Abstract
Rationale
Previous studies have shown that repeated exposure to drugs of abuse is associated with changes in clock genes expression and that mice strains with various mutations in clock genes show alterations in drug-induced behaviors.
Objective
The objective of this study is to characterize the role of the clock gene mPer1 in the development of morphine-induced behaviors and a possible link to histone deacetylase (HDAC) activity.
Methods
In Per1 Brdm1 null mutant mice and wild-type (WT) littermates, we examined whether there were any differences in the development of morphine antinociception, tolerance to antinociception, withdrawal, sensitization to locomotion, and conditioned place preference (CPP).
Results
Per1 Brdm1 mutant mice did not show any difference in morphine antinociception, tolerance development, nor in physical withdrawal signs precipitated by naloxone administration compared to WT. However, morphine-induced locomotor sensitization and CPP were significantly impaired in Per1 Brdm1 mutant mice. Because a very similar dissociation between tolerance and dependence vs. sensitization and CPP was recently observed after the co-administration of morphine and the HDAC inhibitor sodium butyrate (NaBut), we studied a possible link between mPer1 and HDAC activity. As opposed to WT controls, Per1 Brdm1 mutant mice showed significantly enhanced striatal global HDAC activity within the striatum when exposed to a locomotor-sensitizing morphine administration regimen. Furthermore, the administration of the HDAC inhibitor NaBut restored the ability of morphine to promote locomotor sensitization and reward in Per1 Brdm1 mutant mice.
Conclusions
Our results reveal that although the mPer1 gene does not alter morphine-induced antinociception nor withdrawal, it plays a prominent role in the development of morphine-induced behavioral sensitization and reward via inhibitory modulation of striatal HDAC activity. These data suggest that PER1 inhibits deacetylation to promote drug-induced neuroplastic changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past decade, clock genes have been shown to possess other properties than simply acting as circadian core self-regulators sustaining a functional circadian clock (Rosenwasser 2010). By modulating multiple cellular outputs and neurobiological functions, clock genes and clock proteins thus appear to be key players in the preservation of mental health. In particular, numerous studies have demonstrated a role of multiple canonical clock genes (i.e., Per1, Per2, Clock, and NPAS2) in mood (Hampp et al. 2008; McClung 2007; Mendlewicz 2009; Mukherjee et al. 2010) and substance use disorders (Falcon and McClung 2009; Logan et al. 2014; Perreau-Lenz et al. 2007; Perreau-Lenz and Spanagel 2015).
The interplay between clock genes and substances of abuse has been now described repeatedly, especially for psychostimulants, opiates and alcohol (Logan et al. 2014; Perreau-Lenz and Spanagel 2008). Animal studies have shown that different clock genes affect drug-induced behaviors in a different manner, depending on the drug used and/or the behaviors studied. Clock and Per2 mutations enhance cocaine-induced locomotor sensitization, conditioned place preference (CPP), as well as alcohol consumption (Abarca et al. 2002; McClung et al. 2005). On the other hand, Per1 gene mutations lead to hyposensitization (absence of cocaine- and morphine-induced CPP and locomotor sensitization), while enhancing alcohol intake upon stressful stimuli (Abarca et al. 2002, Dong et al. 2011; Perreau-Lenz et al. 2009; Spanagel et al. 2005).
Numerous studies have now shown that clock proteins modulate various neurotransmitter systems involved in synaptic plasticity (for review, see Parekh and McClung 2016). Astroglial glutamate re-uptake is for instance modulated by the genes Clock, Npas2, and Per2 (Beaule et al. 2009; Spanagel et al. 2005). Similarly, clock genes have shown to directly affect dopamine receptors, transporter (Ozburn et al. 2015; Shumay et al. 2012), as well as dopamine metabolizing enzymes (Hampp et al. 2008). In addition, clock genes have shown to modulate nuclear receptor function (Ripperger and Albrecht 2012; Schmutz et al. 2010) as well as cell metabolism (Bellet and Sassone-Corsi 2011). These results led to the hypothesis that alterations in clock gene expression and clock protein function affect numerous processes at the molecular, cellular, and circuit level, hence leading to the development of substance use disorders (Perreau-Lenz et al. 2007).
Opiates, such as morphine, are used extensively for the treatment of severe acute and chronic pain; however, their use (and misuse) for their analgesic effect is plagued with side effects such as dependence and tolerance development (Volkow and McLellan 2016). A better understanding of the underlying mechanisms of these respective effects will thus reveal highly beneficial for public health. Interestingly, clock genes have revealed to be affected by opiate treatment, and, reciprocally, to modulate opiate-induced behaviors. Peak protein expression of Per2 is blunted in rat limbic areas during chronic morphine administration and withdrawal (Hood et al. 2011); and, Per1 and Per2 daily rhythms of messenger RNA (mRNA) expression are affected during and after opioid withdrawal, in both rodents (Ammon et al. 2003; Liu et al. 2005; Garmabi et al. 2016) and humans (Li et al., 2009). Sanchis-Segura and colleagues have also revealed that a sub-chronic 20 mg/kg morphine treatment enhances mPer1 mRNA within the striatum (Sanchis-Segura et al. 2009). Reciprocally, we have shown that a functional mPER2 promotes the development of tolerance to the analgesic effects of morphine and enhances the expression of precipitated withdrawal (Perreau-Lenz et al. 2010). Other studies have provided initial evidence for the involvement of the gene Per1 in the development of morphine-induced locomotor sensitization and morphine-induced CPP (Li et al. 2008; Liu et al. 2007; Liu et al. 2005; Sanchis-Segura et al. 2009). Altogether, these studies reveal the potential implication of Per genes in modulating various opiate-induced effects. These studies shall be completed to characterize fully those effects and underlying mechanisms of action remain to be identified. However, the implication of Per1, in particular, in modulating morphine-induced behaviors still needs to be fully characterized (analgesic, tolerance, sensitization, reward, and dependence).
Epigenetic mechanisms such as DNA methylation and post-translational histone modifications (i.e., histone acetylation) are being currently investigated as part of the cellular mechanisms underlying long-lasting neuroplastic changes triggered by drugs of abuse in neural tissue. Accumulating evidence suggests a modulatory role of histone acetyl transferases (HATs) and histone deacetylases (HDACs) on drug-induced behaviors such as CPP and locomotor sensitization (Adachi and Monteggia 2009; Takizawa and Meshorer 2008). Focusing on the latter, it has been reported that amphetamine- (Kalda et al. 2007; Shen et al. 2008), cocaine- (Kumar et al. 2005; Sanchis-Segura et al. 2009) as well as ethanol- and morphine- (Sanchis-Segura et al. 2009) induced locomotor sensitization is enhanced by histone deacetylase (HDAC) inhibition. Interestingly, Sanchis-Segura et al. (2009) have shown that, as opposed to tolerance and withdrawal, morphine-induced behavioral sensitization and CPP are specifically influenced by chromatin remodeling. Furthermore, mPer1 gene expression is specifically enhanced during locomotor sensitization, and even further enhanced following selective boosting of morphine-induced behavioral sensitization induced by sodium butyrate (NaBut), a class I and II HDAC inhibitor (Sanchis-Segura et al. 2009). Interestingly, class IIa HDACs have shown to regulate circadian clock function (Fogg et al. 2014) and chromatin remodeling is playing a major role in both plasticity and circadian clock-work (Zocchi and Sassone-Corsi 2010). This last piece of evidence suggests that mPer1 gene expression is regulated by inhibiting HDAC and it might represent the missing link between clock genes and drug-induced cellular mechanisms.
In the present study, we examined whether morphine-induced antinociceptive effects, tolerance, and dependence behaviors would be altered in Per1 Brdm1 null mutant mice compared to WT. We first assessed in Per1 Brdm1 null mutant mice and their respective wild-type littermate controls (WT) in the development and expression of morphine-induced tolerance, withdrawal, locomotor sensitization, and CPP. We then studied the role of HDAC activity in Per1 mediated changes in morphine-induced effects.
Material and methods
Animals
In the present study, we used 12 to 16-week-old male Per1 Brdm1 mutant mice, described in Zheng et al. (2001), and their respective wild-type littermates stemming from a heterozygous breeding. All mice were housed individually, kept under a 12-h light/12-h dark conditions (lights were on from 07:00 to 19:00 h) and fed ad libitum. Behavioral experiments started 1 or 2 weeks after the arrival of the animals in the experimental facilities from the breeding colony room.
All experimental procedures were carried out in Mannheim (Germany) following the principles of laboratory animal care as described in the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003) and approved by the Committee on Animal Care and Use (Regierungspräsidium Karlsruhe) in accordance with the local Animal Welfare Act and the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Behavioral procedures
Morphine antinociception and tolerance development
The development of tolerance to the analgesic effects of morphine was studied using two different behavioral procedures: the tail immersion and the hotplate tests as described previously (Perreau-Lenz et al. 2010). All pharmacological treatments and behavioral tests were completed during the light cycle between ZT3 and ZT5 (ZT stands for Zeitgeber Time, where ZT0 and ZT12 correspond to the beginning of the light and the dark cycle phase, respectively).
Naloxone-precipitated withdrawal
In a different cohort of mice, opioid dependence was induced by repeated injections of morphine as previously described (Perreau-Lenz et al. 2010). Briefly, mice received morphine injections twice a day, at ZT3 (3 h after lights on) and ZT15 (3 h after lights off), with incrementing doses (20, 40, 60, 80, and 100 mg/kg, i.p.) for five consecutive days. On the 6th day, mice were injected with morphine (100 mg/kg, i.p.; ZT2) and were individually placed in observation boxes (22 × 22 × 40 cm; Tru Scan Photobeam activity monitors, Coulbourn Instruments, Allentown, USA) after one hour and a half. Thirty minutes later, morphine withdrawal was precipitated by a subcutaneous naloxone injection (1 mg/kg; Sigma-Aldrich) and withdrawal signs were evaluated during 30 min. Two kinds of signs were evaluated: (i) behavioral signs, scored as the number of jumps, rearing, orofacial movements, forepaw tremors and shakes and (ii) physiological signs such as diarrhea and loss of body weight.
Locomotor sensitization
All locomotor sensitization experiments were conducted between ZT4 and ZT6. The protocol chosen was based on the one previously described by Sanchis-Segura et al. (Sanchis-Segura et al. 2009) using the observation boxes mentioned above (Tru Scan Photobeam activity monitors, Coulbourn Instruments, Allentwon, USA) during sessions lasting 1 h each.
Morphine-induced locomotor sensitization was assessed in a first cohort of Per1Brdm1 mutant and WT control mice. Mice were first habituated to the boxes and injected with saline for two successive daily sessions. On the third day, basal locomotion was assessed following saline injection (10 ml/kg; i.p). On the following day, mice were administered 20 mg/kg morphine (i.p; Sigma-Aldrich, Germany) and placed on the observation boxes for 1 h. Morphine-induced locomotion, using the same dose of morphine was then assessed every other day resulting in six morphine sessions (M1-M6). After the 6th morphine session, mice were left untreated for 7 days, and the short-term expression of locomotor sensitization was assessed for 1 h after morphine challenge (20 mg/kg; i.p). One week after an unbiased CPP experiment using custom made two compartment boxes previously described (Abarca et al., 2002; Engblom et al., 2008) (see below), mice were again challenged and long-term expression of sensitization was assessed.
In a second cohort of WT and Per1Brdm1 mutant mice, we assessed the effect of a class I and II HDAC inhibitor (Steliou et al. 2012), NaBut on boosting the development of locomotor sensitization. As in the first experiment, mice were first habituated to the boxes and injected with saline for two successive daily sessions. On the third day, basal locomotion was assessed following saline injection (10 ml/kg; i.p). Mice of each genotype were then separated into two groups and received injections every other day for a total of six sessions. One group of each genotype received simultaneous injections of saline and 20 mg/kg morphine (S+M) whereas the second group received simultaneous injections of 150 mg/kg NaBut and morphine (NB+M) and were then placed in the observation boxes for 1 h. Following the 6th sensitization session, mice were left untreated for 7 days, and the short-term expression of locomotor sensitization was assessed after a single 20 mg/kg morphine injection.
Conditioned place preference test
For the first experiment, we examined whether morphine CPP would be altered in Per1Brdm1 null mutant mice compared to WT littermates. The CPP apparatus consisted of six custom-made gray acrylic chambers (32 cm long × 16 wide × 22 cm high) that had two different sections as previously described in Abarca et al. (2002). In these sections, tiles with two different textures could be placed. The first type of tile, denoted as “Hole” had holes punched into the tile, whereas the second tile had a “Rod” texture. Behavior was monitored by a video-tracking system (Ethovision 2.0, Noldus, The Netherlands), which enabled us to determine locomotion and spatial placement of each mouse. CPP training and testing was conducted over 10 consecutive days, one pre-conditioning day (day 1), eight conditioning days (days 2–9), and a final CPP drug-free test day (day 10). On day 1, mice were placed inside the conditioning chamber with a distinctive floor (a smooth plastic surface covered with soft tissue paper) and were exposed to the apparatus for 30 min. For conditioning (days 2–9), an unbiased pavlovian conditioning procedure was used as described previously (Sanchis-Segura et al. 2009). Briefly, mice of both genotypes were given morphine (20 mg/kg) and vehicle injections on alternate days, with alternating floor type (rod or hole). During this conditioning phase, the animals had access to the entire apparatus but only one floor type (rod or hole) was presented. During the drug session, each mouse received an injection of morphine (20 mg/kg) and was immediately confined to the apparatus for 30 min with a particular floor type, whereas during the vehicle session, animals were injected with equivalent volumes of saline and were exposed to the alternate floor type. Morphine was paired with the “Rod” floor type for half the animals and with the “hole” floor type for the remaining half. These two sessions were repeated over eight consecutive days such that animals received four drug sessions and four vehicle sessions on alternating days. On day 10, a preference test was assessed in unchallenged mice for 15 min in the presence of the two floor type. CPP score was calculated as time spent on the morphine-paired floor—time spent on the saline-paired floor.
In a second CPP experiment, we examined the effects of NaBut (150 mg/kg) on morphine CPP in Per1Brdm1 null mutant mice and WT mice. For these experiments, we used an unbiased protocol; however, the CPP boxes consisted of three distinct compartments: an entry/corridor compartment and two different conditioning compartments that had varying visual and tactile cues (Panlab, Barcelona, Spain). After eight daily 30-min conditioning sessions (four alternating drug sessions with 20 mg/kg morphine with four vehicle sessions), morphine CPP was assessed on day 9 in unchallenged mice given access to the entire apparatus for 15 min.
HDAC enzymatic activity
Wild-type and Per1 Brdm1 mutant mice underwent six i.p. injections of 20 mg/kg morphine or saline 10 ml/kg every other day at ZT5, as described above for the locomotor sensitization protocol. One hour after the 6th morphine or saline injection, mice were sacrificed by cervical dislocation and the striatum and prefrontal cortex was quickly dissected from the brains. Nuclear proteins were extracted using a nuclear protein extraction kit (EpiQuik™ Nuclear Extraction Kit I, Epigentek Group Inc., New York, USA). Striatal tissue was transferred into ice-cold lysis buffer and nuclear proteins were extracted according to kit manufacturer’s instructions. The protein content of the nuclear protein extracts was determined via a Bradford test using the Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories Inc., Hercules, USA). Further, the HDAC enzyme activity was determined in microtitre plates (Rotilabo®-Mikrotest-Plates; Carl Roth GmbH + Co.KG, Karlsruhe, Germany) using the Colorimetric HDAC Activity Assay Kit (BioVision Research Products, Mountain View, USA) following manufacturer’s instructions.
Data analysis and statistics
All data analyses were performed using the software Statistica 6.1 (StatSoft, Inc., Tulsa, OK, USA). Since the data set on the latencies to the first response obtained in the tolerance tests failed to follow a normal distribution, we transformed these data sets using a square root transformation. These transformed data were then analyzed with two-way ANOVAs for repeated measures followed, when appropriate, by Student Newman-Keuls post hoc tests. The degree of tolerance was defined as the difference between the theoretical 100% of tolerance to the analgesic effect of morphine and the percentage of maximum effect observed on D7 over D1, and the following formula was therefore applied: Degree of tolerance (%) = 100%—((100×(LatencyD7–LatencyD0)/(LatencyD1–LatencyD0)). Student’s t tests for independent samples were used to analyze the calculated degrees of tolerance. For analyzing the withdrawal responses, a one-way MANOVA analysis was performed, and group means for each locomotor or physiological withdrawal sign was then assessed using Newman-Keuls post hoc tests. Student’s t tests for independent samples were used to compare global indexes of withdrawal symptomatology. These indexes were calculated assigning weight values to each sign (a weight value of 1 for body weight loss, shakes and rearing, of 1.5 for diarrhea and orofacial movements, 0.8 for jumping, and 0.35 for forepaw tremors) as previously published (Perreau-Lenz et al. 2010). For the locomotor sensitization and CPP experiments, one-way or two-way ANOVAs for repeated measures were applied followed by Newman-Keuls post hoc tests when appropriate.
Results
Role of Per1 in development of morphine-induced behaviors
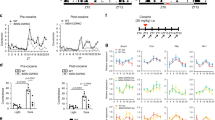
We tested various morphine-induced behaviors in Per1 Brdm1 mutant and WT mice, and results are presented in Fig. 1.
Morphine-induced tolerance (a, b), withdrawal (c, d), locomotor sensitization (e), and CPP (f) in WT and Per1 Brdm1 mutant mice. a, b Tolerance to the analgesic effects of morphine in the tail immersion (a) or hotplate test (b) in WT and Per1 Brdm1 mutant mice. Latencies to tail immersion (a) or to a first response on the hotplate (b) after saline (D0), acute (D1), and repeated morphine injections (D7) did not differ between genotypes (n = 8–10 mice per genotype or n = 16–18 mice per genotype for the tail immersion or hotplate test, respectively). Mean values ± SEM of latencies (s) to a rapid flick of the tail (a) or to the first response (b). c, d After chronic treatment with morphine, naloxone-precipitated withdrawal signs were assessed. No difference was observed between genotypes (c). Global indexes of the intensity of all withdrawal signs (d) were calculated for each subject as described in the Experimental procedure section. Data are expressed as mean value ± SEM (n = 16–18 mice per genotype). e Open-field locomotor activity assessed every other day, for 1 h, after morphine challenge (20 mg/kg; i.p.). The intercepts, slopes, and correlation coefficients of the regression curves were as follows: b(0) = 7735.51, b(1) = 2560.37, r 2 = 0.89 and b(0) = 7759.77, b(1) = 201.22, r 2 = 0.41 for the WT and Per1Brdm1 mutant mice, respectively. As indicated by the slopes of the regression analyses (b1), WT and Per1 Brdm1 mutant mice significantly differed (F Genotype(1,15) = 6.08, p < 0.05) in locomotor enhancement induced by repeated morphine injections treatment. *indicate significant differences from the first morphine injection M1. f Although WT mice showed a clear preference for the morphine-paired compartment, the Per1 Brdm1 mutant mice failed to show such a response. *indicate significant post hoc difference (p < 0.01) from the time spent on saline-paired floor. Data are expressed as mean value ± SEM (n = 8–9 mice per genotype)
Per1 mutation does not affect morphine antinociception or tolerance (Fig. 1 a, b)
Data in latencies presented in Fig. 1 for both the tail immersion (Fig. 1a) and the hotplate (Fig. 1b) test did not follow a normal distribution (Shapiro-Wilk test: W = 0.84 and p < 0.0001, and W = 0.65 and p < 0.0001, respectively). We therefore normalized these data using a square root transformation. A two-way ANOVA for repeated measures performed on these normalized data sets revealed that the chronic morphine treatment significantly enhanced the latencies of response for both tail immersion (F Treatment(2,28) = 37.04, p < 0.0001) and the hotplate test (F Treatment(2,64) = 60.90, p < 0.0001). These data indicated that morphine produced an increase in response and therefore was antinociceptive following acute administration regardless of genotype (p < 0.05). Furthermore, following repeated injections, a decrease in response was observed in both Per1 Brdm1 null mutant and WT mice (p < 0.05). However, Per1 Brdm1 mutant and WT littermates did not react differently to such a treatment (two-way ANOVA for repeated measures: F Treatment×Genotype(2,28) = 1.32, p = 0.28 and F Treatment×Genotype(2,64) = 1.34, p = 0.28, respectively). The degree of tolerance calculated for the tail immersion or the hotplate tolerance tests for the WT (37.87 ± 11.62 and 56.30 ± 10.49%, respectively) and the Per1 Brdm1 mutant mice (38.85 ± 11.62%, respectively) did not significantly differ between genotypes (T 14 = −0.83, p = 0.42 and T 32 = 1.29, p = 0.21, respectively). Thus, the results of this experiment revealed that Per1 Brdm1 mutant mice did not differ from their control littermates in either sensitivity to the acute analgesic effects of morphine, or development of tolerance towards the analgesic effects of morphine.
Per1 mutation does not affect naloxone-precipitated withdrawal (Fig. 1c, d)
Following precipitation of morphine withdrawal, using an acute injection of naloxone, locomotor activity measured in Per1 Brdm1 (1520.13 ± 175.26 cm/30 min) also did not significantly differ (T(16) = −1.30, p = 0.21) from their respective WT littermates (1251.73 ± 110.57 cm/30 min) (Fig. 1c). Furthermore, except for rearing (F Genoytpe(1,23) = 7.5, p = 0.012), each withdrawal sign assessed failed to show significant difference between genotype when analyzed separately (body weight loss: F(1,32) = 0.17, p = 0.68; diarrhea: F(1,32) = 0.06, p = 0.81; jumping: F(1,32) = 1.48, p = 0.23; forepaw tremor: F(1,32) = 0.21, p = 0.65; shakes: F(1,32) = 0.09, p = 0.77; facial movements: F(1,32) = 1.19, p = 0.28). Altogether, the various morphine-induced withdrawal signs assessed did not significantly differ between genotypes (one-way MANOVA: Wilks lambda = 0.62, F Genotype(7,26) = 2.27, p = 0.06). Accordingly, (Fig. 1d), global withdrawal scores (41.28 ± 3.94 and 36.10 ± 4.02 for the WT and the Per1 Brdm1 mutant mice, respectively) also failed to reveal any difference between genotype (T 32 = 0.66, p = 0.51).
Per1 mutation impairs morphine-induced locomotor sensitization and CPP (Fig. 1 e, f)
A clear impairment of the development of behavioral sensitization and CPP was observed in Per1Brdm1 null mutant mice compared to their WT littermates. Repeated morphine treatment differently affected the two genotypes when looking at the development of sensitization to morphine locomotor activity (two-way ANOVA for repeated measures: F Genotype×Treatment(5,75) = 5.21, p < 0.001). Repeated morphine injections induced a typical progressive increase in locomotor activity in WT littermates across the six sessions but failed to do so in the Per1Brdm1 mutant mice. Slopes of the linear regression analyses were also significantly lower (F Genotype(1,15) = 6.08, p < 0.05) in the Per1Brdm1 mutant mice (19.39 ± 445.09) than in WT littermates (1926.71 ± 650.86). Furthermore, the two different genotypes also differed in morphine-induced CPP (two-way ANOVA: F Treatment×Genotype(1,34) = 6.16, p < 0.05)—Per1Brdm1 mutant mice failed to show a preference for the morphine-associated compartment).
Morphine-induced HDAC striatal activity is upregulated in Per1Brdm1 mutant mice
We subsequently assessed whether striatal morphine-induced HDAC activity was differently regulated in WT and Per1 Brdm1 mutant mice. As shown in Fig. 2, HDAC activity was not different in morphine-naïve WT and Per1 Brdm1 mutant mice but was differently and significantly affected following repeated morphine injections depending on the genotype (F Treatment×Genotype(1,38) = 6.06, p < 0.05), with Per1 Brdm1 mutant mice showing higher levels of HDAC activity than WT following morphine treatment. In addition, when each genotype is analyzed separately, HDAC activity of WT mice showed a significant reduction upon morphine treatment as opposed to saline treatment (T 18 = 2.12, p < 0.05). At the opposite, HDAC activity of Per1 Brdm1 mutant mice was enhanced (T 18 = 2.03, p < 0.05). These data indicate that HDAC activity might be involved in the differential effects of repeated morphine injections in WT and Per1 Brdm1 mutant mice.
Striatal histone deacetylase activity induced by morphine treatment in Per1 Brdm1 and WT littermates. HDAC activity is significantly elevated in the striatum in Per1 Brdm1 mutants as compared to WT control littermates (*indicates p < 0.01). Data are expressed as mean value ± SEM (n = 9–11 mice per genotype)
HDAC inhibition restores morphine-induced behavioral sensitization and CPP in Per1Brdm1 mutant mice
In different sets of animals, we further examined the potential mechanism by which the inhibition of CPP and behavioral sensitization was occurring. In these experiments, WT and Per1 Brdm1 null mutant mice received concomitant injections of 20 mg/kg morphine and the HDAC inhibitor NaBut (150 mg/kg; i.p.) or saline.
The effects of NaBut on morphine-induced locomotor sensitization in WT and Per1Brdm1 null mutant mice (Fig. 3a, b)
As shown in Fig. 3a, administration of NaBut enhanced the effect of morphine on the development of locomotor sensitization (three-way ANOVA: F Treatment(1,35) = 9.04, p < 0.01; F Days×Treatment(5,175) = 3.95, p < 0.01). Both genotypes also significantly differed (F Days×Genotype(5,175) = 3.29, p < 0.01), but their reaction to the NaBut treatment did not (F Treatment×Genotype(1,35) = 0.0003, p = 0.99). Post hoc comparisons revealed that repeated morphine injections in saline- and NaBut-pre-treated WT mice led to a progressive enhancement of locomotion. In Per1 Brdm1 mutant mice, this effect was only observed in NaBut-treated mice. Furthermore, a two-way ANOVA of the regression slopes obtained with the linear regression fits of our data revealed a significant effect of the NaBut treatment (F Treatment(1,35) = 7.93, p < 0.01) and a significant genotype effect (F Genotype(1,35) = 5.82, p < 0.05). We thus confirmed an impaired development of morphine-induced locomotor sensitization in the Per1 Brdm1 mutant mice concomitantly treated with saline, and the boosting effect of NaBut on the development of locomotor sensitization (Sanchis-Segura et al. 2009). As shown in Figure3b, we also showed that pre-treatment with NaBut is able to restore the expression of morphine-induced locomotor sensitization in the Per1 Brdm1 mutant mice following a morphine challenge (two-way ANOVA: F Genotype(1.35) = 7.32, p < 0.05; F Treatment(1,35) = 7.71, p < 0.01; F Genotype×Treatment(1,35) = 1.147, p = 0.29).
HDAC inhibitor administration restores locomotor sensitization and CPP in Per1 Brdm1 mutant mice. a NaBut restores the development of locomotor sensitization in Per1 Brdm1 mutant mice. Open-field distance traveled by WT and Per1 Brdm1 mutant mice assessed every other day, for 1 h, after administration of morphine (20 mg/kg; i.p.) concomitant with NaBut (150 mg/kg) or saline administration. Per1 Brdm1 mutant mice only showed locomotor sensitization development when morphine was co-administered with NaBut. The intercepts (b(0), slopes (b(1)) and correlation coefficients (r 2) of the linear regressions were as follows: b(0) = 8864.54, b(1) = 2402.26, r 2 = 0.86 for the WT pre-treated with saline; b(0) = 14,527.27, b(1) = 3418.47, r 2 = 0.93 for the WT pre-treated with NaBut; b(0) = 8298.13, b(1) = 417.43, r 2 = 0.26 for the Per1Brdm1 mutant mice pre-treated with saline; b(0) = 12,704.06, b(1) = 2487.23, r 2 = 0.95 for the Per1Brdm1 mutant mice pre-treated with NaBut. *indicates significant differences from the first morphine injection M1; p < 0.05. b NaBut restores the expression of locomotor sensitization in Per1 Brdm1 mutant mice. Difference on the distance traveled following the acute (M1) and expression (EXP) morphine (20 mg/kg) challenge in mice pre-treated with either saline or NaBut concomitantly. *indicates significant differences from all treated group (p < 0.01 using LSD Fisher pos-hoc test)—data are expressed as mean value ± SEM (n = 10–11 mice per genotype and per group). c NaBut restores CPP in Per1 Brdm1 mutant mice. Morphine-induced CPP was impaired in Per1 Brdm1 mutant mice compared to WT mice, and the administration of NaBut restored the CPP response in the mutants while having no effect in the WT mice. * and # indicate significant (p < 0.05); genotype and treatment differences, respectively—data are expressed as mean value ± SEM (n = 6–8 mice per genotype and per group)
The effects of NaBut on morphine CPP in WT and Per1Brdm1 null mutant mice (Fig. 3c)
Looking at the effects of NaBut on morphine CPP, the overall ANOVA indicated a significant Genotype by Treatment effect (FGenotype×Treatment(1,23) = 13.13, p < 0.001). As in the first CPP experiment, morphine CPP was not evident in Per1 Brdm1 null mutant mice, whereas WT animals spent significantly more time in their drug-paired compartment. However, administration of NaBut restored CPP in Per1 Brdm1 mutant mice as shown in Fig. 3c (two-way ANOVA: F Genotype(1,23) = 1.25, p = 0.3; F Treatment(1,23) = 13.55, p < 0.005; F Genotype×Treatment(1,23) = 13.13, p < 0.001).
Discussion
The present results first show that, as opposed to what we previously observed for the mPer2 gene (Perreau-Lenz et al. 2010), mPer1 does not seem to be involved in the development of morphine-induced tolerance nor naloxone-precipitated withdrawal. Second, we confirmed the involvement of the gene mPer1 in the development of CPP responses to morphine (Liu et al. 2005) and provide evidence for its involvement in the development and expression of morphine-induced locomotor sensitization. Finally, we were able to rescue locomotor sensitization and CPP in Per1 Brdm1 mutant mice using a class I/class II HDAC inhibitor, NaBut, and showed that mutant mice have lower intrinsic striatal HDAC activity upon morphine challenge. Altogether, the present results show an essential role of PER1 in regulating morphine-induced sensitization and CPP specifically and indicate a potential mechanism of action via regulation of striatal HDAC activity (Fig. 4).
PER1 potentiates morphine-induced sensitization and CPP via HDAC regulation. Upon morphine administration, PER1 inhibits HDACs thereby preventing the deacetylation of molecular targets involved in the development of locomotor sensitization and reward. In the Per1 Brdm1 mutant mice, the lack of a functional PER1 protein leads to a desinhibition of deacetylase activity avoiding hyperacetylation of these molecular targets (i.e., ERK, CREB as suggested by Li et al. 2008) and thereby preventing morphine-induced locomotor sensitization and reward. HDAC inhibition, and NaBut administration in particular, would circumvent the inhibitory activity of PER1 on histone deacetylase, thereby leading to the recovery of neuroplastic changes responsible for morphine-induced locomotor sensitization and CPP responses in the mutant mice
The differential involvement of Per1 and Per2 genes in drug-induced behaviors had been reported previously. Thus, cocaine sensitization and CPP is abolished in Per1 Brdm1 mice whereas Per2 Brdm1 mutant mice show enhanced cocaine sensitization and CPP (Abarca et al. 2002). In addition, Per2 Brdm1 mutant mice show enhanced alcohol consumption (Spanagel et al. 2005) while Per1 Brdm1 mice do not differ from their wild-type littermates in alcohol consumption under baseline conditions (Zghoul et al. 2007). As well, the gene Per2, as opposed to Per1, seems to be specifically involved in ethanol brain sensitivity (Perreau-Lenz et al. 2009). The present study adds one more dissociation between these clock genes by showing that, conversely to Per2 (Perreau-Lenz et al. 2010), Per1 does not seem to play a role in morphine tolerance or withdrawal but, similarly to what has been observed for cocaine (Abarca et al. 2002), it is essential for morphine-induced locomotor sensitization and CPP. Regarding the latter, it is noteworthy that Sanchis-Segura et al. (2009) observed that Per1, but not Per2, is upregulated in response to a locomotor-sensitizing morphine treatment and that the degree of Per1 expression in the striatum is proportional to that of this behavioral outcome. A differential role of Per1 and Per2 r in drug-induced behaviors is however not surprising. Hence, there is a mechanistically distinct involvement of mPER1 and mPER2 in the regulation of the core clock mechanism (Zheng et al. 2001) and accumulating evidence shows that Per1 and Per2 transcripts regulate different sets of clock-controlled genes, nuclear receptors (Ripperger et al. 2010; Schmutz et al. 2010), or cell metabolism targets (Zocchi and Sassone-Corsi 2010). Therefore, it seems to be that differential pathways are activated/regulated by the PER1 and PER2 proteins. Environmental stimuli, including the application of drugs of abuse, may thus trigger different neuroplastic changes.
The implication of chromatin remodeling in the regulation of the circadian clock genes and protein expression has become more and more evident in recent years (Aguilar-Arnal and Sassone-Corsi 2013; Doi et al. 2006; Eckel-Mahan and Sassone-Corsi 2013; Tamayo et al. 2015). Acetylation of the Per1 promoter results in enhanced Per1 transcription (Naruse et al. 2004) and similar epigenetic self-regulatory mechanisms were identified for other clock genes such as CLOCK (Grimaldi et al. 2007; Hirayama et al. 2007). Sanchis-Segura and colleagues have shown that HDAC inhibition both boosts morphine-induced locomotor sensitization and enhances Per1 gene expression. These data suggest that Per1 expression might be regulated by histone deacetylases. In our NaBut study (Fig. 3), we could replicate the previous observation that NaBut enhances the development of locomotor sensitization in WT mice. We also observed that co-administration of NaBut and morphine could rescue the sensitization and CPP phenotypes observed in Per1 Brdm1 mutant mice. This observation suggests that morphine-induced sensitization and CPP responses are related to PER1 ability to reduce HDAC activity. Furthermore, WT mice showed significantly lower levels of HDAC activity than Per1 Brdm1 mutant mice following repeated morphine injections, which confirms that PER1 inhibits HDAC activity upon morphine challenge and thereby modulates histone acetylation status.
Per1 mRNA expression is affected by opioids in different parts of the brain (Li et al. 2009; Liu et al. 2005; Garmabi et al. 2016; Piechota et al. 2012). Central inhibition of Per1 mRNA expression attenuated morphine-induced ERK-CREB activation within the hippocampus, frontal cortex, and striatum (Li et al. 2008), and Garmabi et al. (2016) have recently revealed that the upregulation of Per1 mRNA expression within the striatum (induced by constant light circadian rhythm desynchrony) is associated with enhanced morphine intake and preference. Moreover, our preliminary data (presented in supplementary material) show that an acute administration of 10 mg/kg morphine increases Per1 mRNA expression within the striatum (Supplementary Fig. 1). Sanchis-Segura and colleagues have revealed that a sub-chronic 20 mg/kg morphine treatment, similar to our protocol (six intermittent injections every other day from ZT6-ZT10), enhances mPer1 mRNA within the striatum. In summary, these studies demonstrate that mPer1 mRNA expression is influenced by acute and repeated morphine treatment. Reciprocally, Liu and colleagues have showed a functional link between PER1 and extracellular signal-regulated kinase (ERK) activity during morphine treatment (Li et al. 2008; Liu et al. 2007) revealing a first mechanistic hint for PER1.
Here, we propose that stimuli able to trigger Per1 expression (such as repeated morphine administration) could lead to a positive feedback mechanism enhancing histone acetylation through Per1-mediated reduction of HDAC activity and a progressive increase in transcription of Per1, and other target genes (Fig. 4). We further propose that upon morphine administration, PER1 inhibits HDACs thereby preventing the deacetylation of molecular targets involved in the development of locomotor sensitization and reward. In the Per1 Brdm1 mutant mice, the lack of a functional PER1 protein leads to a disinhibition of deacetylase activity avoiding acetylation of these molecular targets and thereby preventing morphine-induced locomotor sensitization and CPP. HDAC inhibition and NaBut administration in particular would circumvent the inhibitory activity of PER1 on histone deacetylase, thereby leading to the recovery of neuroplastic changes responsible for morphine-induced locomotor sensitization and CPP in the mutant mice. Such a proposal is in agreement with findings revealing a functional link between clock-genes epigenetic regulation and cell metabolism (Sahar and Sassone-Corsi 2012).
Several studies have revealed a role of HDAC proteins for driving the behavioral effects of different drugs of abuse (Castino et al. 2015; Godino et al. 2015; Kennedy and Harvey 2015; Sanchis-Segura et al. 2009; Wei et al. 2016) and of natural rewards (Lockett et al. 2014). Most recently, Wei and collaborators (2016) have shown that central administration of the HDAC inhibitor trichostatine A is enhancing the expression of morphine-induced behavioral sensitization. Sanchis-Segura et al. have shown that NaBut was able to specifically affect behavioral sensitization and induce changes in mPer1 gene expression. Our results confirm the efficacy of NaBut in boosting behavioral sensitization.
To our knowledge, there is no evidence thus far for a direct binding of PER1 on any HDAC protein. Binding of HDAC on the Per1 gene promotor region has been shown previously in mice (Naruse et al. 2004). Naruse et al. (2004)) have also shown that transcription of Per1 depends on histone acetylation and deacetylation. Moreover, there is increasing evidence for epigenetic remodeling processes of clock proteins in the regulation of aging, inflammation, and cell metabolism (Masri et al. 2015). Clock protein for instance possesses intrinsic histone acetyltransferase activity, which is counterbalanced by NAD(+)-dependent HDAC activity provided by one component of the sirtuins’ family, SIRT1 (Masri and Sassone-Corsi 2014; Grimaldi et al. 2009). Our results show the potential involvement of PER1 in modulating HDAC activity but the mode of action remains to elucidate.
In summary, the present study confirms and extends the role of Per1 in drug-induced behaviors such as CPP and locomotor sensitization. Further, it provides evidence of a functional link between Per1 transcripts and HDACs. Further studies are still needed to identify the mechanisms by which PER1 protein might alter HDAC activity in the striatum or to identify the genes and protein targets affected by the presently identified PER1-HDAC interaction. In this regard, the downstream genes such as those encoding the mu opioid receptor (Hwang et al. 2010; Hwang et al. 2007), and FOS proteins (Sanchis-Segura et al. 2009) known to be upregulated in response to chronic administration of morphine and HDAC inhibitors are likely candidates.
Abbreviations
- HDAC:
-
Histone deacetylase
- CPP:
-
Conditioned place preference
- Per1:
-
Period 1
- NaBut:
-
Sodium butyrate
References
Abarca C, Albrecht U, Spanagel R (2002) Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A 99:9026–9030
Adachi M, Monteggia LM (2009) Synergistic interactions between histone deacetylase inhibitors and drugs of abuse. Neuropsychopharmacology 34:2619–2620
Aguilar-Arnal L, Sassone-Corsi P (2013) The circadian epigenome: how metabolism talks to chromatin remodeling. Curr Opin Cell Biol 25:170–176
Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V (2003) Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Brain Res Mol Brain Res 112:113–125
Beaule C, Swanstrom A, Leone MJ, Herzog ED (2009) Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One 4:e7476
Bellet MM, Sassone-Corsi P (2011) Mammalian circadian clock and metabolism—the epigenetic link. J Cell Sci 123:3837–3848
Castino MR, Cornish JL, Clemens KJ (2015) Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats. PLoS One 10:e0124796
Doi M, Hirayama J, Sassone-Corsi P (2006) Circadian regulator CLOCK is a histone acetyltransferase. Cell 125:497–508
Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke TK, Lourdusamy A, Smolka MN, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essmann F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, Schumann G (2011) Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. Am J Psychiatry 168:1090–1098
Eckel-Mahan K, Sassone-Corsi P (2013) Epigenetic regulation of the molecular clockwork. Prog Mol Biol Transl Sci 119:29–50
Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R (2008) Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron 59:497–508
Falcon E, McClung CA (2009) A role for the circadian genes in drug addiction. Neuropharmacology 56(Suppl 1):91–96
Fogg PC, O'Neill JS, Dobrzycki T, Calvert S, Lord EC, McIntosh RL, Elliott CJ, Sweeney ST, Hastings MH, Chawla S (2014) Class IIa histone deacetylases are conserved regulators of circadian function. J Biol Chem 289:34341–34348
Garmabi B, Vousooghi N, Vosough M, Yoonessi A, Bakhtazad A, Zarrindast MR (2016) Effect of circadian rhythm disturbance on morphine preference and addiction in male rats: involvement of period genes and dopamine D1 receptor. Neuroscience 322:104–114
Godino A, Jayanthi S, Cadet JL (2015) Epigenetic landscape of amphetamine and methamphetamine addiction in rodents. Epigenetics 10:574–580
Grimaldi B, Nakahata Y, Sahar S, Kaluzova M, Gauthier D, Pham K, Patel N, Hirayama J, Sassone-Corsi P (2007) Chromatin remodeling and circadian control: master regulator CLOCK is an enzyme. Cold Spring Harb Symp Quant Biol 72:105–112
Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P (2009) Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol 41:81–86
Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U (2008) Regulation of monoamine oxidase a by circadian-clock components implies clock influence on mood. Curr Biol 18:678–683
Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P (2007) CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450:1086–1090
Hood S, Cassidy P, Mathewson S, Stewart J, Amir S (2011) Daily morphine injection and withdrawal disrupt 24-h wheel running and PERIOD2 expression patterns in the rat limbic forebrain. Neuroscience 186:65–75
Hwang CK, Kim CS, Kim Do K, Law PY, Wei LN, Loh HH (2010) Up-regulation of the mu-opioid receptor gene is mediated through chromatin remodeling and transcriptional factors in differentiated neuronal cells. Mol Pharmacol 78:58–68
Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, Wei LN, Loh HH (2007) Evidence of endogenous mu opioid receptor regulation by epigenetic control of the promoters. Mol Cell Biol 27:4720–4736
Kalda A, Heidmets LT, Shen HY, Zharkovsky A, Chen JF (2007) Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav Brain Res 181:76–84
Kennedy PJ, Harvey E (2015) Histone deacetylases as potential targets for cocaine addiction. CNS & neurological disorders drug targets 14:764–772
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ (2005) Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48:303–314
Li SX, Liu LJ, Jiang WG, Lu L (2009) Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J Neurochem 109:1668–1679
Li SX, Wang ZR, Li J, Peng ZG, Zhou W, Zhou M, Lu L (2008) Inhibition of Period1 gene attenuates the morphine-induced ERK-CREB activation in frontal cortex, hippocampus, and striatum in mice. Am J Drug Alcohol Abuse 34:673–682
Liu Y, Wang Y, Jiang Z, Wan C, Zhou W, Wang Z (2007) The extracellular signal-regulated kinase signaling pathway is involved in the modulation of morphine-induced reward by mPer1. Neuroscience 146:265–271
Liu Y, Wang Y, Wan C, Zhou W, Peng T, Wang Z, Li G, Cornelisson G, Halberg F (2005) The role of mPer1 in morphine dependence in mice. Neuroscience 130:383–388
Lockett GA, Wilkes F, Helliwell P, Maleszka R (2014) Contrasting effects of histone deacetylase inhibitors on reward and aversive olfactory memories in the honey bee. Insects 5:377–398
Logan RW, Williams WP 3rd, McClung CA (2014) Circadian rhythms and addiction: mechanistic insights and future directions. Behav Neurosci 128:387–412
Masri S, Orozco-Solis R, Aguilar-Arnal L, Cervantes M, Sassone-Corsi P (2015) Coupling circadian rhythms of metabolism and chromatin remodelling. Diabetes Obes Metab 17(Suppl 1):17–22
Masri S, Sassone-Corsi P (2014) Sirtuins and the circadian clock: bridging chromatin and metabolism. Sci Signal 7:re6
McClung CA (2007) Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther 114:222–232
McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ (2005) Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A.
Mendlewicz J (2009) Disruption of the circadian timing systems: molecular mechanisms in mood disorders. CNS Drugs 23(Suppl 2):15–26
Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, Graham A, Gordon E, Enwright JF 3rd, Dileone RJ, Birnbaum SG, Cooper DC, McClung CA (2010) Knockdown of clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. doi:10.1016/j.biopsych.2010.04.031
Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M (2004) Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol Cell Biol 24:6278–6287
National Research Council (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research (The National Academies Collection: Reports funded by National Institutes of Health), Washington (DC)
Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, Arey RN, Mukherjee S, Lyons-Weiler J, Self DW, McClung CA (2015) Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry 77:425–433
Parekh PK, McClung CA (2016) Circadian mechanisms underlying reward-related neurophysiology and synaptic plasticity. Frontiers in psychiatry 6:187
Perreau-Lenz S, Sanchis-Segura C, Leonardi-Essmann F, Schneider M, Spanagel R (2010) Development of morphine-induced tolerance and withdrawal: involvement of the clock gene mPer2. Eur Neuropsychopharmacol 20:509–517
Perreau-Lenz S, Spanagel R (2015) Clock genes x stress x reward interactions in alcohol and substance use disorders. Alcohol 49(4):351–357
Perreau-Lenz S, Spanagel R (2008) The effects of drugs of abuse on clock genes. Drug News Perspect 21:211–217
Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A (2009) Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol 14:253–259
Perreau-Lenz S, Zghoul T, Spanagel R (2007) Clock genes running amok. Clock genes and their role in drug addiction and depression. EMBO Rep 8 Spec No: S20–3.
Piechota M, Korostynski M, Sikora M, Golda S, Dzbek J, Przewlocki R (2012) Common transcriptional effects in the mouse striatum following chronic treatment with heroin and methamphetamine. Genes Brain Behav. doi:10.1111/j.1601-183X.2012.00777.x
Ripperger JA, Albrecht U (2012) REV-ERB-erating nuclear receptor functions in circadian metabolism and physiology. Cell Res 22:1319–1321
Ripperger JA, Schmutz I, Albrecht U (2010) PERsuading nuclear receptors to dance the circadian rhythm. Cell Cycle 9
Rosenwasser AM (2010) Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev 34:1249–1255
Sahar S, Sassone-Corsi P (2012) Circadian rhythms and memory formation: regulation by chromatin remodeling. Front Mol Neurosci 5:37
Sanchis-Segura C, Lopez-Atalaya JP, Barco A (2009) Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology 34:2642–2654
Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U (2010) The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev 24:345–357
Shen HY, Kalda A, Yu L, Ferrara J, Zhu J, Chen JF (2008) Additive effects of histone deacetylase inhibitors and amphetamine on histone H4 acetylation, cAMP responsive element binding protein phosphorylation and DeltaFosB expression in the striatum and locomotor sensitization in mice. Neuroscience 157:644–655
Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, Maloney T, Wong C, Volkow ND (2012) Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Transl Psychiatry 2:e86
Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U (2005) The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11:35–42
Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV (2012) Butyrate histone deacetylase inhibitors. Biores Open Access 1(4):192–198
Takizawa T, Meshorer E (2008) Chromatin and nuclear architecture in the nervous system. Trends Neurosci 31:343–352
Tamayo AG, Duong HA, Robles MS, Mann M, Weitz CJ (2015) Histone monoubiquitination by Clock-Bmal1 complex marks Per1 and Per2 genes for circadian feedback. Nat Struct Mol Biol 22:759–766
Volkow ND, McLellan (2016) Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 374:1253–1263
Wei L, Zhu Y-M, Zhang Y-X, Liang F, Barry DM, Gao H-Y, Li T, Huo F-Q, Yan C-X (2016) Microinjection of histone deacetylase inhibitor into the ventrolateral orbital cortex potentiates morphine induced behavioral sensitization. Brain Res 1646:418–425. doi:10.1016/j.brainres.2016.06.019
Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R (2007) Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology 190:13–19
Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC (2001) Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105:683–694
Zocchi L, Sassone-Corsi P (2010) Joining the dots: from chromatin remodeling to neuronal plasticity. Curr Opin Neurobiol. doi:10.1016/j.conb.2010.04.005
Acknowledgements
We would like to thank sincerely Dr. Taline V. Khroyan for her scientific input and editing advice. We would like to thank Sarah Leixner, Madeleine Saita Claudia Schaeffer for breeding and genotyping the animals. S.P.-L. is supported by the NIAAA (Grant 1R21 AA0023078-01) and by a NARSAD Young Investigator Grant Award 2014 from the Brain & Behavior Research Foundation. All authors gave their informed consent to be included in the present study and have no financial conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Perreau-Lenz, S., Hoelters, LS., Leixner, S. et al. mPer1 promotes morphine-induced locomotor sensitization and conditioned place preference via histone deacetylase activity. Psychopharmacology 234, 1713–1724 (2017). https://doi.org/10.1007/s00213-017-4574-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4574-0