Abstract
Rationale
Repeated intermittent exposure to ketamine has rapid and long-lasting antidepressant effects, but the abuse potential has only been assessed at high doses. Furthermore, while females are more susceptible to depression and more sensitive to ketamine’s antidepressant-like effects, the abuse potential for ketamine in females is unknown.
Objectives
The objectives of this study are to determine the reinforcing properties of low-dose intermittent ketamine in adult rats of both sexes and determine whether cycling gonadal hormones influence females’ response to ketamine. In male rats, we also aimed to determine whether reinstatement to intermittent ketamine is comparable to intermittent cocaine.
Methods
Male rats intravenously self-administered cocaine (0.75 mg/kg/infusion) or ketamine (0.1 mg/kg/infusion) once every fourth day, while intact cycling female rats self-administered ketamine only during preidentified stages of their 4-day estrus cycle, when gonadal hormones are either high (proestrus) or low (diestrus). After acquiring self-administration, rats underwent daily extinction training followed by cue-primed and drug-primed reinstatement to assess drug-seeking behavior.
Results
Diestrus-trained females fail to maintain ketamine self-administration and did not display reinstatement to ketamine-paired cues. Males and proestrus-trained females reinstated to ketamine-paired cues. Ketamine-primed reinstatement was dependent on simultaneous cue presentation. Male rats reinstated to cocaine priming independent of cue presentation.
Conclusion
These findings indicate that females’s responsivity to this dose of ketamine depends on stage of cycle, as only proestrus-trained females and males respond to ketamine’s reinforcing effects under this treatment paradigm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine has a wide variety of clinical applications. While high doses are frequently used as an anesthetic in veterinary and pediatric applications, acute subanesthetic ketamine produces rapid alleviation of major depression symptoms (Berman et al. 2000; Trujillo et al. 2011; Zarate et al. 2006). These findings were groundbreaking because conventional antidepressants that target monoaminergic neurotransmission take several weeks to show an effect, have many undesirable side effects, and are ineffective in a subset of patients. Furthermore, repeated ketamine infusions given intermittently (two to three times a week) under clinical supervision greatly prolong the antidepressant effect (aan het Rot et al. 2010; Murrough et al. 2013). However, illicit and chronic ketamine use is well known for its habit-forming properties, and its abuse is on the rise in many parts of the world (Jansen and Darracot-Cankovic 2001; Morgan and Curran 2012). Recreational users report using the drug intermittently and almost exclusively in novel contexts, such as at raves during the weekend (Jansen 2000). Therefore, there is a critical need to characterize the abuse potential of low-dose, intermittently administered ketamine to better understand how its effects differ from higher doses and to test the safety of repeated infusions.
Preclinical literature using the intravenous self-administration model indicates that male rats will readily self-administer ketamine (Collins and Woods 2007; De Luca and Badiani 2011; van der Kam et al. 2007; Venniro et al. 2015), as will non-human primates (Carroll and Stotz 1983; McCarthy and Harrigan 1976; Young and Woods 1981). The ability of an animal to acquire and maintain ketamine self-administration indicates that the drug may have abuse potential in humans. However, the aforementioned studies used higher doses and daily training sessions, with the exception of Venniro et al. (2015). It still stands that lower doses of ketamine need to be tested and to test for drug-seeking behavior via reinstatement testing after a period of drug-free extinction. The reinstatement model is commonly used to study factors underlying relapse, an insidious characteristic of drug addiction in humans (De Wit and Stewart 1981; Shaham et al. 2003).
Another important factor that has not been sufficiently addressed is the possibility of sex differences in ketamine’s reinforcing properties. In humans, females are more sensitive to ketamine’s withdrawal symptoms (Chen et al. 2014), and preclinical research from our lab and others has shown that female rodents respond to lower doses of ketamine than males in measures of antidepressant-like effects (Carrier and Kabbaj 2013; Franceschelli et al. 2015; Zanos et al. 2016). While ketamine’s abuse potential and reinforcing properties have not been addressed in female rats, a heightened responsiveness to cocaine (Lynch and Carroll 1999, 2000; Swalve et al. 2016), opiates (Cicero et al. 2003; Lynch and Carroll 1999), nicotine (Swalve et al. 2016), cannabinoids (Fattore et al. 2007), and phencyclidine (Carroll et al. 2005; Carroll et al. 2000) have been observed using different self-administration paradigms. Naturally, cycling gonadal hormones may contribute to females’ responsiveness to drugs of abuse, as rats are more sensitive to cocaine during estrus and less responsive during diestrus (Lynch and Carroll 2000). Based on these findings, we hypothesized that females would demonstrate an increased responsiveness to this low dose of ketamine (0.1 mg/kg/infusion) during proestrus (when gonadal hormones are high), and a decreased response when hormones are low (diestrus), compared to males. Additionally, we compared males that have self-administered cocaine either once every fourth day, or daily, to show that a drug with known addictive properties will still induce reinstated drug-seeking behavior when self-administered only once every fourth day.
Materials and methods
Drugs
Ketamine hydrochloride (Ketasthesia®, racemic, Henry Schein) was diluted in 0.9% sterile saline from a 100 mg/mL solution. Cocaine hydrochloride (a gift from NIDA) was dissolved in 0.9% sterile saline. For self-administration experiments, ketamine was administered intravenously at a dose of 0.1 mg/kg/infusion in a 50-μL volume. Cocaine was administered intravenously at 0.75 mg/kg/infusion in a 50-μL volume. For reinstatement experiments, ketamine was injected at a dose of 2.5 mg/kg and cocaine was injected at a dose of 10 mg/kg. Both were administered in a volume of 1 mL/kg, intraperitoneally (i.p.), immediately before being placed in the operant conditioning chambers for the drug-primed reinstatement sessions. This dose of ketamine equates to the therapeutic range previously used to assess antidepressant-like effects of ketamine (Carrier and Kabbaj 2013), and the dose of cocaine has been used previously to trigger cocaine-seeking behaviors after daily self-administration and extinction (Wright et al. 2015).
Subjects
Sixty adult Sprague-Dawley rats (40 males initially weighing 226–250 g and 20 females initially weighing 161–180 g) from Charles River (Raleigh, NC) were used in these experiments. Male and female cohorts were tested separately and housed in separate cubicles throughout the duration of the experiment. Upon arrival, rats were double-housed in 43 × 21.5 × 25.5-cm Plexiglas cages on a 12/12-h reverse light/dark cycle, and food and water were provided ad libitum except during behavioral testing. After a period of 5–6 days of acclimation, females underwent daily vaginal lavage to determine stage of estrous cycle, for two complete cycles before behavioral testing (Becker et al. 2005; Saland et al. 2016). Once cyclicity was established, lavage was carried out every day, no more than 2 h before behavioral testing. Stage of cycle was based on microscopic observation of cell morphology and abundance, as we have previously described (Saland et al. 2016; Stack et al. 2010). Males were handled daily. Immediately following catheterization, all rats were single-housed for the duration of the experiment. Operant training was conducted at least 1 h after the onset of the dark cycle. All experiments were carried out in accordance to the NIH Guide for Care and Use of Laboratory Animals (National Research Council 2011), and all protocols were approved by the Florida State University Institutional Animal Care and Use Committee.
Jugular catheterization and patency test
Surgery was carried out as we have previously described (Wright et al. 2015), with some modifications. Briefly, rats were anesthetized with isoflurane at a rate of 4% for induction and 1–2% for maintenance at 2 L/min, and a heating pad set to approximately 37 °C was used during the procedure and post-operative monitoring. Instech Vascular Access Harnesses (Plymouth Meeting, PA) were connected to indwelling intravenous catheters, which were installed on the right external jugular vein and flushed daily with 0.2-mL heparinized saline (50 U/mL). Patency was tested once a week only on days when they would not have a self-administration session. Xylazine (2.5 mg/kg at a volume of 0.05 mL) was used to test patency, followed by 0.1-mL heparinized saline. If rapid loss of muscle tone was not observed, the catheter was considered to no longer be patent, and a new catheter was installed on the left jugular vein. If a rat failed a second patency test, the rat was removed from the experiment and all data up to that point was not included in the final statistical analyses. Rats were given 3–5 days of post-operative recovery before beginning self-administration training. Ten rats and their data were removed from the study due to failed catheters, 4 diestrus females, 2 proestrus females, and 1 male from experiment 1 and 3 males from experiment 2.
Equipment and operant procedures
Locomotor testing was conducted in a donut-shaped arena 71.2 cm in diameter with four photo-beam sensors and a gridded floor (Med Associates). Self-administration sessions were conducted in operant chambers (30.5 × 24.1 × 21.0 cm; Med Associates) that were housed in sound-attenuating cabinets. Each operant chamber contained a food pellet dispenser and house light on one wall of the operant chamber and two nose-poke holes equipped with lights on the inside on the opposite wall. Syringe pumps (Med Associates, 3.33 rpm, single speed) were housed outside of each cabinet, a tether connected the drug delivery line to the rat’s catheter, and a single-channel 22-ga plastic swivel (Instech Labs) was used to allow full range of motion of the tethered rat. At the beginning of the operant session, the house light would turn on, indicating availability of the drug. Selecting the active hole resulted in the delivery of the reinforcement (initially, sucrose pellets via the pellet dispenser and finally, drug infusions via the syringe pump) paired with a cue light on the inside of the nose-poke hole that remained lit for 20 s. During this 20-s timeout period, the house light remained off and nose pokes were not reinforced, although they were still tallied. Rats self-administered at a fixed-ratio one (FR1) schedule of reinforcement. Selecting the inactive nose-poke hole had no programmed consequences. The number of pellets or infusions, active nose pokes, and inactive nose pokes were counted using Med Associates software. At the end of each drug self-administration session, catheters were flushed with 0.1-mL heparinized saline and rats were returned to their home cages.
Experimental procedures
Novelty response
All rats underwent a 1-h locomotor test as described previously (Wright et al. 2015). Rats were classified as high responders or low responders based on the group median locomotor score to balance group assignment, as individual differences in locomotor activity in a novel environment can influence response to drugs of abuse (Kabbaj 2006). No further analyses were conducted.
Operant training
Rats began operant training with 45-mg sucrose pellets using the procedure described above. The session lasted for 1 h or until 100 pellets were self-administered, whichever occurred first. Rats underwent daily sessions until they reached the minimum criteria of less than 30% variability in active nose pokes between the last two sessions and higher than 70% active than inactive nose pokes. After reaching criteria, they underwent catheterization and began intravenous self-administration procedures.
Experiment 1: ketamine self-administration, extinction, and reinstatement
Once catheterized, male and female rats self-administered 0.1 mg/kg/infusion ketamine during a 2-h session with 50 maximum possible infusions. Selection of the active nose-poke hole would result in an i.v. infusion of 0.1 mg/kg/infusion ketamine. Males were trained once every fourth day. Females were trained only during either proestrus or diestrus, and therefore, they were trained on average once every 3.94 days to accompany their cycle. All rats self-administered for 10 sessions under an FR1 schedule of reinforcement. On days when rats were not scheduled to train, they remained in their home cage and were handled regularly during catheter flushing and/or vaginal lavage.
After 10 sessions of self-administration, rats began extinction training, consisting of 2-h daily sessions, where the syringe pump was turned off, the house light was off, and both active and inactive nose poking had no programmed consequences. Once rats reached criteria for extinction (eight sessions minimum, 70% decrease in active nose pokes from the last 2 days of acquisition, <20% variability in active nose pokes across the last two sessions of extinction), they underwent a cue-primed reinstatement session, where drug-paired cues (which included both the houselight and the cue light) were returned to the operant chamber and rats were tethered, but no intravenous drug was available for infusion. This session was followed by two to four daily sessions of extinction, which was sufficient to return all rats’ responding to levels equivalent to prereinstatement levels. Then, drug-primed reinstatement was tested where ketamine (2.5 mg/kg, i.p.) was administered immediately before the session. The first drug-primed reinstatement session was conducted in the absence of tethering and drug-paired cues. As no reinstated drug-seeking behavior was observed during this session (see “Results” section), all rats then underwent another ketamine-primed reinstatement session where drug-paired cues were returned to the operant chamber and rats were tethered, but no intravenous drug was available for infusion. Females underwent reinstatement testing in the same stage of cycle they trained in during acquisition.
Experiment 2: cocaine self-administration, extinction, and reinstatement
A separate cohort of males was used to test that cocaine self-administered once every fourth day will induce drug-seeking behavior. The time frame for experiment 2 was identical to experiment 1. After sucrose pellet training and catheterization, male rats self-administered cocaine (0.75 mg/kg/infusion) at an FR1 schedule of reinforcement once every fourth day for 10 sessions. Ten daily extinction sessions followed, where the houselight and cue light were absent and the syringe pump was turned off, but active and inactive nose-poke selections were recorded. After extinction, drug-seeking behavior was tested, first by cue-primed reinstatement, then by cocaine-primed reinstatement. All rats received an injection of cocaine (10 mg/kg i.p.) before testing, first in the absence of cues, then in the presence of cues, as was carried out in experiment 1. At least 2 days of extinction sessions were conducted in between each reinstatement test, as this was sufficient to return all rats’ responding to levels comparable to extinction before reinstatement. Additionally, a separate cohort of male rats self-administered cocaine (0.75 mg/kg/infusion) daily under an FR1 schedule for 10 sessions. This experiment was carried out as a comparison to cocaine self-administered every fourth day.
Statistical analysis
Ketamine self-administration data were analyzed by mixed-model ANOVA, with sex/cycle (male, proestrus, diestrus) as the between-subject factor and sessions as the within-subject factor. Ketamine reinstatement sessions were analyzed by mixed-model ANOVA, with sex/cycle as the between-subject factor and session (extinction vs reinstatement session) as the within-subject factor. Cocaine self-administration data were analyzed by mixed-model ANOVA with nose poking (active or inactive) and sessions as within-subject factors. For all mixed ANOVA tests, the random effects included variation of individual animals, while fixed effects included sex/cycle, session, and active or inactive nose poking, where appropriate. Active nose pokes during cocaine reinstatement sessions were compared to the previous extinction session by paired t test. Significance was set at α = 0.05. The Holm-Bonferroni method (Holm 1979) was used to adjust p values of family-wise comparisons within each figure. GraphPad Prism 6.0 and R (3.3.1) with the nlme package (Pinheiro et al. 2016) were used for all statistical analyses and figures.
Results
The last 3 days of sucrose pellet training were analyzed due to the fact that some rats required 4 or 5 days of training to reach criteria for advancement. All cohorts acquired sucrose pellet training similarly (data not shown). Statistical analysis comparing sugar pellets received by males and females in experiment 1 showed an interaction between session and sex (F (1, 44) = 14.44, p = 0.0004). Post hoc analysis revealed a significant difference on the first session analyzed (p = 0.042), but importantly, there were no differences between males and females on subsequent sessions. Therefore, males and females acquired this operant task similarly.
Experiment 1: ketamine self-administration, extinction, and reinstatement
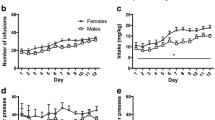
After catheterization, male and female rats underwent 10 sessions of ketamine self-administration under an FR1 schedule, once every fourth day. Figure 1a shows the number of infusions taken by males, proestrus-trained females, and diestrus-trained females. An interaction between sex/cycle and session was observed (F (2, 204) = 6.77, p = 0.0014). Upon examination of the parameter estimates, diestrus females experienced a decrease of 2.1 infusions per session, compared to proestrus (0.2) and males (0.8). Taken together with the intercepts, the model indicates that diestrus females failed to acquire and maintain ketamine self-administration compared to levels observed in proestrus females and males.
Experiment 1, acquisition and extinction of ketamine self-administration once every fourth day. a Number of infusions received during ten 2-h sessions in males, diestrus-trained females, and proestrus-trained females. Diestrus females had a significantly lower intake than males and proestrus females. b Above, active nose pokes during acquisition and daily extinction training; below, inactive nose pokes. Data are presented as mean + SEM, n = 6–9 per group
Daily extinction training followed acquisition. Some females reached criteria for extinction early and therefore moved on to the next phase of the experiment sooner in order to catch them in the cycle they were trained in for acquisition. Therefore, only the first eight extinction sessions for both males and females were analyzed. Figure 1b shows the active (above) and inactive (below) nose pokes for males, proestrus-trained females, and diestrus-trained females during both acquisition and extinction phases. There was a significant interaction between active/inactive nose poking and sex/cycle (F (2, 339) = 10.318, p < 0.0001). Examination of the parameter estimates of the model revealed that diestrus-trained females had 0.1 fewer active than inactive nose pokes, proestrus-trained females had 3.7 more active nose pokes than inactive, and males had 6.8 more active than inactive nose pokes. It appears that while the model describes some differences between groups, there was no main effect of session, indicating that across the course of extinction, their response was stable.
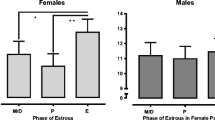
Drug-paired cues (the houselight and the cue light) were returned to the operant chambers for a single cue-primed reinstatement test, shown in Fig. 2a. An interaction between sex/cycle and session was observed (F (2, 20) = 5.20, p = 0.015). Post hoc analyses revealed that males and proestrus females had higher active nose pokes compared to extinction (p = 0.0001 for males, trending for proestrus with p = 0.07, respectively), but this was not observed in diestrus females (p = 0.821). Furthermore, diestrus females were significantly lower than males (p = 0.005), while there were no differences between males and proestrus females. Therefore, while males reliably reinstate to cue-primed drug seeking after intermittent ketamine self-administration, females’ reduced level of drug-seeking behavior is driven primarily by females that trained and tested in diestrus.
Experiment 1, cue- and ketamine-primed reinstatement following extinction from ketamine self-administered once every fourth day. a Active nose pokes during cue-primed reinstatement. b Ketamine-primed reinstatement (2.5 mg/kg, i.p.) in the absence of drug-paired cues. c Ketamine-primed reinstatement in the presence of cues. Data are presented as mean + SEM. Ext refers to the extinction session prior to each respective reinstatement session. Ket refers to ketamine. In c, *p < 0.05, main effect of session D, n = 6–9 per group
After 2–4 days of extinction (sufficient to return rats to levels of nose pokes not different from prereinstatement levels and to test females in their correct stage of cycle), a 2.5-mg/kg injection of ketamine was given to each rat before a session with no cue presentation to determine if ketamine alone would reinstate drug-seeking behaviors, seen in Fig. 2b. There was a main effect of sex/cycle (F (2, 20) = 8.016, p = 0.003) and a trend for session (F (1, 22) = 4.158, p = 0.053) but no interaction. Post hoc analysis revealed significant differences between groups on the last extinction session (p = 0.0003, 0.039 for males vs diestrus and proestrus vs diestrus, respectively). Males had a significantly lower response during ketamine-primed reinstatement than their last extinction session (p = 0.026), while there were no differences in active nose pokes from diestrus or proestrus females, indicating that ketamine alone is not sufficient to reinstate drug-seeking behavior. Following this session, rats were then retested with both 2.5 mg/kg ketamine and the presentation of cues during the session, shown in Fig. 2c. A main effect of session was observed (F (2, 22) = 8.229, p = 0.009), but no main effect of group or an interaction was observed, indicating an overall increase in active nose pokes. This increase in active nose pokes is indicative of drug-seeking behavior in response to 2.5 mg/kg ketamine, but only when cues were also present, suggesting that ketamine-seeking behavior is dependent on the presentation of drug-paired cues.
Experiment 2: cocaine self-administration, extinction, and reinstatement
Results of males’ cocaine acquisition once every fourth day are shown in Fig. 3a and once every day are shown in Fig. 3b. Both groups maintain stable levels of infusions through ten sessions, whether daily or once every fourth day. Active and inactive nose pokes during acquisition once every fourth day and extinction are shown in Fig. 3c. Acquisition and extinction were analyzed separately. There was a significant main effect of session and nose pokes (F (1, 150) = 7.367, p = 0.0074; F (1, 150) = 13.280, p = 0.0004, respectively), but not an interaction during acquisition, indicating a significant difference between active and inactive nose pokes across the duration of acquisition. During extinction, a significant interaction between nose pokes and session was observed (F (1, 149) = 9.577, p = 0.0024). By observing Fig. 3c, it is clear that inactive and active nose pokes were initially different (i.e., session 1) but rapidly become indistinguishable, indicative of extinction.
Experiment 2, acquisition of cocaine self-administration daily or once every fourth day in males, followed by extinction and reinstatement. a Number of infusions received during ten 2-h sessions by males that self-administered cocaine once every fourth day. b Number of infusions received by males with daily cocaine self-administration. c Active and inactive nose pokes during once every fourth day acquisition and daily extinction training. d Cue-primed reinstatement, e cocaine-primed reinstatement in the absence of cues, and f cocaine-primed reinstatement in the presence of cues. Ext refers to the last extinction session prior to each respective reinstatement session. Coc refers to cocaine. Data are presented as mean + SEM. In c, ***p < 0.001, main effect of nose pokes; in d–f, *p < 0.05 compared to extinction session, n = 5–8 per group
Drug-paired cues were returned to the operant chamber to test for cue-primed reinstatement, shown in Fig. 3d. There was a significant increase in active nose pokes between the two trials (t (7) = 5.856, p = 0.002). Cocaine-primed reinstatement in the absence of cues is shown in Fig. 3e. There was a significant increase in active nose pokes between the two trials (t (7) = 2.877, p = 0.028). Cocaine-primed reinstatement was then tested in the presence of cues, shown in Fig. 3f. There was a significant increase in active nose pokes between the two trials (t (7) = 3.268, p = 0.028). Taken together, as expected, cocaine self-administering male rats reliably reinstated to cue presentation and cocaine exposure with or without simultaneous cue presentation, indicating that cocaine self-administered once every fourth day is sufficient to reinstate drug-seeking behavior after a period of extinction.
Discussion
The purpose of this study was to characterize the abuse potential and reinforcing properties of low-dose, intermittent ketamine (0.1 mg/kg/infusion, once every fourth day) using an intravenous self-administration paradigm. Female rats that only trained during diestrus failed to acquire ketamine self-administration and rapidly decrease their intake, compared to proestrus-trained females and males. After extinction, both males and proestrus females reinstated ketamine-seeking behavior upon ketamine reexposure, but only when drug-paired cues were also present, as ketamine in the absence of cues did not reinstate drug seeking. Furthermore, males and proestrus-trained (but not diestrus-trained) females displayed ketamine-seeking behavior upon presentation of drug-paired cues alone (i.e., without ketamine). Finally, males that self-administered cocaine once every fourth day had similar rates of infusions as males that self-administered cocaine daily. They also robustly reinstated drug-seeking behavior upon reexposure to cocaine.
This study aims to characterize the reinforcing properties of intermittent ketamine exposure in both male and female rats at 0.1 mg/kg/infusion. Several other preclinical studies have demonstrated that self-administration of ketamine at higher doses, as well as similar drug analogs, can be readily acquired (Botanas et al. 2015; Collins and Woods 2007; De Luca and Badiani 2011; Huang et al. 2015; van der Kam et al. 2007), but these studies utilized daily self-administration paradigms, and they do not include females in their experimental designs. Our results build upon these findings, showing that male and female rats in proestrus will acquire response at FR1, even when self-administering a low dose of ketamine (0.1 mg/kg/infusion) once every fourth day. Additionally, this level of exposure is sufficient to reinstate drug-seeking behavior. There is limited research on intermittent ketamine exposure. Trujillo et al. (2008) was the first to show that weekly, repeated, non-contingent exposure to ketamine at 20 and 50 mg/kg resulted in an augmented locomotor response known as sensitization in male rats, which is indicative of heightened activation of the reward circuitry and predictive of abuse potential. More recently, Venniro et al. (2015) demonstrated that males maintained similar levels of intake with weekly self-administration of 0.5 mg/kg/infusion ketamine as they do daily. Our results complement these findings not only by assessing different facets of reinstatement, which has not been tested before, but also by subjecting rats to shorter time periods between sessions, which reflect the typical length of time between administrations in human clinical populations. In addition, it allows us to take advantage of the 4-day estrous cycle in female rats to tease apart the contribution of gonadal hormones to ketamine-seeking behavior.
Previous work from our lab indicates that the gonadal hormones estradiol and progesterone are responsible for female rats’ increased sensitivity to the antidepressant-like effects of ketamine (Carrier and Kabbaj 2013). In our study, we found that females trained and tested in diestrus did not acquire ketamine self-administration at this dose nor did they display reinstated drug seeking. Contrastingly, both males and females trained and tested in proestrus acquire self-administration of ketamine and display drug-seeking behavior. These results are not surprising because others have shown that gonadal hormones influence females’ self-administration of other drugs of abuse such as cocaine (Lynch and Carroll 2000), heroin (Lynch and Carroll 1999), and methamphetamine (Cox et al. 2013). Future research to understand the interaction of gonadal hormone fluctuations and ketamine is warranted.
When rats were challenged with an injection of 2.5 mg/kg ketamine (a dose that exerts antidepressant-like effects in females but is subthreshold for males (Carrier and Kabbaj 2013)), this alone (i.e., without simultaneous cue presentation) was not sufficient to reinstate ketamine-seeking behavior. However, when a challenge injection is followed by a session with cue presentation, both are sufficient to reinstate ketamine-seeking behavior (Fig. 2c). Our data complements previous findings showing that the reinforcing effects of ketamine are tied to the drug-paired environment. Male rats will not self-administer ketamine in the absence of cues, indicating that the acute subjective effects of ketamine alone are not reinforcing (Venniro et al. 2015). Furthermore, males self-administered more ketamine in novel cages compared to their home cage (De Luca and Badiani 2011). This preference for novel drug-paired environments is observed in human ketamine users too, as users report taking ketamine in novel, non-residential environments to enhance an experience (Morgan and Curran 2012). Comparing our ketamine results with males that self-administered cocaine under a similar regimen, we show that males will maintain cocaine self-administration when only given access once every fourth day as they do with ketamine. However, these rats did reinstate cocaine-seeking behavior when reexposed to cocaine in the absence of drug-paired cues, whereas ketamine alone did not reinstate ketamine-seeking behavior in males. This speaks to the known salient reinforcing properties of cocaine, even when exposed non-contingently by an experimenter, and suggests that ketamine on its own does not possess as strong of an incentive salience as cocaine in males. Future studies are required to characterize female rats’ propensity to reinstate drug-seeking behaviors under a similar time frame of exposure to cocaine and other drugs of abuse.
It is important to note that while the dose of ketamine used to assess drug-seeking behavior in this study is similar to what is used in preclinical studies assessing ketamine’s antidepressant-like effects (Carrier and Kabbaj 2013), testing multiple doses for the priming injection will provide more insight into cycle- and sex-dependent differences in sensitivity to ketamine. Additionally, more doses need to be tested for acquisition, as it is still unknown whether females would increase their intake or have a greater propensity to reinstate relative to males at higher or lower doses. This is the first study, to date, to investigate the reinforcing properties of ketamine self-administered at a low dose (0.1 mg/kg/infusion), intermittently (once every fourth day), in males and intact cycling females using an extinction-reinstatement paradigm. We conclude that the estrous cycle contributes to the increased propensity to reinstate ketamine-seeking behavior in proestrus females at this dose, possibly via gonadal hormone fluctuations. Additionally, since both males and proestrus-trained females readily self-administer and reinstate to ketamine- and drug-paired cues, even low doses of chronic ketamine must be carefully studied before considering its therapeutic potential.
References
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67(2):139–145. doi:10.1016/j.biopsych.2009.08.038
Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, ... Young E (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146(4): 1650–1673. doi:10.1210/en.2004-1142
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354
Botanas CJ, de la Pena JB, Dela Pena IJ, Tampus R, Yoon R, Kim HJ et al (2015) Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: evidence of its abuse potential. Pharmacol Biochem Behav 133:31–36. doi:10.1016/j.pbb.2015.03.007
Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. doi:10.1016/j.neuropharm.2012.12.009
Carroll ME, Batulis DK, Landry KL, Morgan AD (2005) Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology 180(3):414–426. doi:10.1007/s00213-005-2182-x
Carroll ME, Roth ME, Voeller RK, Nguyen PD (2000) Acquisition of oral phencyclidine self-administration in rhesus monkeys: effect of sex. Psychopharmacology 149(4):401–408
Carroll ME, Stotz DC (1983) Oral d-amphetamine and ketamine self-administration by rhesus monkeys: effects of food deprivation. J Pharmacol Exp Ther 227(1):28–34
Chen WY, Huang MC, Lin SK (2014) Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 9:39. doi:10.1186/1747-597X-9-39
Cicero TJ, Aylward SC, Meyer ER (2003) Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74(3):541–549
Collins GT, Woods JH (2007) Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther 323(2):599–605. doi:10.1124/jpet.107.123042
Cox BM, Young AB, See RE, Reichel CM (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38(10):2343–2353. doi:10.1016/j.psyneuen.2013.05.005
De Luca MT, Badiani A (2011) Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology 214(2):549–556. doi:10.1007/s00213-010-2062-x
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75(2):134–143
Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W (2007) Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152(5):795–804. doi:10.1038/sj.bjp.0707465
Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60. doi:10.1016/j.neuroscience.2015.01.008
Holm S (1979) A simple sequential rejective method procedure. Scand J Stat 6:65–70
Huang X, Huang K, Zheng W, Beveridge TJ, Yang S, Li X ... Liu Y (2015) The effects of GSK-3beta blockade on ketamine self-administration and relapse to drug-seeking behavior in rats. Drug Alcohol Depend 147: 257–265. doi:10.1016/j.drugalcdep.2014.10.028
Jansen KL (2000) A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs 32(4):419–433. doi:10.1080/02791072.2000.10400244
Jansen KL, Darracot-Cankovic R (2001) The nonmedical use of ketamine, part two: a review of problem use and dependence. J Psychoactive Drugs 33(2):151–158. doi:10.1080/02791072.2001.10400480
Kabbaj M (2006) Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets 5(5):513–520
Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144(1):77–82
Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology 148(2):196–200
McCarthy D, Harrigan S (1976) Dependence producing capacity of ketamine in Macaca mulatta. Anaesthesiology 399:160–168
Morgan CJ, Curran HV (2012) Ketamine use: a review. Addiction 107(1):27–38. doi:10.1111/j.1360-0443.2011.03576.x
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74(4):250–256. doi:10.1016/j.biopsych.2012.06.022
National Research Council (2011) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US). doi:10.17226/12910
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: linear and nonlinear mixed effects models. R package version 3.1–128, http://CRAN.R-project.org/packages=nlme
Saland SK, Schoepfer KJ, Kabbaj M (2016) Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep 6:21322. doi:10.1038/srep21322
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168(1–2):3–20. doi:10.1007/s00213-002-1224-x
Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M (2010) Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology 35(2):570–580. doi:10.1038/npp.2009.163
Swalve N, Smethells JR, Carroll ME (2016) Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology 233(6):1005–1013. doi:10.1007/s00213-015-4183-8
Trujillo KA, Smith ML, Sullivan B, Heller CY, Garcia C, Bates M (2011) The neurobehavioral pharmacology of ketamine: implications for drug abuse, addiction, and psychiatric disorders. ILAR J 52(3):366–378. doi:10.1093/ilar.52.3.366
Trujillo KA, Zamora JJ, Warmoth KP (2008) Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry 63(2):178–183. doi:10.1016/j.biopsych.2007.02.014
van der Kam EL, de Vry J, Tzschentke TM (2007) Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol 18(8):717–724. doi:10.1097/FBP.0b013e3282f18d58
Venniro M, Mutti A, Chiamulera C (2015) Pharmacological and non-pharmacological factors that regulate the acquisition of ketamine self-administration in rats. Psychopharmacology 232(24):4505–4514. doi:10.1007/s00213-015-4077-9
Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC ... Kabbaj M (2015) Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci 35(23): 8948–8958. doi:10.1523/jneurosci.5227-14.2015
Young AM, Woods JH (1981) Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther 218(3):720–727
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI … Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604): 481–486. doi: 10.1038/nature17998
Zarate CA Jr., Singh JB, Carlson PL, Brutsche NE, Ameli R, Luckenbaugh DA ... Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8): 856–864. doi:10.1001/archpsyc.63.8.856
Acknowledgements
This research was supported by R01MH 087583 and R01MH 099085 to MK. Special thanks to Samantha Pavlock, Elsa Johnson, and Amanda Dossat for their technical assistance. KNW and MK designed the experiments. KNW, CES, and MNA carried out the behavioral testing. KNW, NCB, and MK performed the data analysis. KNW and MK wrote the manuscript. All authors provided editorial input for the final draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols were approved by the Florida State University Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wright, K.N., Strong, C.E., Addonizio, M.N. et al. Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology 234, 393–401 (2017). https://doi.org/10.1007/s00213-016-4470-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4470-z