005) and reduced resting functional connectivity between the insular cortex and sensorimotor cortices in smokers (Addicott et al. 2015) are associated with increased probability of drug relapse. Similarly, AI lesions in rats potentiate cocaine-seeking behavior after forced abstinence (Pelloux et al. 2013). Thus, we speculate that tonic AI hypoactivity may contribute to heightened CS-induced AI activation in cocaine users (Kühn and Gallinat 2011) and increased drug context-induced AI activation and smoking behavior in cigarette smokers (McClernon et al. 2016). Consistent with this, transient AI inactivation reliably inhibits CS- and drug context-induced drug-seeking behavior (present study; Cosme et al. 2015; Pushparaj et al. 2015a), and it can reduce impulsive behavior (Pushparaj et al. 2015b; Ishii et al. 2012, but see St. Onge and Floresco 2010) in rats. Based on similar observations in the lOFC (Fuchs et al. 2004; Lasseter et al. 2009), we propose that neural activity in the AI in response to drug context- or CS-related sensory cortical inputs encourages prepotent responses, such as drug-seeking behavior, and this neural response may be magnified by tonic AI hypoactivity and/or related neuroadaptations in chronic drug users.
The finding that pharmacological inactivation of the AI after cocaine memory reactivation failed to alter subsequent drug context-induced reinstatement of cocaine-seeking behavior was not due to insufficient memory destabilization or BM dosing. Studies from our laboratory indicate that 15-min exposure to the cocaine-paired context results in reliable cocaine memory destabilization and subsequent memory reconsolidation in our paradigm (Fuchs et al. 2009; Ramirez et al. 2009; Wells et al. 2011; Wells et al. 2013; Arguello et al. 2014). In addition, the BM dose used in the present study disrupted the expression of cocaine-seeking behavior after infusion into the AI (Fig. 2b) and has been shown to inhibit cocaine memory reconsolidation upon infusion into the BLA or the dorsal hippocampus (DH) in our paradigm (Fuchs et al. 2009; Ramirez et al. 2009). Despite the use of identical methodology, responding during the memory reactivation session was slightly greater in AI-cannulated rats in the present study (Fig. 4) than in BLA- or DH-cannulated rats in our previous memory reconsolidation studies (20–30/15-min session; Fuchs et al. 2009; Ramirez et al. 2009). However, the magnitude of cocaine-seeking behavior, and thus the sensitivity for detecting a BM effect, at test was identical across these studies. Based on these considerations, we conclude that the functional integrity of the AI is not necessary for cocaine memory reconsolidation and thus for lasting contextual stimulus-control over cocaine-seeking behavior under the current experimental parameters.
The absence of an AI inactivation effect on cocaine memory reconsolidation (Fig. 4) was unexpected, given that the AI plays an important role in the reconsolidation of Pavlovian context-amphetamine memories (Contreras et al. 2012). Specifically, intra-AI administration of the protein synthesis inhibitor, anisomycin, at the time of drug memory reconsolidation [i.e., immediately after a conditioned place preference (CPP) test] produces long-lasting inhibition in amphetamine CPP concomitant with a decrease in AI zif268 immunoreactivity. The effect on zif268 immunoreactivity is notable because zif268 expression in the BLA is known to play a fundamental role in cocaine memory reconsolidation both in Pavlovian models of conditioned drug effects and in instrumental models of cue-induced drug seeking (Lee et al. 2005; Lee et al. 2006; Théberge et al. 2010). Accordingly, the apparent discrepancy between these and the present findings may reflect that the AI selectively contributes to memory reconsolidation in Pavlovian paradigms. The AI is not unique in this respect; the nucleus accumbens (NAc) appears to mediate cocaine memory reconsolidation similarly in Pavlovian, but not in instrumental, paradigms (Miller and Marshall 2005; Théberge et al. 2010; Wells et al. 2013). Methodological differences and consequent dissimilarities in memory age, strength, and structure (i.e., Pavlovian context-drug memories versus instrumental context-response-drug associative memories) in these paradigms may lead to the differential recruitment of the AI and other brain regions. However, our findings do not rule out the possibility that the AI may play a role in instrumental memory reconsolidation under other experimental conditions or that it may regulate phenomena, like memory destabilization, that prompt memory reconsolidation.
Conclusions
The AI plays a general role in the expression of cocaine-seeking behavior elicited by exposure to a drug-associated context (present study) or explicit CS (Di Pietro et al. 2006; Cosme et al. 2015), and it appears to promote selectively the reconsolidation of drug memories established in Pavlovian (Contreras et al. 2012), but not in instrumental (present study), models. The AI is in an ideal position to influence multiple hedonic, motivational, and executive cognitive functions that bring about drug relapse through its outputs directed at several brain regions, including the dorsomedial PFC, lOFC, BLA, hippocampus, and NAc (Naqvi et al. 2014). The AI may be particularly important for the retrieval of cue/drug-related emotional associations and the anticipation of impending drug experiences (Lovero et al. 2009). AI input regarding conscious emotional state may be utilized by the BLA to assess the motivational effects of drug-predictive stimuli based on predicted outcome value (Fuchs and See 2002; Naqvi et al. 2014). Tight functional connection between the AI and BLA can also be inferred based on the similar, cue type-independent involvement of these brain regions in cue-induced cocaine-seeking behavior, but not in drug-primed reinstatement (Grimm and See 2000; Fuchs et al. 2005; Cosme et al. 2015). The AI also functions as a detector of salient events and signals information to the dorsomedial PFC and lOFC upon exposure to cocaine-paired environmental stimuli, and thus, it guides executive functions that impact response selection (Lasseter et al. 2010; Menon and Uddin 2010; Khani and Rainer 2016). Finally, excitatory input from the AI to the NAc core increases the vigor of drug-seeking behavior despite negative consequences, as indicated by a decrease in quinine-laced alcohol-seeking behavior following optogenetic inhibition of AI neurons that project to the NAc core (Seif et al. 2013). This suggests that the AI is a critical hub of information processing within the relapse circuitry. Therefore, future studies will need to identify critical neural pathways through which the AI supports cue-induced relapse to cocaine-seeking behavior and cocaine-induced plasticity, as well as possible sex differences in these pathways, in order to increase our neurobiological understanding of drug addiction.
References
Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ (2015) Increased functional connectivity in an insula-based network is associated with improved smoking cessation outcomes. Neuropsychopharmacology 40:2648–2656
Arguello AA, Hodges MA, Wells AM et al (2014) Involvement of amygdalar protein kinase A, but not calcium/calmodulin-dependent protein kinase II, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology 231:55–65
Barak S, Liu F, Ben Hamida S et al (2013) Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci 16:1111–1117
Belin-Rauscent A, Daniel ML, Puaud M, Jupp B, Sawiak S, Howett D, McKenzie C, Caprioli D, Besson M, Robbins TW, Everitt BJ, Dalley JW, Belin D (2016) From impulses to maladaptive actions: the insula is a neurobiological gate for the development of compulsive behavior. Mol Psychiatry 21(4):491–499
Childress AR, Mozley PD, McElgin W et al (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18
Clark L, Bechara A, Damasio H et al (2008) Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 131:1311–1322
Contreras M, Ceric F, Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318:655–658
Contreras M, Billeke P, Vicencio S et al (2012) A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology 37:2101–2108
Cosme CV, Gutman AL, LaLumiere RT (2015) The dorsal agranular insular cortex regulates the cued reinstatement of cocaine-seeking, but not food-seeking, behavior in rats. Neuropsychopharmacology 40:2425–2433
Di Pietro NC, Black YD, Kantak KM (2006) Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci 24:3285–3298
Ehrman RN, Robbins SJ, Childress AR, O’Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107:523–529
Foltin RW, Haney M (2000) Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology 149:24–33
Franklin TR, Acton PD, Maldjian JA et al (2002) Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 51:134–142
Fuchs RA, See RE (2002) Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology 160:425–433
Fuchs RA, Fuchs RA, Evans KA, Parker MP, See RE (2004) Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci 24(29):6600–6610
Fuchs RA, Evans KA, Ledford CC et al (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rat. Neuropsychopharmacology 30(2):296–309
Fuchs RA, Eaddy JL, Su Z-I, Bell GH (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498
Fuchs RA, Bell GH, Ramirez DR et al (2009) Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30:889–900
García-DeLaTorre P, Rodriguez-Ortiz CJ, Arreguin-Martinez JL, Cruz-Castañeda P, Bermúdez-Rattoni F (2009) Simultaneous but not independent anisomycin infusions in insular cortex and amygdala hinder stabilization of taste memory when updated. Learn Mem 16(9):514–519
García-DeLaTorre P, Rodríguez-Ortiz CJ, Balderas I, Bermúdez-Rattoni F (2010) Differential participation of temporal structures in the consolidation and reconsolidation of taste aversion extinction. Eur J Neurosci 32(6):1018–1023
Grant S, London ED, Newlin DB et al (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A 93:12040–12045
Grimm JW, See RE (2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22:473–479
Ishii H, Ohara S, Tobler PN et al (2012) Inactivating anterior insular cortex reduces risk taking. J Neurosci 32:16031–16039
Khani A, Rainer G (2016) Neural and neurochemical basis of reinforcement-guided decision making. J Neurophysiol 116:724–741
Kobilo T, Hazvi S, Dudai Y (2007) Role of cortical cannabinoid CB1 receptor in conditioned taste aversion memory. Eur J Neurosci 25(11):3417–3421
Kühn S, Gallinat J (2011) Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 33:1318–1326
Lasseter HC, Ramirez DR, Xie X, Fuchs RA (2009) Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci 30:1370–1381
Lasseter HC, Xie X, Ramirez DR, Fuchs RA (2010) Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci 3:101–117
Lee JLC, Di Ciano P, Thomas KL, Everitt BJ (2005) Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47:795–801
Lee JLC, Milton AL, Everitt BJ (2006) Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26:5881–5887
Liu P, Zhang J, Li M, Sui N (2016) Distinctive roles of 5-aza-2'-deoxycytidine in anterior agranular insular and basolateral amygdala in reconsolidation of aversive memory associated with morphine in rats. Front Behav Neurosci. doi:10.3389/fnbeh.2016.00050
Lovero KL, Simmons AN, Aron JL, Paulus MP (2009) Anterior insular cortex anticipates impending stimulus significant. NeuroImage 45:976–983
McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, Addicott MA, Chou YH, Chen NK, Hallyburton MB, DeVito AM (2016) Hippocampal and insular response to smoking-related environments: neuroimaging evidence for drug-context effects in nicotine dependence. Neuropsychopharmacology 41(3):877–885
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:665–667
Milekic MH, Brown SD, Castellini C, Alberini CM (2006) Persistent disruption of an established morphine conditioned place preference. J Neurosci 26:3010–3020
Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873–884
Moreno-López L, Catena A, Fernández-Serrano MJ et al (2012) Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend 125:208–214
Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726
Naqvi NH, Gaznick N, Tranel D, Bechara A (2014) The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci 1316:53–70
National Research Council (2011) Guide for the care and use of laboratory animals: eight edition. The National Academies Press, Washington, DC. doi:10.17266/12910
Paulus MP, Tapert SF, Schuckit MA (2005) Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry 62:761–768
Paxinos G, Watson C (2014) The rat brain in stereotaxic coordinates, seventh edition. Academic Press, San Diego, United States
Pelloux Y, Murray JE, Everitt BJ (2013) Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci 38(7):3018–3026
Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, Le Foll B (2013) Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology 38(4):690–698
Pushparaj A, Kim AS, Musiol M et al (2015a) Involvement of the rostral agranular insular cortex in nicotine self-administration in rats. Behav Brain Res 290:77–83
Pushparaj A, Kim AS, Musiol M et al (2015b) Differential involvement of the agranular vs granular insular cortex in the acquisition and performance of choice behavior in a rodent gambling task. Neuropsychopharmacology 40:2832–2842
Ramirez DR, Bell GH, Lasseter HC et al (2009) Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30:901–912
Rohsenow DJ, Niaura RS, Childress AR et al (1990) Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict 25:957–993
Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK (2009) A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci 10(12):885–892
Seif T, Chang S-J, Simms JA et al (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16:1094–1100
Sorg BA (2012) Reconsolidation of drug memories. Neurosci Biobehav Rev 36:1400–1417
Sorg BA, Todd RP, Slaker M, Churchill L (2015) Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology 92:25–33
St. Onge JR, Floresco SB (2010) Prefrontal cortical contribution to risk-based decision making. Cereb Cortex 20:1816–1828
Stehberg J, Levy D, Zangen A (2009) Impairment of aversive memory reconsolidaiton by localized intracranial electrical stimulation. Eur J Neurosci 29:964–969
Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009) Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56(Suppl 1):186–195
Théberge FRM, Milton AL, Belin D et al (2010) The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem 17:444–453
Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8:262–275
Wang Y, Zhang TY, Xin J, Li T, Yu H, Li N, Chen ZY (2012) Differential involvement of brain-derived neurotrophic factor in reconsolidation and consolidation of conditioned taste aversion memory. PLoS One 7(11):e49942
Wells AM, Lasseter HC, Xie X et al (2011) Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine-memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem 18:693–702
Wells AM, Arguello AA, Xie X et al (2013) Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine-memory reconsolidation in rats. Neuropsychopharmacology 38:753–762
Zubedat S, Akirav I (2017) The involvement of cannabinoids and mTOR in the reconsolidation of an emotional memory in the hippocampal-amygdala-insular circuit. Eur Neuropsychopharmacol. doi:10.1016/j.euroneuro.2017.01.011
Acknowledgements
This work was supported by NIDA grants R01 DA017673 and DA025646.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols for the housing and treatment of animals were approved by the Institutional Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences 2011).
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Arguello, A.A., Wang, R., Lyons, C.M. et al. Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology 234, 2431–2441 (2017). https://doi.org/10.1007/s00213-017-4632-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4632-7